Abstract
Study Objectives:
Insomnia is prevalent in patients with chronic obstructive pulmonary disease (COPD), and benzodiazepine receptor agonists (BZRAs) are the most commonly used drugs despite their adverse effects on respiratory function. The aim of this study was to investigate whether the use of BZRAs was associated with an increased risk of respiratory failure (RF) in COPD patients.
Design:
Matched case-control study.
Setting:
National Health Insurance Research Database (NHIRD) in Taiwan.
Participants:
The case group consisted of 2,434 COPD patients with RF, and the control group consisted of 2,434 COPD patients without RF, matched for age, sex, and date of enrollment.
Measurements and Results:
Exposure to BZRAs during the 180-day period preceding the index date was analyzed and compared in the case and control groups. Conditional logistic regression was performed, and the use of BZRAs was associated with an increased risk of RF (adjusted odds ratio [aOR] 1.56, 95% confidence interval [CI] 1.14–2.13). In subgroup analysis, we found that the benzodiazepine (BZD) users had a higher risk of RF (aOR 1.58, 95% CI 1.14–2.20), whereas the risk in non-benzodiazepine (non-BZD) users was insignificant (aOR 0.85, 95% CI 0.51–1.44). A greater than 2-fold increase in risk was found in those who received two or more kinds of BZRAs and those using a combination of BZD and non-BZD medications.
Conclusions:
The use of benzodiazepine receptor agonists was a significant risk factor for respiratory failure in patients with chronic obstructive pulmonary disease (COPD). Compared to benzodiazepine, the prescription of non-benzodiazepine may be safer for the management of insomnia in COPD patients.
Citation:
Chen SJ, Yeh CM, Chao TF, Liu CJ, Wang KL, Chen TJ, Chou P, Wang FD. The use of benzodiazepine receptor agonists and risk of respiratory failure in patients with chronic obstructive pulmonary disease: a nationwide population-based case-control study. SLEEP 2015;38(7):1045–1050.
Keywords: benzodiazepine receptor agonists, respiratory failure, chronic obstructive pulmonary disease, Taiwan National Health Insurance Research Database
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a progressive disease, and is one of the leading causes of morbidity and mortality worldwide.1 Several comorbidities accompany COPD, including unexplained weight loss, cardiovascular disease, peripheral muscle weakness, cognitive impairment, anxiety, depression, and sleep disorders.2 According to previous studies, 17% to 50% of COPD patients have sleep difficulties, characterized by a longer latency to falling asleep and frequent awakenings.3 These sleep disturbances increase daytime tiredness/sleepiness and impair quality of life.
Many sedatives and hypnotics have been employed for the management of sleep disorders. Benzodiazepine receptor agonists (BZRAs) are among the most commonly used pharmacological agents for treating patients with insomnia, including those with comorbid COPD.4 BZRAs are a group of drugs that include the traditional benzodiazepines (BZDs), as well as a newer group of more selective BZRAs called non-benzodiaze-pines (non-BZDs). Benzodiazepine receptors are expressed in the plasma membrane of neurons throughout the central nervous system (CNS) and peripheral nervous system. By binding to these receptors, CNS function is suppressed and sedation is achieved. However, the main problem associated with the use of BZRAs is respiratory depression, which may worsen sleep-related hypoventilation, especially in patients with underlying pulmonary diseases.4 Although BZRAs may have adverse effects on pulmonary function and were recommended to be avoided in patients with severe COPD,5 evidence on whether the use of BZRAs is associated with poor clinical outcomes in COPD patients is limited. It is a very important clinical and public health issue, because insomnia is prevalent in COPD patients and BZRAs are widely prescribed. The aim of this study was to investigate whether the use of BZRAs is a significant risk factor for the development of respiratory failure (RF) in COPD patients.
METHODS
Database
We conducted a retrospective nationwide study, based on information from the National Health Insurance Research Database (NHIRD) released by the Taiwan National Health Research Institutes (NHRI). The National Health Insurance (NHI) system is a mandatory universal health insurance program, which offers comprehensive medical care coverage to nearly all Taiwanese residents (the coverage rate was 99.6% at the end of 2010; http://www.nhi.gov.tw/english/index.aspx). The NHIRD is a cohort dataset containing all the medical claims data for 1,000,000 beneficiaries, who were randomly sampled from the 25.68 million enrollees under the NHI program. This random sample has been confirmed by the NHRI to be representative of the Taiwanese population. In this cohort dataset, the original patient identification numbers have been encrypted to protect privacy, but the encryption procedure was consistent, so that the linkage of the claims belonging to the same patient is feasible within the NHI database, and they can be followed continuously. This database, with a large sample size, provided an excellent opportunity to study the association between the use of BZRAs and the risk of RF among COPD patients.
Identification of COPD Cohort
From January 1, 2001, to December 31, 2005, a total of 19,788 patients (age ≥ 45 years) with newly diagnosed COPD were identified from the NHIRD. The diagnosis of COPD was based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (491.x, 492.x, or 496). To ensure the accuracy of diagnosis, we included patients with COPD only when it was a primary discharge diagnosis, or was repeatedly confirmed more than twice in an outpatient department within 12 months. Patients had to receive respiratory medications to be included in the study, and patients with a combined diagnosis of asthma were excluded.6
Case and Control Groups
Among the COPD cohort, patients who experienced RF necessitating the use of mechanical ventilation were identified as the case group. The index date for case patients was defined as the date when RF occurred. On the same index date, one control patient without RF, matched for age and sex with the case patient, was selected for inclusion in the control group. Controls were selected by risk set sampling.7 The study period was the 180 days preceding the index date. Exposure to BZRAs during the 180-day study period was analyzed and compared in the case and control groups. We used inpatient and outpatient diagnosis to identify comorbidities and calculate Charlson Comorbidity Index scores.8 Information on COPD medication use including bronchodilators, corticosteroids, and theophyllines was collected. Healthcare utilization was estimated by measuring the annual number of outpatient physician visits and hospitalizations. We used acute exacerbation of COPD (ICD-9 codes 491.21) necessitating emergency department (ED) visits as a proxy measure of disease severity. A flow chart of patient enrollment is shown in Figure 1.
Figure 1.
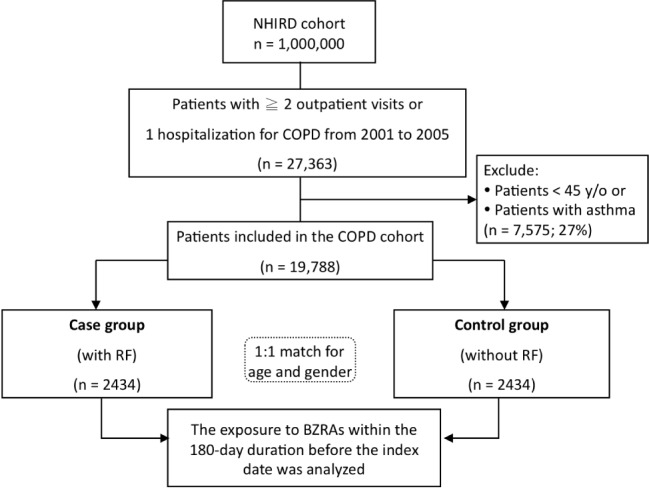
Flow chart of the enrollment of study patients. A total of 4,868 COPD patients were identified as the study population. Exposure to BZRAs during the 180-day period preceding the index date was analyzed and compared in the case and control groups. BZRAs, benzodiazepines receptor agonists; COPD, chronic obstructive pulmonary disease; RF, respiratory failure.
Exposure to BZRAs
BZRA exposure status was determined from prescription claims data. BZRAs included those listed in the Anatomical Therapeutic Chemical (ATC) classification system in the section on benzodiazepine derivatives (N03AE, N05BA, and N05CD) and benzodiazepine-related drugs (N05CF).9 Defined daily dose (DDD)—that is, “the assumed average maintenance dose per day for a drug used for its main indication in adults”9—was used as a dose standard unit for various BZRAs.10
Categorization of BZRA Users
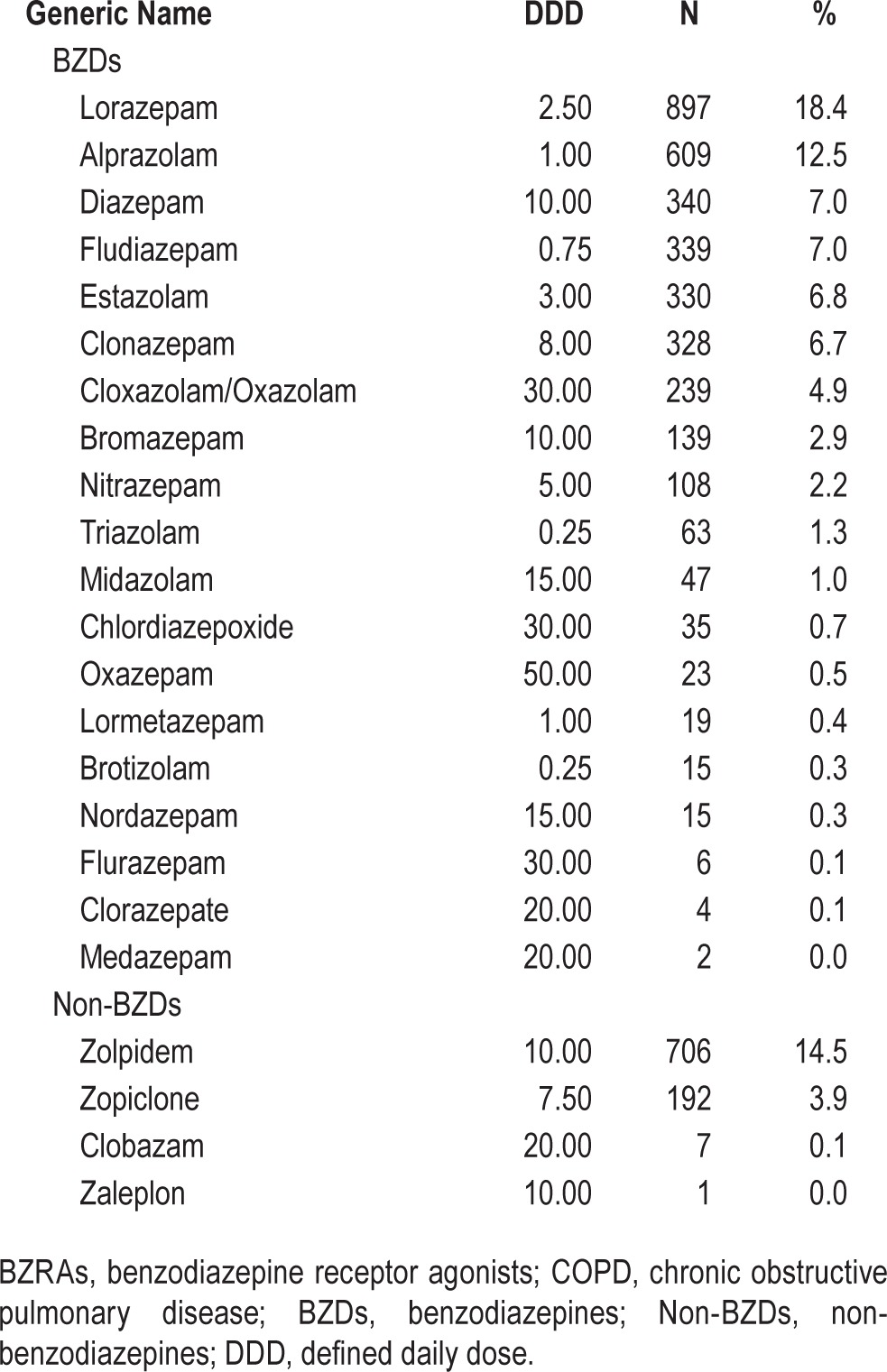
BZRA users were further categorized according to the timing, number, and subclass of BZRAs used. Based on the timing of exposure, patients were defined as new users if they received their first ever BZRA prescription within the 30 days preceding the index date, and the others were defined as chronic users. According to the various generic drugs listed in Table 1, the number of BZRAs used was calculated for each patient. Furthermore, users were classified as “BZDs alone,” “non-BZDs alone,” or “mixed” users.
Table 1.
Frequency of BZRAs use among the 4,968 COPD patients.
Statistical Analysis
The SAS (version 9.2; SAS Institute, Inc., Gary, NC, USA) and SPSS (version 15, SPSS Inc., Chicago, IL, USA) statistical packages were used to analyze the data. The differences between categorical variables were compared using the McNemar test. Continuous variables were assessed using the two-sample t-test for normally distributed continuous variables, and the Mann-Whitney rank-sum test for skewed variables.
To estimate the risk of RF associated with the use of BZRAs, we performed conditional logistic regression to derive odds ratios (ORs) and 95% confidence intervals (CIs). In addition, we used propensity score as a covariate to be adjusted in the model to reduce the effect of selection bias resulting from treatment assignment.11 A propensity score was quantified for the likelihood of receiving BZRAs by using a multivariable logistic regression model conditional on baseline characteristics. These characteristics included age, gender, comorbidities (e.g., hypertension, diabetes mellitus, congestive heart failure, coronary artery disease, chronic kidney disease, ischemic stroke, malignancy, liver cirrhosis, and mood disorder), Charlson Comorbidity Index score, COPD medications, annual healthcare utilization, and acute exacerbation. We examined the variance inflation factors to check whether the model exhibited multicollinearity. A value > 10 was deemed indicative of a serious multicollinearity problem.12 A two-sided P value < 0.05 was considered statistically significant in all statistical analyses.
RESULTS
Patient Characteristics and Use of BZRAs
The baseline characteristics of patients with and without RF are shown in Table 2. Patients with RF had more comorbidities and higher Charlson Comorbidity Index scores than patients without RF. During the 180-day study period, a total of 1,590 (32.7%) patients received BZRA prescriptions. Table 1 provides the counts and proportions of BZRAs used, based on generic names. Lorazepam (18.4%), zolpidem (14.5%), alprazolam (12.5%), diazepam (7.0%), and fludiazepam (7.0%) were the most frequently used BZRAs in the study population.
Table 2.
Baseline characteristics of the COPD patients with and without RF.

Association between Use of BZRAs and Risk of RF in COPD Patients
The association between the use of BZRAs and risk of RF represented by ORs is shown in Table 3. BZRA users had an increased risk of RF in comparison to “non-users” (OR 1.56, 95% CI 1.14–2.13) after adjustment for differences in covariates and propensity score. When we excluded the less- prescribed drugs and only focused on the most used BZDs (lorazepam and alprazolam) and non-BZDs (zolpidem and zopiclone) in our study, users were still at a higher risk of RF (OR 1.76, 95% CI 1.23–2.53).
Table 3.
Association between BZRAs use and RF among COPD patients (n = 4,868).

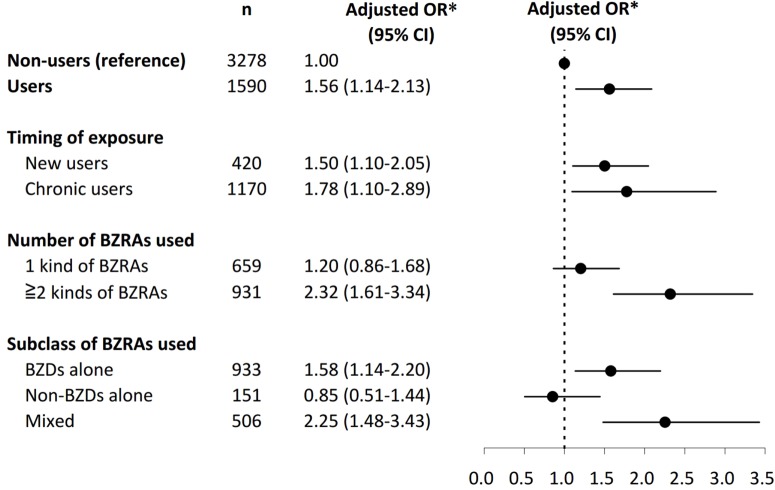
In the subgroup analysis (Figure 2), both new users and chronic users of BZRAs had an increased risk of RF as compared to non-users, with adjusted ORs of 1.50 (95% CI 1.10–2.05) and 1.78 (95% CI 1.10–2.89), respectively. With regard to the number of different kinds of BZRAs used, patients who received > 2 kinds of BZRAs were at a higher risk of RF (OR 2.32, 95% CI 1.61–3.34), whereas the risk was not significant for those who received only one kind of BZRA (OR 1.20, 95% CI 0.86–1.68). With respect to the subclass of BZRAs used, significant associations were evident between RF and BZRAs for “BZDs alone” users (OR 1.58, 95% CI 1.14–2.20) and “mixed” users (OR 2.25, 95% CI 1.48–3.43), but not for “non-BZDs alone” users (OR 0.85, 95% CI 0.51–1.44).
Figure 2.
Association between the use of BZRAs and risk of RF. In the subgroup analysis, both new users and chronic users of BZRAs were associated with an increased risk of RF as compared to non-users. With regard to the number of different kinds of BZRAs used, patients who received more than 2 kinds of BZRAs were at a higher risk of RF, whereas the risk was not significant for those who only received one kind of BZRAs. With respect to the subclass of BZRAs used, significant associations were evident between RF and BZRAs for “BZDs alone” users and “mixed” users, but not for “non-BZDs alone” users. BZDs, benzodiazepines; BZRAs, benzodiazepine receptor agonists; CI, confidence interval; non-BZDs, non-benzodiazepines; OR, odds ratio; RF, respiratory failure. *Adjustment for variables with P values < 0.05 between 2 groups in Table 2, and propensity score for the likelihood of receiving BZRAs during the study period
DISCUSSION
Main Findings
In this population-based matched case-control study which enrolled a total of 4,868 COPD patients, we investigated the association between the use of BZRAs and risk of RF. The main findings were as follows: (1) The use of BZRAs was a signifi-cant risk factor for RF in COPD patients. The risk increased more than 2-fold in those who received two or more kinds of BZRAs, or those who used a combination of BZDs and non-BZDs. (2) With regard to subclasses of BZRAs, BZD users were associated with a 58% increased risk of RF, whereas the risk in users of non-BZDs was not significant.
Effects of BZRAs on Respiratory Function
COPD patients are susceptible to sleeping difficulties, and successful management of insomnia is particularly important because it improves patients' quality of life. BZRAs are commonly used to treat patients with insomnia, despite the concern of respiratory suppression, which could predispose COPD patients to RF. Previous studies have demonstrated that the natural drops in minute ventilation and increased airway resistance during sleep are more pronounced in patients with COPD.13 In addition, BZRAs induce respiratory depression, which could persist for several hours, and COPD patients may take twice as long to return to their baseline levels of pulmonary function.4 Taken together, these results suggest that the use of BZRAs in COPD patients may be dangerous. However, we found a striking absence of large-scale longitudinal observational studies investigating this important issue.
Although there have been a number of studies evaluating the risks associated with BZRAs use in COPD patients,4,13 most of the endpoints of these studies focused on changes in respiratory parameters, not on clinical adverse events.3 Furthermore, most of the studies were limited to a small sample size. To the best of our knowledge, the present study is the first to show that the use of BZRAs is associated with an increased risk of RF in COPD patients, and the results may fill a gap in the literature.
However, it should be noted that insomnia tends to be more prevalent and severe in advanced COPD patients.13 Therefore, a plausible alternate explanation for the association is that increased severity of COPD is the cause of increased use of BZRAs.14 Although we have adjusted important baseline differences, including disease severity, between the case and the control groups, some residual unmeasured confounders may still exist. Further investigation is required to confirm the relationship between the use of BZRAs and RF in COPD patients.
Risks of RF Associated with BZDs and non-BZDs
One of the major findings of the present study was that the use of BZDs was associated with an increased risk of RF, whereas the use of non-BZDs seemed to be safe. It may be that BZDs and non-BZDs have different impacts on respiratory function. From previous studies, we recognized that different BZD receptor subtypes mediate different effects. The traditional BZDs, which have a broad range of affinity for BZD receptors in the CNS and peripheral tissues, are thought to be able to reduce upper airway muscle activation and inhibit the hypoglossal and the recurrent laryngeal nerves.15 Together, these effects could lead to pulmonary muscle relaxation and airway obstruction.15 In contrast, the newer generation of BZRAs (non-BZDs) may have fewer effects on pulmonary function, due to their higher specificity for BZD receptors.16,17 The results of the present study support the hypothesis that non-BZDs may be less likely to further compromise pulmonary function in COPD patients than traditional BZDs, and may be associated with a more favorable safety profile.
Clinical Applications
Our findings indicate that we should pay close attention to the risk of RF resulting from the use of BZRAs in COPD patients. If the prescription of BZRAs is necessary, the newer generation of BZRAs, i.e., non-BZDs, may be safer than the traditional ones. In addition, we should avoid long-term or combination use of these drugs, because the risk of RF is much higher under such conditions. However, it should be noted that the sample size of the non-BZDs subgroup was relatively small, and a large scale, prospective trial is necessary to confirm these findings.
Limitations and Strengths of the Study
This study had several limitations. First, as it was a retrospective observational study, it could not be established whether the association between the use of BZRAs and risk of RF was a causal relationship. Second, information about functional status, body mass index, and personal habits such as alcohol use, smoking, and physical activity were not available in the database used. However, these are common limitations of secondary data analysis. Finally, pulmonary function test data, which is the recommended test for classification of COPD severity,18 was lacking. As an alternative, we used “acute exacerbation” as a proxy measure of disease severity.19–21
Despite these limitations, there were several important strengths. First, this was the first study to specifically examine the association between the use of BZRAs and risk of RF by using a nationwide cohort of COPD patients. The NHIRD includes all hospital admissions, outpatient visits, and prescription claims data in Taiwan, and was therefore not affected by selection bias from selective inclusion of specific hospitals or health insurance systems. Second, we used propensity score in the multivariable logistic model to reduce selection bias derived from treatment assignment.
CONCLUSION
The current study demonstrated that the use of BZRAs is an important risk factor for RF in COPD patients. Compared to BZDs, the prescription of non-BZDs may be safer for the management of insomia in COPD patients.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported in part by grants from the National Science Council (NSC98-2410-H-010-003-MY2), and Taipei Veterans General Hospital (V99C1-140, V99A-153, and V100D-002-3, V101D-001-2). The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–51. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urbano F, Mohsenin V. Chronic obstructive pulmonary disease and sleep: the interaction. Panminerva Med. 2006;48:223–30. [PubMed] [Google Scholar]
- 3.Stege G, Vos PJ, van den Elshout FJ, et al. Sleep, hypnotics and chronic obstructive pulmonary disease. Respir Med. 2008;102:801–14. doi: 10.1016/j.rmed.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 4.George CF, Bayliff CD. Management of insomnia in patients with chronic obstructive pulmonary disease. Drugs. 2003;63:379–87. doi: 10.2165/00003495-200363040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Celli BR, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 6.Lee TA, Pickard AS, Au DH, et al. Risk for death associated with medications for recently diagnosed chronic obstructive pulmonary disease. Ann Intern Med. 2008;149:380–90. doi: 10.7326/0003-4819-149-6-200809160-00004. [DOI] [PubMed] [Google Scholar]
- 7.Wacholder S, McLaughlin JK, Silverman DT, et al. Selection of controls in case-control studies. I. Principles. Am J Epidemiol. 1992;135:1019–28. doi: 10.1093/oxfordjournals.aje.a116396. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 9.Guideline for ATC Classification and DDD Assignment. Oslo, Norway: World Health Organization, Collaborating Centre for Drug Statistic Methodology; 2009. [Google Scholar]
- 10.Merlo J, Wessling A, Melander A. Comparison of dose standard units for drug utilisation studies. Eur J Clin Pharmacol. 1996;50:27–30. doi: 10.1007/s002280050064. [DOI] [PubMed] [Google Scholar]
- 11.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 12.Allison PD. Logistic Regression using SAS Systems: Theory and Applications. 2nd ed. Cary, NC: SAS Institute; 2001. [Google Scholar]
- 13.Roth T. Hypnotic use for insomnia management in chronic obstructive pulmonary disease. Sleep Med. 2009;10:19–25. doi: 10.1016/j.sleep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44:332–40. doi: 10.1183/09031936.00008014. [DOI] [PubMed] [Google Scholar]
- 15.Altose MD, Hudgel DW. The pharmacology of respiratory depressants and stimulants. Clin Chest Med. 1986;7:481–94. [PubMed] [Google Scholar]
- 16.George CF. Perspectives on the management of insomnia in patients with chronic respiratory disorders. Sleep. 2000;23(Suppl 1):S31–5. discussion S36–8. [PubMed] [Google Scholar]
- 17.Ebert B, Wafford KA, Deacon S. Treating insomnia: current and investigational pharmacological approaches. Pharmacol Ther. 2006;112:612–29. doi: 10.1016/j.pharmthera.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 19.Matkovic Z, Huerta A, Soler N, et al. Predictors of adverse outcome in patients hospitalised for exacerbation of chronic obstructive pulmonary disease. Respiration. 2012;84:17–26. doi: 10.1159/000335467. [DOI] [PubMed] [Google Scholar]
- 20.Celli BR. Predictors of mortality in COPD. Respir Med. 2010;104:773–9. doi: 10.1016/j.rmed.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]