Abstract
Study Objective:
To examine the effect of sleep deprivation compared to sleep, immediately after experimental trauma stimuli on the development of intrusive memories to that trauma stimuli.
Design:
Participants were exposed to a film with traumatic content (trauma film). The immediate response to the trauma film was assessed, followed by either total sleep deprivation (sleep deprived group, N = 20) or sleep as usual (sleep group, N = 22). Twelve hours after the film viewing the initial psychological effect of the trauma film was measured and for the subsequent 6 days intrusive emotional memories related to the trauma film were recorded in daily life.
Setting:
Academic sleep laboratory and participants' home environment.
Participants:
Healthy paid volunteers.
Measurements and results:
On the first day after the trauma film, the psychological effect as assessed by the Impact of Event Scale – Revised was lower in the sleep deprived group compared to the sleep group. In addition, the sleep deprived group reported fewer intrusive emotional memories (mean 2.28, standard deviation [SD] 2.91) compared to the sleep group (mean 3.76, SD 3.35). Because habitual sleep/circadian patterns, psychological health, and immediate effect of the trauma film were similar at baseline for participants of both groups, the results cannot be accounted for by pre-existing inequalities between groups.
Conclusions:
Our findings suggest that sleep deprivation on one night, rather than sleeping, reduces emotional effect and intrusive memories following exposure to experimental trauma.
Citation:
Porcheret K, Holmes EA, Goodwin GM, Foster RG, Wulff K. Psychological effect of an analogue traumatic event reduced by sleep deprivation. SLEEP 2015;38(7):1017–1025.
Keywords: analogue trauma, emotional memory, intrusive memory, posttraumatic stress disorder, sleep deprivation
INTRODUCTION
Sleep promotes the consolidation of emotional memory,1–4 suggesting sleeping after an emotional event will result in emotional, autobiographical memories, regardless of how pleasant or distressing the event. Following a psychological trauma, some people can develop “flashbacks” and intrusive memories to that event.5 Such intrusive memories are autobiographical,6 emotionally tagged,7 and image-based8 memories, which reappear spontaneously in a person's consciousness; i.e., they are a type of memory that springs to mind involuntarily compared to those that are deliberately retrieved.9 Intrusive memories can become debilitating as a result of their horrific or distressing content relating to the original traumatic situation10; their frequent recurrence bringing back associated emotions; and in their more extreme forms presenting as so-called dissociative ”flashbacks” and nightmares.5 Re-experiencing the trauma in the form of intrusive memories is a hallmark of both acute stress disorder (ASD), when posttraumatic symptomatology occurs in the first month following a trauma,5 and posttraumatic stress disorder (PTSD),5 when symptoms persist beyond the first month following a trauma. Some studies suggest that early re-experiencing may predict later PTSD,11 whereas others relate early re-experiencing not only to PTSD but to a variety of later psychiatric diagnoses.12 For example, Bryant and colleagues12 found that chronic PTSD syndrome eventually developed in 36% of those individuals who received an initial diagnosis of ASD, whereas symptoms of any psychiatric disorder including PTSD developed in 65%.12 It is noteworthy that intrusive memories have also been noted in other psychiatric disorders, such as depression.13–15
The use of sleep deprivation immediately after trauma as a preventive measure against the development of posttraumatic intrusive memories has previously been considered, though never directly tested.16 Short periods of sleep post-learning have been shown to be sufficient to enhance emotional memory for years after initial learning,16 suggesting indirectly that the immediate prevention of sleep after trauma may be enough to impair long-term emotional memories. Sleep deprivation compared to sleep has been shown to reduce fear responses to aversive material (motor vehicle accidents),17 while not effecting explicit memory recall of the encoding material. However the situation remains unclear. When healthy subjects were required to suppress memories of an aversive film, subsequent sleep deprivation resulted in enhanced fear responses.18 Moreover, sleep, specifically rapid eye movement (REM) sleep, has been reported to diminish,19,20 maintain,21 and enhance22,23 emotional reactivity to affective material.
Clinically, retrospective self-reported sleep disturbances both before24 and after25 trauma have been found to be predictive for the development of PTSD and other psychiatric disorders, whereas objective sleep recordings showed fragmented REM sleep within 1 mo after trauma to be associated with subthreshold or established PTSD 2 mo following the trauma.26,27 However, these studies assessed sleep disturbances in the weeks before and after the trauma, rather than effects emerging from total sleep deprivation (i.e., on the first night after trauma). Prolonged insufficient sleep causes cumulative deficits in memory and mood over time, which differ from only one night of full sleep deprivation.28 For example, prolonged sleep restriction of 4 h per night over 2 w causes lapses in behavioural alertness and deficits in working memory equivalent to 2 nights of full sleep deprivation.28 Furthermore, 1 w of insufficient sleep leads to a cumulative increase in fatigue, sleepiness, and feelings of tension, anxiety, and confusion,29 which is likely to be due to accumulated changes in the brain's arousal and neuroendocrine stress system.30 In contrast, and perhaps counterintuitively, we predict that acute total sleep deprivation would offer the opportunity to affect memory consolidation mechanisms after experimental trauma, and thus restrict the encoding of negative emotional material.31 Currently, however, the effect of total sleep deprivation immediately after trauma on the generation of intrusive memories is unknown.
Experimentally it is unethical to subject people to real-life trauma. Therefore, we used an experimental analogue of witnessing a traumatic event known as the trauma film paradigm.32 Participants viewed a film with content designed to relate to PTSD diagnostic criteria for a traumatic event, such as scenes related to suicide and a car crash.5 The use of traumatic film footage has long been used as an analogue of exposure to psychological stress.33 This paradigm has been shown to increase physiological stress responses including skin conductance and heart rate; alter mood; and critically induce intrusive memories to the trauma stimuli.32 Thus, the trauma film paradigm is a model of a specific feature of posttraumatic symptomatology, intrusive memories. Using this paradigm, we hypothesize that the frequency of intrusive memories would be reduced by a full night of sleep deprivation immediately following traumatic stimuli.
The aim of the current study was to test experimentally whether sleep as usual versus sleep deprivation immediately after exposure to an analogue traumatic event influenced the development of psychological phenomena with hypothesized relevance to posttraumatic symptomatology, in particular intrusive memories of the traumatic material. Importantly, the trauma film paradigm allowed prospective self-reports of intrusive memories, assessment of emotionality before and after watching the film, and inclusion of only good, regular sleepers.
METHODS AND MATERIAL
Participants
Forty-two healthy student volunteers aged 18–25 y were randomly assigned to either the sleep deprivation group (n = 20, 14 females, mean age: 21.45 ± 1.70 y) or sleep group (n = 22, 15 females, mean age: 21.59 ± 2.17 y). All participants were non-smokers, consumed no more than moderate levels of caffeine (≤ 300 mg/day), had no personal or family history of mental health problems, alcohol, or drug abuse as assessed with the Mini International Neuropsychological Interview (MINI).34 Participants were excluded if they had an extreme diurnal preference as assessed by the Morningness-Eveningness Questionnaire (MEQ35; score of > 69 or < 31), poor sleep quality as assessed by the Pittsburgh Sleep Quality Index (PSQI36; score of > 6), or regularly went to bed later than 02:00. None of the participants were taking any medication (with the exception of the contraceptive pill) and none of the female participants were pregnant. None of the participants had previously taken part in another research study using similar film footage. All participants provided their written informed consent and received an honorarium for their participation. The study was conducted in accordance with the Declaration of Helsinki and approved by the Milton Keynes NHS Research Ethics Committee.
Experimental Design
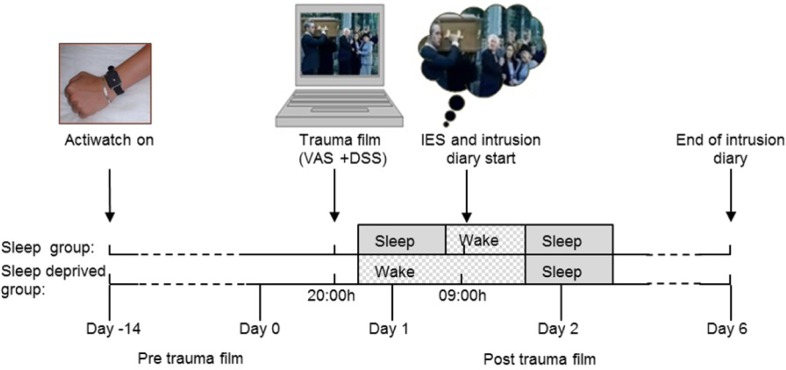
Before the participants watched the trauma film (day −14 to day 0) they were assessed for baseline trait measures of chronotype, mood and personality, and sleep profiles were monitored (Figure 1). Chronotype was evaluated subjectively using the MEQ.35 Sleep profiles were determined actigraphically for 10 to 14 days preceding the trauma film. Mood and personality were assessed using the Profile of Mood States (POMS37) and the Eysenck Personality Questionnaire (EPQ38), respectively.
Figure 1.
Both groups watched the trauma film in the evening of day 0. The sleep deprived group remained awake until 19:00 the next day (end of day 1), while the sleep group slept. The psychological effect of the trauma film was assessed for both groups on the morning of day 1 using the Impact of Event Scale (IES-R). From day 1, all participants kept a self-reported intrusion diary (intrusive memories and analogue flashbacks) for 6 days, during which they returned to their normal lives. Both groups were also assessed 2 weeks before the experiment (day −14 to day 0) to ensure regular sleep-wake timings (actigraphy), no extreme chronotype (Morningness-Eveningness Questionnaire) or abnormal mood and personality traits (questionnaires, see Experimental Procedures for details). To assess the immediate reaction to the trauma film exposure, the participants completed visual analogue mood scales (VAS) and the dissociative state scale (DSS) before and after watching the film. The participants rated their personal relevance and familiarity of the film clips after watching the film.
All participants watched the trauma film between 19:00 and 21:00 on day 0, in the Sleep/Circadian Neuroscience Laboratory at the John Radcliffe Hospital in Oxford. Immediately before and after watching the film, participants rated their mood using visual analogue scales (VAS), and completed the Dissociative State Scale (DSS39) to assess dissociate state (i.e., their sense of detachment from time, space, and the self). Next, familiarity with the clips used in the trauma film, and the personal relevance of the content of the clips were rated using a seven-point scale. The sleep deprived group were monitored until 19:00 the following day in the sleep laboratory. The sleep group returned home with instructions to not watch any television or listen to any music before going to bed.
On day 1, 12 h after the trauma film viewing, both groups completed the revised version of the Impact of Event Scale– Revised (IES-R40) and received instructions to begin recording intrusive memories relating to the film in an intrusion diary in the same environment in which they viewed the trauma film. Both groups continued to record intrusive memories experienced once they returned to their daily life for a period of 6 days in total. For 3 days prior and throughout the experiment the participants were asked to refrain from consuming any caffeine or alcohol, and were screened for drugs of abuse. During the day preceding the trauma film the participants were asked not to nap, which was controlled by continuous actigraphic monitoring (day −14 to day 1).
Film With Traumatic Content (Trauma Film)
The film consisted of a 15 min 01sec compilation of traumatic and distressing clips. There were six clips in total from films and television adverts, depicting scenes such as a suicide, bullying, injury, and cutting to the face, which have been used previously.41–43 Before viewing the film (upon recruitment into the study) the participants were made aware it contained distressing content. Participants were instructed to give the film their full attention, not to close their eyes or look away and to imagine that they were there at the scene watching the events unfold in front of their eyes. Participants were told they were free to stop the film at any time, but none did. The film was shown on a laptop computer with a 15-inch screen, with the room lights turned off.
Sleep Deprivation
During the sleep deprivation period in the sleep laboratory: illumination of the room was kept to between 165–276 lux using artificial fluorescent lights; participants were allowed water ad libitum, and they had a choice of snacks every 2 h (a sandwich or a piece of fruit); the use of computers, TV, DVDs, music, or leaving the sleep laboratory were not allowed; participants were allowed to walk around, talk to the researchers, read, and play board games. In the morning the participants were allowed a shower. All the participants were continually monitored by staff trained to prevent napping and to ensure compliance and wellbeing.
Assessments
Impact of Event Scale – Revised
The IES-R40 comprised 22 items assessing the three aspects of PTSD symptomatology: intrusions, avoidance, and hyper-arousal. A global score of the IES-R was used giving a score in the range of 0 to 88. In line with previous research,43 the IES-R used the trauma film as an index event, and used “restful state” for items 2 and 15.
Intrusive Emotional Memories (Intrusion Diary)
The intrusion diary consisted of a pen and paper diary used on a daily basis to (1) report all intrusive memories or indicate zero for no intrusions on a given day; (2) give detailed content of each intrusion, so that it could be verified as matching a scene in the trauma film; (3) rate the level of distress experienced with the intrusion from 0 “not at all” to 10 “extremely”; and (4) to confirm the intrusion involved mental imagery. Intrusive memories were defined as spontaneously occurring (i.e., not deliberately or effortfully retrieved) image-based memories of the trauma film.43 Participants were given standardized verbal and written instructions about the nature of intrusive memories: “It may be that over the next few days images about the film you have just seen will pop into your mind. Mental images can often take the form of pictures in the mind's eye, but can actually include any of the five senses, so you can imagine sounds too. Mental images can be fuzzy or fragmented as well as clear, they can also be brief and very fleeting”. The frequency of intrusive memories was calculated as the total number of image-based intrusions relating to the trauma film over the following 6 days. For day-by-day analysis, intrusive memories were analyzed separately for day 1 and day 2, as well as being pooled for 2-day periods.
Mood Visual Analogue Scale (VAS)
Negative affect was assessed by VAS anchored from “not at all” (0) to “very” (100), to give a composite mood score calculated by averaging the scores for sad, hopeless, and depressed (items used in previous trauma film studies).43
Dissociative State Scale (DSS)
The 19-item version of the DSS39 was used to assess the dissociative state of the participants, using ratings from “not at all” to “considerably” for a list of questions including “do things seem to be moving in slow motion?” and “do you space out, or in some other way, lose track of what is going on?” The score for each question was summated to give a score from 0 to 57.
Baseline Questionnaires
The Mood Disorder Questionnaire44 score was taken as the number of symptoms reported on the first question of the questionnaire. An average score of all the EPQ38 subscales was used. The POMS37 was used to calculated total mood disturbance score: (tension-anxiety + depression-dejection + anger-hostility + fatigue + confusion) – (vigour-activity).45
Actigraphy
The participants wore an Actiwatch with integrated light sensor (AWL; CamNtech Ltd., Cambridge, UK) on their nondominant wrist and kept a standardized diary of bed times and daily activities. Actigraphy data were sampled in 1-min epochs and analyzed with version 7 of the Actiwatch activity and sleep analysis software (CamNtech Ltd.). Rest-activity patterns were annotated using diary data, “Bed time” and “Get-up time” were manually entered and “Sleep start” and “Sleep end” calculated automatically using medium sensitivity.
Statistical Analysis
All statistical analyses were assisted by a statistician and performed using R version 2.12.0 (2010-10-15, Copyright © 2010, The R Foundation for Statistical Computing). Simple group comparisons of continuous variables were carried out by performing Wilcoxon rank-sum test. Repeated measures were taken on individuals, so that when statistical models were fitted it was important to take into account correlation within individuals. This was done by fitting two-level generalized linear models with the R-package lme4, binomial regression being used to fit proportional responses, and Poisson regression being used to fit count data. Pre-film and post-film mood scores were analyzed with an analysis of variance (ANOVA), as these data fitted a normal distribution. All model fitting was validated by carrying out the usual goodness-of-fit tests and all fits quoted were satisfactory.
The primary outcome measure (frequency of intrusive memories) for one participant in the sleep deprived group was identified as an extreme outlier (10 times above the mean), so was excluded from the dataset.46 One non-sleep deprived participants failed to complete the POMS; three participants (two sleep deprived participants, one non-sleep deprived participant) failed to wear the Actiwatch for a sufficient period of time; and one sleep deprived and one non-sleep deprived participant did not return their intrusion diary so were not included in these analyses.
RESULTS
Participant Characteristics
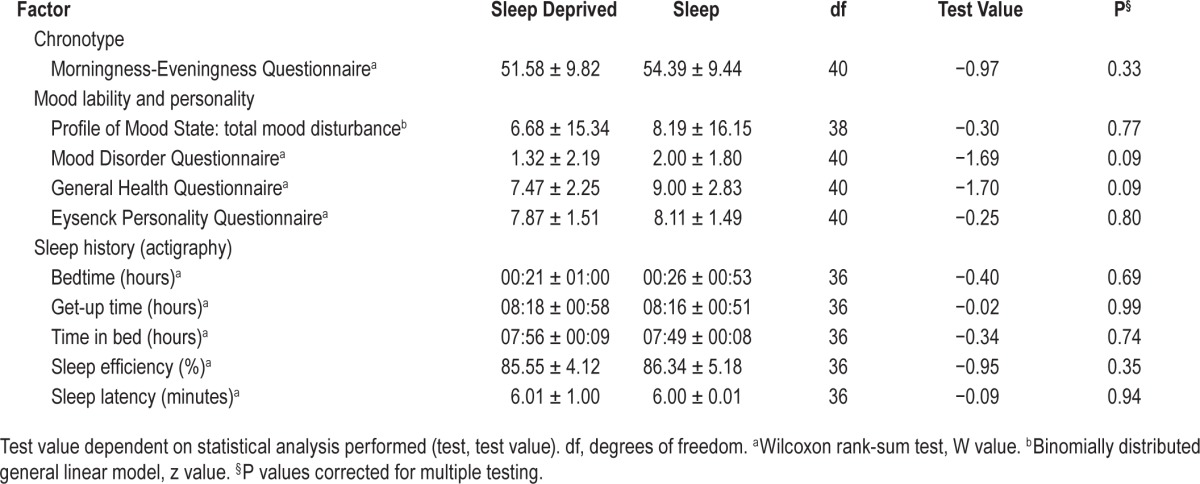
All participants showed a good sleep profile for 10–14 days preceding the trauma film (averaged across days, sleep duration: mean 07:52 h, SD 00:38 h; sleep efficiency mean 85.78%, SD 4.77%). On average, the participants showed an intermediate chronotype (MEQ: mean 53.30, SD 9.58), and low trait mood scores (POMS: mean 7.12, SD 15.56). The two experimental groups showed no significant differences between any of the baseline factors assessed (Table 1). Thus, data confirmed the absence of sleep or circadian disturbance among the participants and the groups were matched for trait mood scores before watching the trauma film.
Table 1.
Mean (± standard deviation of the mean) shown for each factor that was matched for group at baseline before the trauma film.
Response to Trauma Film
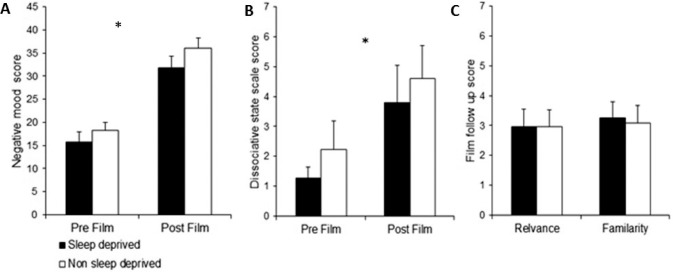
Both groups showed a negative mood response to the trauma film, which critically was not different between the groups (ANOVA, Film effect: F = 85.68, P < 0.001, Group/Film in -teraction: F = 0.247, P = 0.622; Figure 2A). After watching the trauma film, state dissociation increased to the same extent in both groups (two-level binomial generalized linear model (GLM) fitted, Group effect: z = 1.04, P = 0.30, Time effect: z = 2.97, P = 0.003, Group/Time interaction: z = −1.22, P = 0.22; Figure 2B). The groups also did not differ in the degree of familiarity with the film clips used (binomial GLM fitted, z = −0.37, P = 0.71; Figure 2C) nor the personal rele -vance of the content of the clips (binomial GLM fitted, z = 0.02, P = 0.99; Figure 2C). Therefore, both groups reported a similar experience of the analogue traumatic event.
Figure 2.
(A) Change in emotional reaction to trauma film: repeated measure analysis of mood visual analogue scores before and after trauma film, demonstrating a significant increase in negative affect, which was equivalent for both groups. (B) Change in state dissociation to trauma film: repeated measure analysis of state dissociation, demonstrating a significant increase in mean state dissociation after watching the trauma film for both groups, but no difference between groups. *P < 0.05; error bars represent standard error of the mean. (C) Personal relevance and familiarity of trauma film clips: no difference between groups in terms of mean personal relevance of content of film clips and familiarity of film clips.
Psychological Effect of Trauma Film
On day 1 post-trauma film, a significantly lower score was reported on the IES-R by the sleep deprived group (mean = 8.47, SD = 5.31) compared to the sleep group (mean = 11.52, SD = 6.64), reflecting a lower effect of the trauma film (binomial GLM fitted, z = 3.27, P = 0.001; Figure 3).
Figure 3.
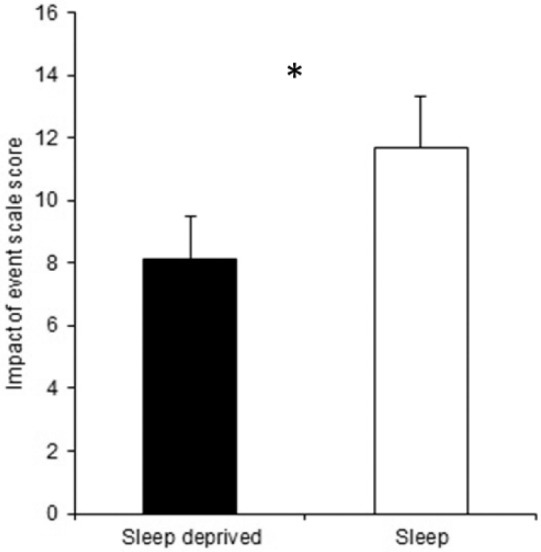
The sleep deprived group showed a significantly lower mean global score on the Impact of Event Scale-Revised on day 1 after the analogue psychologically traumatic event. *P < 0.05; error bars represent standard error of the mean.
Intrusive Memories to Trauma Film Over 1 Week
Overall, a total of 120 intrusive memories were reported by the participants. The content of the intrusive memories reported were highly matched (93%) in content to clips from the trauma film. In terms of the intrusions' content, 23.3% related to the clip containing self-mutilation (facial cutting), 13.3% to a clip depicting a car crash, and 50.8% to three clips all involving different scenes of suicide. The remaining 7% were relevant to only one specific scene of suicide. Examples of intrusive memories reported include “I saw a boy lying dead behind a desk”, “saw image of old man hanging from ceiling”, and “hear water running and see guy shaving and blood dripping”. Seventy-six percent of intrusive memories were reported to be triggered by an event in daily life for example “passing cemetery” or “washing hands in the sink”.
Over the 6 days following the trauma film, significantly fewer intrusive memories in daily life were reported by the sleep deprived group (mean 2.28, SD 2.91) compared to the sleep group (mean 3.76, SD 3.35; Poisson GLM fitted, z = 2.61, P = 0.009). The frequency of intrusive memories was found to decline over the 6 days following the trauma film (Figure 4A). The sleep deprived participants reported fewer intrusive memories for the first 2-day period compared to the sleep group (Poisson GLM fitted, Group effect: z = 2.905, P = 0.004). The intensity of the intrusions was similar between groups as measured in the level of distress reported when experiencing an intrusive memory (Poisson GLM fitted, z = 1.2, P = 0.23; Figure 4B).
Figure 4.
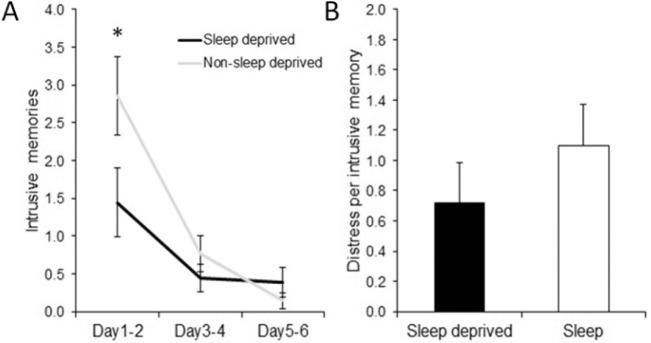
(A) Day-by-day profile of intrusive memories: The average number of intrusive memories for each 2-day period for each group. The sleep deprived group show a significantly lower frequency of intrusive memories for the first 2-day period. (B) Level of distress: no difference between groups in the mean level of distress reported when experiencing an intrusive memory. *P < 0.05, error bars represent standard error of the mean.
Post hoc analysis of the first 2 days following the trauma film was carried out to ensure the group difference in intrusive memory frequency was unlikely to be driven by potential fatigue experienced by the sleep deprived individuals. The number of intrusive memories on day 1 within the sleep deprived group (mean 0.94, SD 1.56) was significantly lower compared to the sleep group (mean 1.81, SD 1.57, Poisson GLM fitted z = 2.23, P = 0.03). On day 2, a non-significant trend of fewer intrusive memories being reported by the sleep deprived group (mean 0.50, SD 0.71) compared to the sleep group (mean 1.05, SD 0.97) was also found (Poisson GLM fitted z = 1.87, P = 0.0616). If the group difference was being driven by fatigue-related effects, an increase in intrusive memories would have been expected. However, no such increase was seen between the number of intrusive memories reported for day 1 and day 2 for the sleep deprived group (day 1 mean 0.94, SD 1.55, day 2 mean 0.5, SD 0.70, Poisson GLM fitted z = −1.54, P = 0.123).
DISCUSSION
This experimental study is the first that demonstrates a benefit of sleep deprivation during the first night following an analogue psychological trauma against the development of intrusive memories. Keeping subjects awake, as compared to letting them sleep, resulted in a lower psychological effect of the analogue traumatic event and fewer intrusive emotional memories subsequently. These findings provide evidence that initial sleep may actually worsen outcomes to psychologically traumatic stimuli and paradoxically, sleep deprivation on the first night may be advantageous for the amelioration of analogue trauma-related responses.
The possibility of a beneficial effect of sleep deprivation compared to sleep appears counterintuitive. Deprivation of sleep is generally considered a problem, because chronic deficit of sleep is associated with poor health,47–50 whereas short-term sleep deprivation is associated with a range of deleterious effects particularly on cognitive functions.51 Usually sleep improves memory performance and positively enhances the consolidation of some aspects of emotional memory, when tested by recall and recognition paradigms.1–4 However, when memories become intrusive (i.e., flashback-type memories) it may be better not to remember in this manner. Therefore, not sleeping immediately following a psychologically traumatic event may actually be helpful in this regard. That is, sleep deprivation may prevent the sleep dependent memory enhancement52–54 by disrupting the consolidation of aspects of the trauma film memory that could otherwise spring back to mind involuntarily as emotional intrusive memories.
Although clinically important, intrusive emotional memories are poorly understood. Current theories suggest that they are generated by a shift in the way an event is processed or laid down from focusing on the meaning and context of the event towards sensory impressions.10,55,56 Intrusive memories by their very nature involuntarily spring to mind without a deliberate attempt of retrieval. Laboratory-based studies have found that performance on recall and recognition tests of memory of the trauma film is not correlated to the frequency of intrusive memories.57,58 This underscores the need to better understand factors such as sleep, which may selectively affect intrusive emotional memories, and future studies should differentiate the effect on different types of memory.
The effect of sleep on emotional memory has been tested primarily on explicit memory (intentionally remembered),1–4,59 but there is growing evidence that sleep deprivation preferentially affects implicit memory (unintentionally remembered) over explicit memory. Using motor vehicle accident films, Kuriyama and colleagues17 have shown that implicit memory (self-reported fear and skin conductance), but not explicit memory, was disrupted following sleep deprivation. Baran and colleagues21 have shown that implicit memory (emotional reaction) for a set of negative pictures was reduced following a period of wake, but preserved following sleep. Taken together, these studies emphasize that not sleeping following an emotional event may differentially impair implicit, compared to explicit, memory of that event. Thus, in our study it is possible that fewer intrusive memories are reported following sleep deprivation as a result of disruption of implicit memory of the trauma film. It would be useful to test the level of explicit, emotionally unrelated memory recall related to the content of the film. We did not utilize declarative memory tests in the current study, but previous research has shown that verbal or visual recall tests for a similar analogue trauma film did not appear to relate to the frequency of intrusive memories reported.57,58 However, these studies did not involve a period of sleep deprivation, and thus, future research should include measures of explicit memory of the film to ensure that conscious recognition of the film is not affected by sleep deprivation.
Alternatively, the difference in intrusive memory frequency seen between the groups could be due to the additional period of wakefulness experienced by the sleep deprived group after the film, compared to the sleep group. It could be argued that “length of time awake” after the traumatic event affects the development of intrusive memory production. However, preliminary data (unpublished, Wulff et al.) do not support this possibility because no difference was found in the frequency of intrusive memories between two groups with different lengths of time between the analogue traumatic exposure and concurrently aligned recording times of intrusive memory.
Within this study we sought to identify factors other than sleep deprivation that may have influenced our key measures (IES, intrusive memory frequency). Immediately after the trauma film the effect of the film on mood and state dissociation were assessed. This key manipulation check showed that mood deteriorated and dissociation increased, confirming the negative effect of the trauma film. Critically, however, no differences between the groups were found, indicating that both groups had the same initial experience of the trauma film before sleep deprivation occurred.
Groups were also well matched at baseline for participant characteristics, sleep, and chronotype, indicating that trait characteristics of the participants cannot account for the differences seen between the groups. We note that participants in the sleep group returned home to sleep in their familiar environment following the analogue psychological trauma rather than sleeping in the sleep laboratory to avoid disturbed sleep due to a novel environment (“first night” effect). It is possible that this may have altered the sleep participants' reaction to the study and be a component in which sleep deprivation in a sleep laboratory environment is beneficial.
Regardless of the mechanism, our findings indicate that sleep deprivation in a sleep laboratory in the immediate aftermath of an analogue psychological trauma manipulation could be important for moderating the psychological response to experimental trauma. The extent to which these findings have implications for pathological responses to real-world trauma is not known. This is an area of research that requires extensive investigation to fully elucidate the role of sleep in the response to psychological trauma. Such research however may be particularly valuable as sedatives are still readily prescribed to encourage sleep following trauma,60 even though their use is contraindicated in acutely traumatized people.61
This analogue study has some limitations, and the first concerns the suitability of an experimentally induced “trauma” using a film with traumatic content as an analogue stressor of real-life trauma. Although intrusive memories are often maintained long term after a real-life trauma, the trauma film as an experimental stressor has been shown to reliably produce short-term, intrusive memories.32 The induction of intrusive memories for a transient period is sufficient to investigate mechanisms of intrusive memory generation. Future research should ensure continuous screening of sleep quality and quantity utilizing sleep diaries and actigraphy before and during the post-trauma film period. A continuation of sleep measurements following the trauma film would allow an estimation of the effect of potential sleep disturbances during this period on the frequency of intrusive memories. Furthermore, the collection of dream reports relating to the trauma film would be of particular interest, because distressing and recurrent dreams relating to a trauma are also diagnostic criteria for both ASD and PTSD.5
The context in which the study participants processed the trauma film may also have influenced their outcome. Recently Kuriyama and colleagues18 have shown that directed forgetting (i.e., deliberately trying to forget) an aversive film increased fear responses following sleep deprivation. Thus, future studies must consider the way in which participants process such experimental traumatic stimuli. Finally, the participants of the sleep-as-usual group returned home to sleep, whereas the sleep deprived participants remained in the sleep laboratory. Although this difference in environment may have altered the experience of the groups, sleeping in an unfamiliar environment is well known to lower sleep quality, while remaining awake in the hospital setting is consistent with the potential use of sleep deprivation in clinical practise.
In conclusion, this study has demonstrated that a period of sleep deprivation on the first night (rather than sleep as usual) after viewing experimental trauma results in a lower psychological impact, and fewer intrusive memories of that event. Our findings support the hypothesis that in some situations of high emotional distress, and perhaps counterintuitively, sleep deprivation on the first night after an experimental trauma may be beneficial, a suggestion that warrants further investigation.
DISCLOSURE STATEMENT
This was not an industry supported study. These experiments were partly supported by a Wellcome Trust Strategic Award [098461/Z/12/Z] to the Sleep and Circadian Neuroscience Institute (SCNi) and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust, Oxford University (KW and RGF [REF A90305 and A92181] and EAH). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. Emily A. Holmes is also supported by the Medical Research Council (United Kingdom) intramural programme [MRC-A060-5PR50] and a Wellcome Trust Clinical Fellowship [WT088217]. Dr. Goodwin has received research support from Bailly Thomas, Medical Research Council, NIHR, Wellcome Trust, and Servier; holds shares in P1vital; and has served as consultant, advisor or CME speaker for AstraZeneca, BMS, Boehringer Ingelheim, Cephalon/Teva Janssen-Cilag, Eli Lilly, Lundbeck, Otsuka, P1Vital, Roche, Servier, Shering Plough, Shire, Sunovion, and Takeda. The other authors have indicated no financial conflicts of interest. This work was performed at the University of Oxford, Oxford, UK.
ACKNOWLEDGMENTS
The authors thank Stephanie Halford, Iona Alexander, Benita Middleton, Laura Hoppitt, and Ella James for comments on this manuscript, Daniel Lunn for statistical consultation, and the research assistants for the their help with the sleep deprivation including: Emma Wams, Emma Hirons, Bernard Maybury, Julia Pakpoor, Manaka Paranathala, and Emily Tweed.
Footnotes
A commentary on this article appears in this issue on page 997.
REFERENCES
- 1.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–9. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. 2008;19:781–8. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–66. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17:891–8. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistial Manual of Mental Disorders. Fifth ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 6.Conway MA. Sensory-perceptual episodic memory and its context: autobiographical memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1375–84. doi: 10.1098/rstb.2001.0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackmann A, Ehlers A, Speckens A, Clark DM. Characteristics and content of intrusive memories in PTSD and their changes with treatment. J Trauma Stress. 2004;17:231–40. doi: 10.1023/B:JOTS.0000029266.88369.fd. [DOI] [PubMed] [Google Scholar]
- 8.Holmes EA, Grey N, Young KAD. Intrusive images and “hotspots” of trauma memories in Posttraumatic Stress Disorder: an exploratory investigation of emotions and cognitive themes. J Behav Ther Exp Psychiatry. 2005;36:3–17. doi: 10.1016/j.jbtep.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Kvavilashvili L. Solving the mystery of intrusive flashbacks in posttraumatic stress disorder: comment on Brewin (2014) Psychol Bull. 2014;140:98–104. doi: 10.1037/a0034677. [DOI] [PubMed] [Google Scholar]
- 10.Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behav Res Ther. 2000;38:319–45. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 11.O'Donnell ML, Elliott P, Lau W, Creamer M. PTSD symptom trajectories: from early to chronic response. Behav Res Ther. 2007;45:601–6. doi: 10.1016/j.brat.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Bryant RA, Creamer M, O'Donnell M, Silove D, McFarlane AC. The capacity of acute stress disorder to predict posttraumatic psychiatric disorders. J Psychiatr Res. 2012;46:168–73. doi: 10.1016/j.jpsychires.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Kuyken W, Brewin CR. Intrusive memories of childhood abuse during depressive episodes. Behav Res Ther. 1994;32:525–8. doi: 10.1016/0005-7967(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 14.Holmes EA, Mathews A. Mental imagery in emotion and emotional disorders. Clin Psychol Rev. 2010;30:349–62. doi: 10.1016/j.cpr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Parry L, O'Kearney R. A comparison of the quality of intrusive memories in post-traumatic stress disorder and depression. Memory. 2014;22:408–25. doi: 10.1080/09658211.2013.795975. [DOI] [PubMed] [Google Scholar]
- 16.Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biol Psychiatry. 2006;60:788–90. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 17.Kuriyama K, Soshi T, Kim Y. Sleep deprivation facilitates extinction of implicit fear generalization and physiological response to fear. Biol Psychiatry. 2010;68:991–8. doi: 10.1016/j.biopsych.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Kuriyama K, Honma M, Yoshiike T, Kim Y. Memory suppression trades prolonged fear and sleep-dependent fear plasticity for the avoidance of current fear. Sci Rep. 2013;3:2227. doi: 10.1038/srep02227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–97. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 20.Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–48. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baran B, Pace-Schott EF, Ericson C, Spencer RMC. Processing of emotional reactivity and emotional memory over sleep. J Neurosci. 2012;32:1035–42. doi: 10.1523/JNEUROSCI.2532-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner U, Fischer S, Born J. Changes in emotional responses to aversive pictures across periods rich in slow-wave sleep versus rapid eye movement sleep. Psychosom Med. 2002;64:627–34. doi: 10.1097/01.psy.0000021940.35402.51. [DOI] [PubMed] [Google Scholar]
- 23.Groch S, Wilhelm I, Diekelmann S, Born J. The role of REM sleep in the processing of emotional memories: evidence from behavior and event-related potentials. Neurobiol Learn Mem. 2013;99:1–9. doi: 10.1016/j.nlm.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Bryant RA, Creamer M, O'Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33:69–74. doi: 10.1093/sleep/33.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159:855–7. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- 26.Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696–701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- 27.Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20:893–901. doi: 10.1002/jts.20246. [DOI] [PubMed] [Google Scholar]
- 28.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;2:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 29.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 30.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Nader K, Einarsson EO. Memory reconsolidation: an update. Ann N Y Acad Sci. 2010;1191:27–41. doi: 10.1111/j.1749-6632.2010.05443.x. [DOI] [PubMed] [Google Scholar]
- 32.Holmes EA, Bourne C. Inducing and modulating intrusive emotional memories: a review of the trauma film paradigm. Acta Psychol (Amst) 2008;127:553–66. doi: 10.1016/j.actpsy.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Horowitz MJ. Psychic trauma. Return of images after a stress film. Arch Gen Psychiatry. 1969;20:552–9. doi: 10.1001/archpsyc.1969.01740170056008. [DOI] [PubMed] [Google Scholar]
- 34.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. quiz 34–57. [PubMed] [Google Scholar]
- 35.Horne JA, Ostberg A. self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 36.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 37.McNair DM, Lorr M, Droppleman LF. Profile of mood states manual (revision) San Diego: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 38.Eysenck HJ, Eysenck SBG. Manual of the Eysenck personality Inventory. London: University of London Press; 1964. [Google Scholar]
- 39.Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–36. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 40.Weiss DS, Marmar CR. The Impact of Event Scale – Revised. In: Wilson JP, Keane TM, editors. Assessing Psychological Trauma and PTSD: A Handbook for Practitioners. New York: Guildford Press; 1997. pp. 399–411. [Google Scholar]
- 41.Lang TJ, Moulds ML, Holmes EA. Reducing depressive intrusions via a computerized cognitive bias modification of appraisals task: developing a cognitive vaccine. Behav Res Ther. 2009;47:139–45. doi: 10.1016/j.brat.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Deeprose C, Zhang S, Dejong H, Dalgleish T, Holmes EA. Imagery in the aftermath of viewing a traumatic film: using cognitive tasks to modulate the development of involuntary memory. J Behav Ther Exp Psychiatry. 2012;43:758–64. doi: 10.1016/j.jbtep.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmes EA, James EL, Coode-Bate T, Deeprose C. Can playing the computer game “Tetris” reduce the build-up of flashbacks for trauma? A proposal from cognitive science. PLoS ONE. 2009;4:e4153. doi: 10.1371/journal.pone.0004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirschfeld RMA, Williams JBW, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873–5. doi: 10.1176/appi.ajp.157.11.1873. [DOI] [PubMed] [Google Scholar]
- 45.Markus CR, Firk C. Differential effects of tri-allelic 5-HTTLPR polymorphisms in healthy subjects on mood and stress performance after tryptophan challenge. Neuropsychopharmacology. 2009;34:2667–74. doi: 10.1038/npp.2009.92. [DOI] [PubMed] [Google Scholar]
- 46.Barnett V, Lewis T. Chichester: Wiley; 1994. Outliers in statistical data. 3rd ed. Wiley series in probability and mathematical statistics Applied probability and statistics. [Google Scholar]
- 47.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–61. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–66. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–28. [PMC free article] [PubMed] [Google Scholar]
- 52.Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 53.Walker MP. Sleep-dependent memory processing. Harv Rev Psychiatry. 2008;16:287–98. doi: 10.1080/10673220802432517. [DOI] [PubMed] [Google Scholar]
- 54.Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brewin CR, Holmes EA. Psychological theories of posttraumatic stress disorder. Clin Psychol Rev. 2003;23:339–76. doi: 10.1016/s0272-7358(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 56.Brewin CR, Dalgleish T, Joseph S. A dual representation theory of posttraumatic stress disorder. Psychol Rev. 1996;103:670–86. doi: 10.1037/0033-295x.103.4.670. [DOI] [PubMed] [Google Scholar]
- 57.Nixon RD, Nehmy T, Seymour M. The effect of cognitive load and hyperarousal on negative intrusive memories. Behav Res Ther. 2007;45:2652–63. doi: 10.1016/j.brat.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Holmes EA, Brewin CR, Hennessy RG. Trauma films, information processing, and intrusive memory development. J Exp Psychol Gen. 2004;133:3–22. doi: 10.1037/0096-3445.133.1.3. [DOI] [PubMed] [Google Scholar]
- 59.Payne JD, Kensinger EA. Sleep leads to changes in the emotional memory trace: evidence from FMRI. J Cogn Neurosci. 2011;23:1285–97. doi: 10.1162/jocn.2010.21526. [DOI] [PubMed] [Google Scholar]
- 60.Zohar J, Juven-Wetzler A, Sonnino R, Cwikel-Hamzany S, Balaban E, Cohen H. New insights into secondary prevention in post-traumatic stress disorder. Dialogues Clin Neurosci. 2011;13:301–9. doi: 10.31887/DCNS.2011.13.2/jzohar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The Management of Post-traumatic Stress Working Group. VA/DoD Clinical practise guildlines for management of post-traumatic stress. Department of Veteran Affairs and Department of Defense; 2010. [Google Scholar]