Abstract
Study Objectives:
The attenuation of heart rate recovery after maximal exercise (ΔHRR) is independently impaired by obstructive sleep apnea (OSA) and metabolic syndrome (MetS). Therefore, we tested the hypotheses: (1) MetS + OSA restrains ΔHRR; and (2) Sympathetic hyperactivation is involved in this impairment.
Design:
Cross-sectional study.
Participants:
We studied 60 outpatients in whom MetS had been newly diagnosed (ATP III), divided according to apnea-hypopnea index (AHI) ≥ 15 events/h in MetS + OSA (n = 30, 49 ± 1.7 y) and AHI < 15 events/h in MetS - OSA (n = 30, 46 ± 1.4 y). Normal age-matched healthy control subjects (C) without MetS and OSA were also enrolled (n = 16, 46 ± 1.7 y).
Interventions:
Polysomnography, microneurography, cardiopulmonary exercise test.
Measurements and Results:
We evaluated OSA (AHI - polysomnography), muscle sympathetic nerve activity (MSNA - microneurography) and cardiac autonomic activity (LF = low frequency, HF = high frequency, LF/HF = sympathovagal balance) based on spectral analysis of heart rate (HR) variability. ΔHRR was calculated (peak HR minus HR at first, second, and fourth minute of recovery) after cardiopulmonary exercise test. MetS + OSA had higher MSNA and LF, and lower HF than MetS - OSA and C. Similar impairment occurred in MetS - OSA versus C (interaction, P < 0.01). MetS + OSA had attenuated ΔHRR at first, second, and at fourth minute than did C, and attenuated ΔHRR at fourth minute than did MetS - OSA (interaction, P < 0.001). Compared with C, MetS - OSA had attenuated ΔHRR at second and fourth min (interaction, P < 0.001). Further analysis showed association of the ΔHRR (first, second, and fourth minute) and AHI, MSNA, LF and HF components (P < 0.05 for all associations).
Conclusions:
The attenuation of heart rate recovery after maximal exercise is impaired to a greater degree where metabolic syndrome (MetS) is associated with moderate to severe obstructive sleep apnea (OSA) than by MetS with no or mild or no OSA. This is at least partly explained by sympathetic hyperactivity.
Citation:
Cepeda FX, Toschi-Dias E, Maki-Nunes C, Rondon MU, Alves MJ, Braga AM, Martinez DG, Drager LF, Lorenzi-Filho G, Negrao CE, Trombetta IC. Obstructive sleep apnea impairs postexercise sympathovagal balance in patients with metabolic syndrome. SLEEP 2015;38(7):1059–1066.
Keywords: heart rate recovery, heart rate variability, muscle sympathetic nerve activity
INTRODUCTION
Patients with metabolic syndrome (MetS) often have obstructive sleep apnea (OSA).1–4 The reverse is also true, the probability being very high (9.1 times greater) that patients with OSA have MetS.5 The overlap of OSA and MetS leads to additional cardiovascular risk in these patients. Strong evidence shows that the substrates to developing cardiovascular disease are linked to autonomic dysfunction.6,7 The reasons for the increased cardiovascular risk in MetS are not completely understood, and in addition to metabolic disturbances, sympathetic hyperactivation and depressed baroreflex are involved in the pathophysiological basis.6 However, OSA in patients with MetS causes further sympathetic hyperactivation8 and decreased baroreflex sensitivity.4 Moreover and interestingly, augmented chemoreflex sensitivity occurs only when both conditions are present.9
Evaluation of hemodynamic changes during cardiopulmo-nary exercise testing and during the recovery period has been considered a simple and useful marker for identification of autonomic dysfunction.10 The inability to increase the heart rate (HR reserve) or to facilitate heart rate recovery after maximal exercise (ΔHRR) has been consistently associated with long-term increases in mortality in different subsets of patients and even in a population of adults without evidence of cardiovascular disease.10–13 Although the reduction in heart rate during the postexercise period has been mostly attributed to parasym-pathetic recovery,14 the restoration of the heart rate after exercise termination to the baseline levels takes a few minutes and depends on the interaction of parasympathetic reactivation and sympathetic withdrawal.15 However, because the heart rate response is subject to parasympathetic and sympathetic control, it is legitimate to question whether the attenuation of heart rate recovery is a consequence of an imbalance between these two cardiac controls.
Several studies have demonstrated attenuated ΔHRR in patients with either OSA16,17 or MetS.18,19 However, whether the overlap of both conditions further impairs ΔHRR and the mechanisms underlying this imbalance are still not clear. Considering that patients with MetS and OSA have sympathetic hyperactivation at baseline conditions, we sought to investigate the effect of this autonomic dysfunction on the ΔHRR.
Thus, the current study aimed to investigate whether the overlap of OSA in subjects with MetS further delays the ΔHRR, and whether sympathetic hyperactivation would be associated with this impairment.
MATERIALS AND METHODS
Study Population
We invited outpatients from the Heart Institute (InCor) to participate in the study immediately after receiving a diagnosis of MetS providing they met the criteria described next. Thus, patients with MetS were eligible if they had at least three of the five diagnostic criteria, as proposed by the Adult Treatment Panel III Report,20 as follows: (1) waist circumference ≥ 102 cm in men and ≥ 88 cm in women; (2) fasting triglyceride level ≥ 150 mg/dL (> 1.69 mmol/L); (3) high-density lipoprotein (HDL) cholesterol < 40 mg/dL (< 1.03 mmol/L) in men and < 50 mg/dL (< 1.29 mmol/L) in women; (4) blood pressure (BP) ≥ 130 or 85 mmHg; and (5) fasting glucose leve1 ≥ l00 mg/dL (≥ 6.1 mmol/L). In addition, patients were eligible for enrollment if they were sedentary, i.e., had no regular physical activity (> 3 days/w, > 30 min/day) for the previous 6 mo, were between 30 and 60 y of age, were taking no medications, were nonsmokers, had no history of excessive alcohol consumption, and had no evidence of pulmonary or cardiovascular disease (no history of prior invasive cardiac procedures or congestive heart failure, congenital or valvular heart disease, the pre-excitation syndrome, or left bundle-branch block) and musculoskeletal disorders that would preclude maximal aerobic exercise test.
After clinical examination and blood tests, all patients who met the inclusion criteria and agreed to participate in the study underwent nocturnal polysomnography to detect the presence of OSA.
Moderate to severe OSA was characterized by an apnea/ hypopnea index (AHI) ≥ 15 events/h by polysomnography.4,8,9 Therefore patients were divided according to AHI into MetS with moderate-severe OSA (MetS + OSA) and MetS with no or mild OSA (MetS - OSA). Healthy, sedentary normal weight subjects without either MetS or OSA (AHI < 5 events/h) were recruited as a control group (C). Approximately 60% of the studied patients had also participated in other autonomic evaluation studies.4–9 We continued subject recruitment in order to achieve the number required by sample size calculation for the current study.
The study was approved by the Scientific Commission of the Heart Institute (InCor), and by the Ethics in Research Commission of the Clinical Hospital, University of São Paulo (#1222/05), and each subject gave written consent.
Experimental Design
The evaluations were performed on different days with intervals of between 2 days and 1 w between them. On the first visit, total serum cholesterol, triglycerides, HDL cholesterol (enzymatic method), and plasma glucose (standard glucose oxidase method) concentrations were assayed from a venous blood sample after overnight fasting. On the second visit, clinical and physical examinations were undertaken and standard blood pressure (BP), body mass index (BMI), and waist circumference (WC) were measured. After MetS was confirmed nocturnal polysomnography was performed. Finally, autonomic control and cardiopulmonary exercise tests were conducted.
Procedures and Measures
Sleep Study
All subjects underwent a standard overnight polysomnography using an Embla digital system (17 channels, EMBLA, Flaga hf. Medical Devices, Reykjavik, Iceland) as previously described.21 The polysomnography started at approximately 22:00 (lights off) and finished at approximately 06:00 (lights on). The AHI was calculated as the total number of respiratory events (apneas plus hypopneas) per hour of sleep. Apnea was defined as complete cessation of airflow for at least 10 sec, and hypopnea was defined as a significant reduction (> 50%) in respiratory signals for at least 10 sec associated with 3% oxygen desaturation.22
Autonomic Control Evaluation
The subjects were instructed to refrain from eating 2 h before the test, and to abstain from caffeine and physical activity for the 48 h leading up to the evaluation. The experimental protocol was performed in the morning in a quiet, temperature-controlled (22°C) room. Then, muscle sympathetic nerve activity and heart rate were recorded over 10 min with the patients lying supine.
Muscle Sympathetic Nerve Activity (MSNA). The MSNA was directly measured through a multiunit postganglionic efferent from the peroneal nerve using the microneurography technique, which consists of the impaction of a tungsten micro-electrode to the peroneal nerve.23 The neurogram was recorded using a software program (WinDaq Software, Transonic Systems, Dataq Instruments Inc, Akron, OH, USA).
Power Spectrum Analysis of HR Variability. HR was recorded through lead II of an electrocardiogram (EKG) to evaluate power spectrum analysis of HR variability. Cardiovascular fluctuations of the R-R interval underwent autoregressive spectral decomposition to the automatic quantification of the center frequency and the power of each relevant component in absolute value (expressed in milliseconds squared) as well as in normalized units (nu). Components of the frequency band of 0.04 to 0.15 Hz are considered low frequency (LF), which reflects both cardiac sympathetic and parasympathetic modulation.21 The components ranging between 0.15 and 0.40 Hz, synchronized with the breath, are considered high frequency (HF) and are components that reflect parasympathetic modulation.24 The normalization procedure was performed by dividing the power of the LF or HF component by the total spectral power from which the power of the very low frequency (VLF, range: 0 to 0.04Hz) component had been subtracted and by multiplying the result by 100.25,26 Furthermore, the ratio LF and HF (LF/HF) was calculated to obtain the sympathovagal balance.
Cardiopulmonary Exercise Test
The subjects were instructed to eat a light meal 1–2 h before the test, and to abstain from caffeine and physical activity 48 h leading up to evaluation. Before each test, the gas analyzers were calibrated using gases of known concentrations, while the flow meter was calibrated using a 3-L syringe. The ventilatory parameters during graded exercise were collected after complete adaptation to the mouthpiece and nose clip. Oxygen consumption (VO2) and carbon dioxide output (CO2) were obtained on a breath-by-breath basis and expressed as 30-sec averages using an indirect calorimetry system (Vmax, series 229 – Sensor Medics Corporation, Buena Vista, CA, USA).
Functional Capacity. The functional capacity was determined by maximal oxygen consumption (peak VO2) achieved at the end of the exercise period.
Peak O2 Pulse. The peak O2 pulse was calculated by peak VO2 (mL/kg/min) divided by peak HR (bpm) multiplied by 100.27
HR was continuously recorded at rest, during graded exercise test, and at the recovery period using a 12-lead electrocardiogram (EKG, Marquette Medical Systems, Inc. CardioSoft, Milwaukee, WI, USA). The bicycle (Ergoline ViaSprint 150P, Bitz, Germany) maximal cardiopulmonary exercise test was performed using a ramp protocol with 10- to 20-W increments every minute up to exhaustion.
HR Reserve. The HR reserve was calculated as peak HR minus resting HR before exercise.13
Both of the following criteria were used to define maximal effort achievement: (1) when the subject no longer maintained the bicycle cadence at 60 rpm and was unable to continue exercising despite verbal encouragement, demonstrating evidence of exhaustion, and (2) maximal respiratory exchange ratio (RER) > 1.10.28 Because subjects without clinically signifi-cant heart disease can exhibit exercise-induced hypotension, caused by delayed in the postexercise increase in systemic vascular resistance following prolonged high-intensity exercise, we chose an active recovery protocol similar to that in other studies.29–31Thus, in the recovery period, the first minute (50% of the maximum peak workload) and the second minute (25% of the maximum peak workload) were active with velocity at 60 rpm, whereas the third and fourth minutes were passive, with the patients still sitting on the bicycle.
HR Recovery. ΔHRR was evaluated by EKG and defined as the difference between peak HR and HR at the first, second, and fourth minute postexercise during the recovery period.10
Statistical Analysis
The data are presented as mean ± standard error. A chi-square (χ2) test was used to assess categorical data differences. The Kolmogorov–Smirnov and Levine tests were used to assess the normality and homogeneity of distribution of each variable studied. Physical characteristics, hemodynamic and autonomic data were compared using one-way analysis of variance followed by Scheffé post hoc multiple comparisons or Kruskall-Wallis test. Values of P < 0.05 were considered statistically significant. The univariate correlation (Pearson correlation) between ΔHRR at the first, second, and fourth minute with AHI, MSNA, LF, HF, and LF/HF were conducted to test the association.
RESULTS
Eighty-two consecutive patients who received a recent diagnosis of MetS were initially invited to participate in the study. Seven declined to participate, four were smokers, and two were pregnant. From those who are eligible and agreed to participate, nine patients were excluded because they did not reach the maximal effort achievement criteria in the cardiopulmo-nary exercise test (six in MetS + OSA and three in MetS - OSA group). Finally, there were 60 eligible patients for the analyses. They were allocated into two groups according to AHI obtained by nocturnal polysomnography: (1) with moderate-severe OSA (MetS + OSA; n = 30); and (2) with mild or no OSA (MetS - OSA; n = 30). For the healthy C group we selected 21 individuals without MetS. From these five were excluded because they were found to have OSA (AHI ≥ 5 events/h) on polysomnography. Thus, 16 healthy subjects without MetS or OSA were also studied (C).
Baseline characteristics of the study groups are shown in Table 1. Sex distribution and age were similar among groups. Patients with MetS + OSA and MetS - OSA were similar, and had higher BMI, WC, glucose, triglycerides, and systolic and diastolic blood pressure and lower levels of HDL-c than the C group (Table 1). Similarly, both MetS groups had a similar prevalence of all MetS risk factors.
Table 1.
Physical characteristics, metabolic syndrome diagnostic criteria measurements and polysomnography measurements.
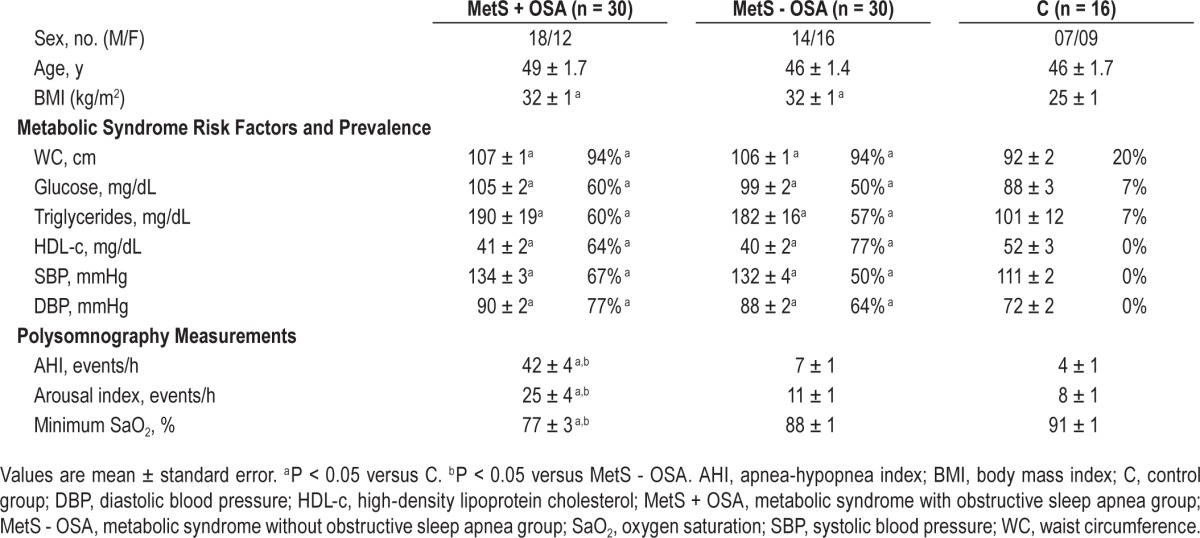
Regarding the nocturnal polysomnography measurements, no differences were found in sleep efficiency, stage 1, stage 2, stage 3 and in rapid eye movement stage of sleep among groups (data not shown). However, AHI and arousal index were higher and minimum oxygen saturation was lower in the MetS + OSA group compared with that in the MetS - OSA and C groups (Table 1).
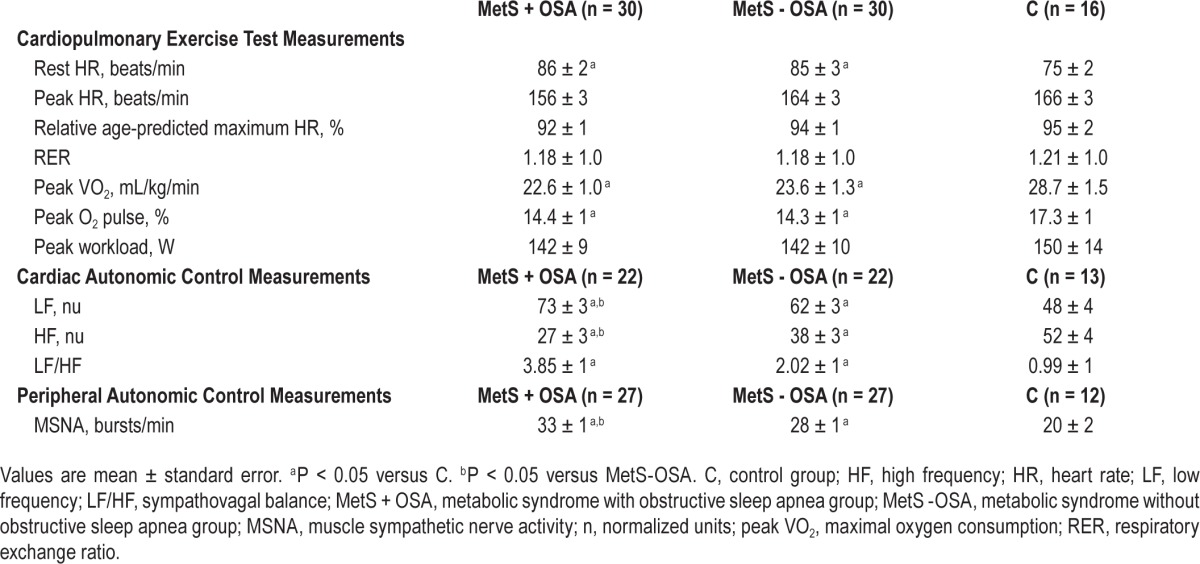
Table 2 shows cardiopulmonary exercise test measurements and cardiac and peripheral autonomic control measurements. Both MetS groups are similar and had higher resting HR and lower peak VO2 and peak O2 pulse than C. No differences in peak HR, relative age-predicted maximum HR, RER, and peak workload were found among groups. MSNA and LF were significantly higher and HF lower in MetS + OSA compared to that in MetS - OSA and C groups. In 10 patients (three in MetS + OSA, three in MetS - OSA, and four in C group) we were not able to obtain a satisfactory nerve recording. Similarly, 19 subjects (eight in MetS + OSA, eight in MetS - OSA, and three in C group) were excluded from the power spectrum analysis of heart rate variability because their spontaneous respiratory rate was below the required high frequency band (HF band). The comparisons between MetS - OSA and C showed that MSNA and LF were significantly higher and HF significantly lower in MetS - OSA patients than in C. LF/HF was similar in both MetS groups and higher than that in C (Table 2).
Table 2.
Cardiopulmonary exercise test results, cardiac and peripheral autonomic control measurements.
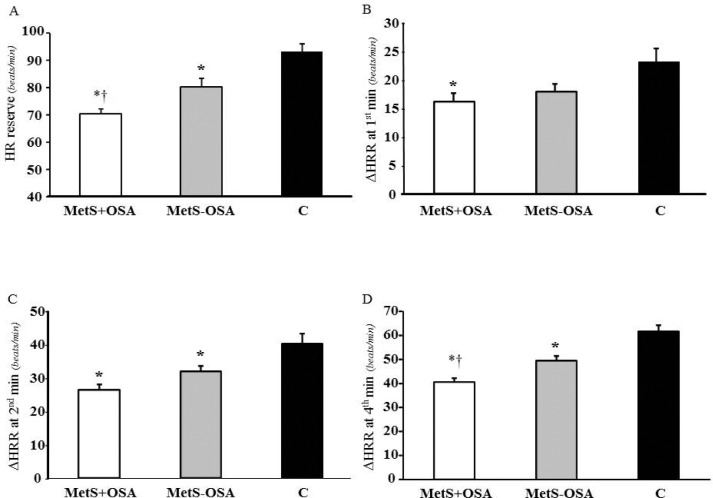
The HR reserve and ΔHRR at first, second, and fourth minute postexercise are shown in Figure 1 (panel A, B, C, and D, respectively). The HR reserve was attenuated in MetS + OSA relative to MetS - OSA and both were attenuated relative to C (70 ± 3, 80 ± 3 and 93 ± 3 bpm, interaction P < 0.001, respectively). Interestingly, MetS + OSA had similar ΔHRR at first minute to MetS - OSA, but this was attenuated compared with C (16 ± 2, 18 ± 1 and 24 ± 2 bpm, interaction P = 0.008). ΔHRR at second minute (26 ± 2, 32 ± 2, and 40 ± 3 bpm, interaction P < 0.001) and ΔHRR at fourth minute (40 ± 2, 50 ± 2, and 61 ± 3 bpm, interaction P < 0.001), were attenuated in both MetS groups compared with C. However, only in the ΔHRR at fourth minute MetS + OSA was attenuated compared with MetS - OSA.
Figure 1.
(A) Heart rate reserve (HR reserve) and (B) attenuation of the heart rate recovery after exercise (ΔHRR) at first, (C) second and (D) fourth minute in patients with metabolic syndrome and obstructive sleep apnea (MetS + OSA), without obstructive sleep apnea (MetS - OSA) and in control subjects (C). *P ≤ 0.05 versus C, †P ≤ 0.05 versus MetS - OSA.
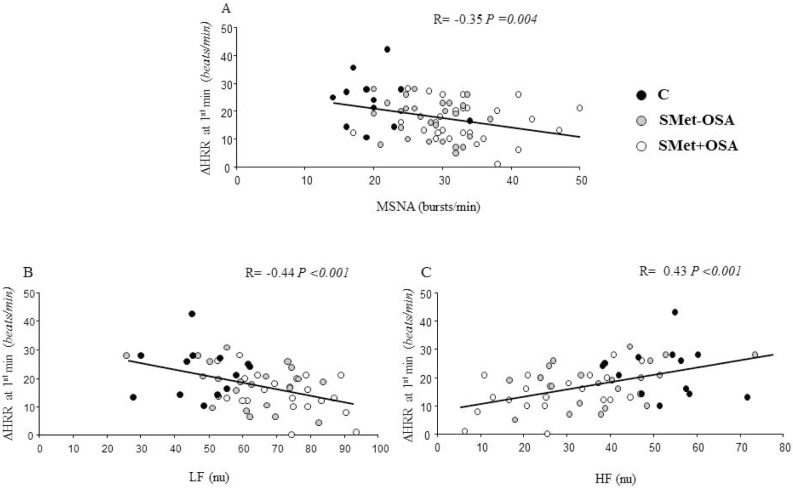
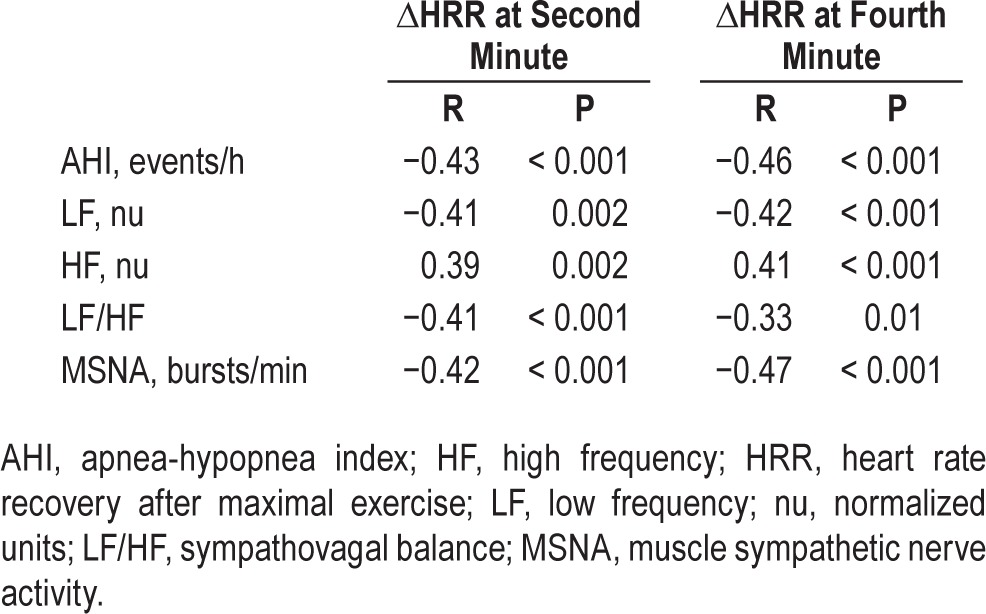
Further analysis showed significant correlations between ΔHRR at first minute and AHI (R = −0.27, P = 0.03) and LF/ HF (R = −0.41, P = 0.001). In addition, there were correla -tions between ΔHRR at first minute and MSNA, LF, and HF (Figure 2). Similarly, ΔHRR either at second minute or at fourth minute were correlations with AHI, LF, HF, LF/HF, and MSNA (Table 3).
Figure 2.
Pearson correlation between ΔHRR at first minute and (A) muscle sympathetic nerve activity (MSNA), (B) low frequency (LF), and (C) high frequency (HF) in all patients with metabolic syndrome and in normal control subjects.
Table 3.
Univariate linear regression.
DISCUSSION
To the best of our knowledge, this is the first study to investigate the HR responses to exercise in individuals with a new diagnosis of MetS and untreated OSA. The main finding of the study is that patients with both MetS and moderate to severe OSA had a more attenuated HR reserve and ΔHRR after maximal exercise than seen with MetS with no or mild OSA relative to the control group.
Previous observational studies demonstrated that patients with MetS had reduced HRR during the postexercise period.32,33 In addition, Spies et al.34 reported that the MetS was associated with impaired ΔHRR independently of diabetes, hypertension, and obesity in patients with coronary heart disease. In the current investigation, we found that patients with MetS and OSA have greater MSNA, greater LH component, and lower HF component than do MetS patients. In addition, there is an inverse association between ΔHRR and MSNA and LF component.
OSA is quite common in patients with MetS and we have provided additional findings3 suggesting that OSA may further contribute to impaired ΔHRR after exercise training in patients with MetS. The importance of our results is that diminished ΔHRR after maximal exercise test, mainly the rate of decrease in HR during the first 2 min of recovery,35 is a powerful predictor of cardiovascular disease and mortality.10–12,14 Thus, the evaluation of HR recovery is a simple and important tool with prognostic value to evaluate the integrity of the sympathovagal balance. It was established that ΔHRR less than 12 beats/min at the first minute of recovery10 and less than 22 beats/min12 at the second minute of recovery following exercise is associated with increased mortality.
The notion that OSA could further impair ΔHRR after progressive maximal exercise in patients with MetS seems to be linked to a sympathovagal imbalance. Although impaired ΔHRR has been thought of as an important tool to assess para-sympathetic control,14 in patients with increased baseline sympathetic tonus, it is reasonable to expect that this sympathetic exacerbation will restrain the recovery of the HR even at the first minute after exercise. Our results show that the overlap of OSA in patients with MetS could have an additional impairment over the vagal modulation, as well as an additional increase over cardiac and muscular sympathetic modulation.
Accumulated evidence shows that sympathetic outflow is an independent predictor of mortality in patients with heart failure.7 It is possible that the same occurs in patients with MetS, especially those with OSA. The explanation of the increased sympathetic outflow is out of the scope of the current study. However, someone could suggest candidates for such an autonomic alteration. Obesity36 and high levels of insulin37 and blood pressure34 can be the key to the beginning of sympathetic hyperactivity in MetS. Nevertheless, the association of OSA causes an overburden, because several acute pathophysiological reflexes occur during sleep in patients with OSA. OSA and exaggerated oxygen saturation reduction are important triggers of sympathetic discharge. In this regard, we have recently reported that the chemoreflex control may play an important role in the augmented sympathetic nerve activity in patients with MetS plus OSA.9 During sleep, the activation of the peripheral and central chemoreflex by hypoxemia and hypercapnia during the cessation of airflow results in a persistent increased chemoreflex sensitivity9 with consequent activation of the sympathetic nerve activity in MetS with patients with OSA. Our study brings to light the importance of sympathetic hyperactivity in restraining early HR recovery in patients with MetS, with or without OSA. The inverse association between ΔHRR at first, second, and fourth minute with sympathetic cardiac modulation and peripheral muscle sympathetic activity may reinforce this idea. In the current study, we found a correlation between ΔHRR and sympathetic activity even at the first minute of the recovery period after maximal exercise. Our results are in accordance with several previous studies, which showed that sympathetic markers are associated with attenuation of HRR either in normal subjects,39 in obese children,40 in anabolic steroids users,41 or in patients with myocardial infarction.42
In the current study, the sympathovagal imbalance toward sympathetic control may explain the diminished HR reserve in patients with MetS and OSA. In fact, HR reserve has been used to estimate sympathetic activity,39 and HR reserve < 89 bpm has been associated with cardiac diseases.13 Age affects HR reserve and it is a potential confounder. However, in the current study there were no differences among groups in age.
With or without the presence of OSA, patients with MetS exhibited increased resting HR, decreased peak VO2, and lower peak O2 pulse relative to controls, suggesting increased cardiovascular risk. Indeed, previous studies have provided convincing evidence of a worse prognosis in individuals with reduced functional capacity regardless of associated risk factors.39
Our study has several limitations. First, we studied patients with a recent diagnosis and who were untreated for MetS. There are positive and negative aspects regarding these subjects. The positive aspect is that there was no drug interference in our results. The negative aspect is that we cannot extrapolate these results to patients receiving pharmacologic treatment. Second, we did not include a group of patients with only OSA. Nevertheless, the objective of the current study was to investigate whether OSA would exacerbate the delay in the HRR after the cardiopulmonary exercise test in patients with MetS. Because occult OSA is as very common risk factor in patients with MetS, reaching a prevalence of 60%,3,4 and knowing that OSA is extremely deleterious, causing significant autonomic dysfunction such as baroreflex and chemoreflex control alterations,4,8 it would be reasonable to address its pathophysiological effect on cardiovascular responses to exercise, which largely depend on autonomic adjustments. Finally, we did not perform the autonomic measurements of MSNA and power spectrum analysis of HR variability during the postexercise recovery period. We did these measurements on a different day than the cardiopulmonary exercise test, with the subjects lying in the supine position. We did this because it is impossible to get the microelectrode needle impact on the peroneal nerve immediately after exercise. The power spectrum analysis of heart rate variability was evaluated at rest to avoid the influence of the increase on the respiratory rate and on tidal volume caused by maximal exercise, which exert significant influences on the amplitude of the high frequency component of HR variability. R–R interval variability declines as respiratory rate increases, but increases as tidal volume increases.44,45
In conclusion, the coexistence of MetS and moderate to severe OSA is associated with greater delay in recovery of HR after exercise than MetS with no or mild OSA. This response is associated with a sympathovagal imbalance toward the sympathetic component. These findings suggest that these patients are at increased risk of cardiovascular disease.
DISCLOSURE STATEMENT
This was not an industry-supported study. This study was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP #2011/17533-6) and, in part, by Fundação Zerbini. The authors have indicated no financial conflicts of interest. The work was performed at the Heart Institute (InCor), University of São Paulo Medical School, São Paulo, Brazil. There was no off-label or investigational use.
REFERENCES
- 1.Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9:211–24. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Venkateswaran S, Shankar P. The prevalence of syndrome Z (the interaction of obstructive sleep apnoea with the metabolic syndrome) in a teaching hospital in Singapore. Postgrad Med J. 2007;83:329–31. doi: 10.1136/pgmj.2006.051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drager LF, Lopes HF, Maki-Nunes C, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5:12065. doi: 10.1371/journal.pone.0012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trombetta IC, Somers VK, Maki-Nunes C, et al. Consequences of comorbid sleep apnea in the metabolic syndrome implications for cardiovascular risk. Sleep. 2010;33:1193–9. doi: 10.1093/sleep/33.9.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Grassi G, Dell'Oro R, Quarti-Trevano F, et al. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005;48:1359–65. doi: 10.1007/s00125-005-1798-z. [DOI] [PubMed] [Google Scholar]
- 7.Barretto AC, Santos AC, Munhoz R, et al. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. 2009;135:302–7. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 8.Toschi-Dias E, Trombetta IC, Dias da Silva VJ, et al. Time delay of baroreflex control and oscillatory pattern of sympathetic activity in patients with metabolic syndrome and obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2013;304:H1038–44. doi: 10.1152/ajpheart.00848.2012. [DOI] [PubMed] [Google Scholar]
- 9.Trombetta IC, Maki-Nunes C, Toschi-Dias E, et al. Obstructive sleep apnea is associated with increased chemoreflex sensitivity in patients with metabolic syndrome. Sleep. 2013;36:41–9. doi: 10.5665/sleep.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;28:1351–7. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 11.Adabag AS, Grandits GA, Prineas RJ, et al. Relation of heart rate parameters during exercise test to sudden death and all-cause mortality in asymptomatic men. Am J Cardiol. 2008;15:1437–43. doi: 10.1016/j.amjcard.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehabil. 2007;14:215–21. doi: 10.1097/HJR.0b013e328088cb92. [DOI] [PubMed] [Google Scholar]
- 13.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetière P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;12:1951–8. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 14.Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–8. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 15.Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–35. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 16.Hargens TA, Guill SG, Zedalis D, Gregg JM, Nickols-Richardson SM, Herbert WG. Attenuated heart rate recovery following exercise testing in overweight young men with untreated obstructive sleep apnea. Sleep. 2008;31:104–10. doi: 10.1093/sleep/31.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeder MT, Münzer T, Rickli H, et al. Association between heart rate recovery and severity of obstructive sleep apnea syndrome. Sleep Med. 2008;9:753–61. doi: 10.1016/j.sleep.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Sung J, Choi YH, Park JB. Metabolic syndrome is associated with delayed heart rate recovery after exercise. J Korean Med Sci. 2006;21:621–6. doi: 10.3346/jkms.2006.21.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deniz F, Katircibasi MT, Pamukcu B, Binici S, Sanisoglu SY. Association of metabolic syndrome with impaired heart rate recovery and low exercise capacity in young male adults. Clin Endocrinol. 2007;66:218–23. doi: 10.1111/j.1365-2265.2006.02711.x. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 21.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–8. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 22.American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events. Westchester, IL: American Academy of Sleep Medicine; 2007. [PMC free article] [PubMed] [Google Scholar]
- 23.Fagius J, Wallin BG. Long-term variability and reproducibility of human muscle nerve activity at rest, as reassessed after a decade. Clin Auton Res. 1993;3:201–5. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- 24.Task Force of the European Society of Cardiology, and the North American Society of Pacing Electrophysiology. Heart rate variability: standards measurements, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 25.Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A. A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation. 1994;90:1826–31. doi: 10.1161/01.cir.90.4.1826. [DOI] [PubMed] [Google Scholar]
- 26.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympathovagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 27.Cohen-Solal, Barnier P, Pessione F, et al. Comparison of the long term prognostic value of peak exercise oxygen pulse and peak oxygen uptake in patients with chronic heart failure. Heart. 1997;78:572–6. doi: 10.1136/hrt.78.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balady GJ, Arena R, Sietsema K, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 29.Wilks DC, Rank M, Christle J, Langhof H, Siegrist M, Halle M. An inpatient lifestyle-change programme improves heart rate recovery in overweight and obese children and adolescents (LOGIC Trial) Eur J Prev Cardiol. 2012;21:876–83. doi: 10.1177/2047487312465691. [DOI] [PubMed] [Google Scholar]
- 30.Maeder MT, Ammann P, Rickli H, Brunner-La Rocca HP. Impact of the exercise mode on heart rate recovery after maximal exercise. Eur J Appl Physiol. 2009;105:247–55. doi: 10.1007/s00421-008-0896-2. [DOI] [PubMed] [Google Scholar]
- 31.Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132:552–5. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- 32.Cohen-Solal A, Barnier P, Pessione F, et al. Comparison of the long term prognostic value of peak exercise oxygen pulse and peak oxygen uptake in patients with chronic heart failure. Heart. 1997;78:572–6. doi: 10.1136/hrt.78.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kizilbash MA, Carnethon MR, Chan C, Jacobs DR, Sidney S, Liu K. The temporal relationship between heart rate recovery immediately after exercise and the metabolic syndrome: the CARDIA study. Eur Heart J. 2006;27:1592–6. doi: 10.1093/eurheartj/ehl043. [DOI] [PubMed] [Google Scholar]
- 34.Spies C, Otte C, Kanaya A, Pipkin SS, Schiller NB, Whooley MA. Association of metabolic syndrome with exercise capacity and heart rate recovery in patients with coronary heart disease in the heart and soul study. Am J Cardiol. 2005;95:1175–9. doi: 10.1016/j.amjcard.2005.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipinski MJ, Vetrovec GW, Froelicher VF. Importance of the first two minutes of heart rate recovery after exercise treadmill testing in predicting mortality and the presence of coronary artery disease in men. Am J Cardiol. 2004;93:445–9. doi: 10.1016/j.amjcard.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro MM, Trombetta IC, Batalha LT, et al. Muscle sympathetic nerve activity and hemodynamic alterations in middle-aged obese women. Braz J Med Biol Res. 2001;34:475–8. doi: 10.1590/s0100-879x2001000400006. [DOI] [PubMed] [Google Scholar]
- 37.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–52. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laterza MC, de Matos LD, Trombetta IC, et al. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension. 2007;49:1298–306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- 39.Perini R, Orizio C, Comandè A, Castellano M, Beschi M, Veicsteinas A. Plasma norepinephrine and heart rate dynamics during recovery from submaximal exercise in man. Eur J Appl Physiol Occup Physiol. 1989;58:879–83. doi: 10.1007/BF02332222. [DOI] [PubMed] [Google Scholar]
- 40.Prado DM, Silva AG, Trombetta IC, et al. Exercise training associated with diet improves heart rate recovery and cardiac autonomic nervous system activity in obese children. Int J Sports Med. 2010;31:860–5. doi: 10.1055/s-0030-1267158. [DOI] [PubMed] [Google Scholar]
- 41.dos Santos MR, Dias RG, Laterza MC, et al. Impaired post exercise heart rate recovery in anabolic steroid users. Int J Sports Med. 2013;34:931–5. doi: 10.1055/s-0032-1331741. [DOI] [PubMed] [Google Scholar]
- 42.Ushijima A, Fukuma N, Kato Y, Aisu N, Mizuno K. Sympathetic excitation during exercise as a cause of attenuated heart rate recovery in patients with myocardial infarction. J Nippon Med Sch. 2009;76:76–83. doi: 10.1272/jnms.76.76. [DOI] [PubMed] [Google Scholar]
- 43.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;14:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 44.Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R–R interval power spectra is largely ignored. J Appl Physiol. 1993;75:2310–7. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch JA, Bishop B. Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol. 1981;241:620–9. doi: 10.1152/ajpheart.1981.241.4.H620. [DOI] [PubMed] [Google Scholar]