Abstract
Objective
This study aims to examine the association between dietary changes and improvement of metabolic syndrome components in Mexican postmenopausal women receiving two different nutrition interventions.
Methods
Women (n = 118) with metabolic syndrome were randomly assigned to group 1 (n = 63; structured hypocaloric diet) or group 2 (n = 55; behavioral therapy). Metabolic and nutrition assessment was performed at baseline and after 2, 4, and 6 months of intervention. Dietary changes throughout the study and achievement of cardioprotective dietary goals were assessed at the end of the intervention.
Results
There was a significant increase in the number of women who met recommended servings for fruits/vegetables, low-fat dairy, and sugars in both groups. In group 1, elimination of high-energy refined grains increased the probability of having normal fasting glucose (relative risk, 1.514; 95% CI, 0.989-2.316; P = 0.035). In this group, women who met the low-fat dairy goal at the end of the study had lower diastolic blood pressure (P = 0.012) and higher high-density lipoprotein cholesterol (P = 0.001). In group 2, women who met the high-fat dairy goal had greater probability of having normal fasting glucose (relative risk, 1.915; 95% CI, 1.123-3.266; P = 0.026). In all women, exclusion of high-fat dairy decreased by 60% the probability of having impaired fasting glucose (relative risk, 0.40; 95% CI, 0.181-0.906; P = 0.028).
Conclusions
Both strategies promote achievement of cardioprotective dietary goals for fruits/vegetables, sugars, soda and sweetened beverages, low-fat dairy, and high-energy refined grains, and improve some metabolic syndrome components. Elimination of high-fat dairy decreases the risk of impaired fasting glucose. Dietary strategies should be flexible and individualized based on metabolic profile.
Key Words: Cardiovascular risk, Menopause, Eating behaviors, Food servings, Cardioprotective diet
Metabolic syndrome (MetS) is a cluster of interrelated metabolic abnormalities characterized by abdominal obesity, hypertension, high levels of triglycerides (TG), low high-density lipoprotein (HDL) cholesterol levels, impaired glucose metabolism, and a prothrombotic and proinflammatory state. The prevalence of MetS in the adult population is 20% to 30% in most countries.1 In Mexico, its prevalence has been reported to be 36.8%, with a higher prevalence in women (42.2%). In women aged 50 to 59 years, most of whom have already gone through menopause, the prevalence of obesity and abdominal obesity is highest.2 Because of hormonal and metabolic changes in these women, body weight increases and visceral fat accumulates. Insulin, total cholesterol, low-density lipoprotein (LDL) cholesterol, and HDL cholesterol concentrations are usually altered in postmenopausal women, with a 60% higher risk of having MetS.3
Even for women receiving pharmacologic treatment, lifestyle changes are not only the starting strategy but also the foundation for MetS treatment. Lifestyle management of MetS should focus not on transient modifications but on permanent changes.4 Lifestyle changes that focus on diet and physical activity can successfully modify both metabolic and cardiovascular risk factors.5,6
Promotion of healthy dietary behaviors has become the cornerstone of the prevention and treatment of obesity and MetS. A variety of individual foods and nutrients (eg, fats, meat, fruits, vegetables, fish, and dietary fiber) have been reported to be associated with MetS, but evidence is still scarce.7
A Western dietary pattern has been associated with increased risk of MetS. On the other hand, a diet characterized by higher intakes of fruits/vegetables, whole grains, fish, low-fat milk, and dairy have shown favorable effects on metabolic abnormalities.4,8-12 Mediterranean and Dietary Approaches to Stop Hypertension dietary patterns have been associated with benefits for some MetS components.4,13-15
The effects of changes in specific dietary behaviors on MetS components within a clinical intervention have not been studied in depth. In a recent clinical trial, Perichart-Perera et al16 compared a structured hypocaloric diet with a behavioral therapy (BT) intervention in Mexican postmenopausal women with MetS. An important and significant decrease in MetS prevalence was observed in each group after 6 months, and significant improvements in weight and waist circumference were found in both groups. Differences in metabolic outcomes were observed between groups. The remaining question is whether achievement of specific target dietary behaviors within a nutrition intervention results in positive metabolic outcomes. This article presents the results of a dietary analysis within a clinical trial to evaluate the association between positive dietary changes and improvement of individual MetS components in postmenopausal women.
METHODS
Ethics and study participants
This study was conducted according to the guidelines laid down in the Declaration of Helsinki. The Institutional Review Board at the Instituto Nacional de Perinatología “Isidro Espinosa de los Reyes” (Mexico City, Mexico) approved all procedures involving human participants. A written informed consent form was obtained from all participants.
Participants
Postmenopausal women were included if they were overweight or obese (body mass index ≥25 kg/m2) and had MetS (three or more Adult Treatment Panel III risk criteria).17 Women were excluded if they had type 2 diabetes mellitus (T2DM) or a fasting glucose concentration higher than 126 mg/dL (7.0 mmol/L), had thyroid problems, or were taking medications that may affect nutrient metabolism or absorption.
Design
Between 2005 and 2009, women were selected by convenience at the postmenopausal clinic of the Instituto Nacional de Perinatología “Isidro Espinosa de los Reyes,” where they were receiving medical treatment.
During the first visit, a clinical dietitian—using a random number list and sequentially numbered files—randomly assigned women (simple randomization) to study groups (parallel design). Both the dietitian and gynecologists were blinded to allocation schedule. The recruitment protocol included an initial visit where suitability for randomization was evaluated and women were invited to participate.
Women were allocated into two groups:
Group 1: women received a structured hypocaloric diet with serving sizes based on cardioprotective dietary recommendations (25%-35% fat intake; <7% saturated fat intake; increased intake of monounsaturated and polyunsaturated fat; fiber 25-30 g/d; high intake of fruits/vegetables [≥5 servings daily], whole grains, low-fat animal products, healthy fats [avocado, canola oil, and seeds]; and low intake of high-energy refined grains, red and processed meats, added sugars, and soda and sweetened beverages). They received lifestyle recommendations and established a physical activity goal of 30 minutes three times a week.
Group 2: women did not receive a structured diet but received a BT intervention. They were evaluated using a traffic-light questionnaire (details of dietary risk assessment were published recently), where dietary and lifestyle behaviors associated with cardiovascular disease were included.16 The main objective was to gradually promote a change in these behaviors from the high-risk category (red light) to the low-risk category (green light). Based on the risk factors detected, short-term goals were established for the next visit. To improve success in goal achievement, we used other behavioral strategies such as problem solving and stimulus control.
In both groups, initial visits lasted 60 to 70 minutes, and follow-up visits were 45 minutes long. All measurements (metabolic assessment: blood pressure, fasting glucose, total cholesterol, HDL cholesterol, LDL cholesterol, and TG; nutrition assessment: weight, height, waist circumference, and 24-h food recalls) were performed at baseline and after 2, 4, and 6 months of intervention. Both groups received routine medical care and the same individual nutrition education at each visit.
Dietary assessment
Dietary assessment was performed using a five-step multiple-pass 24-hour recall.18 Estimation of food servings was performed using measuring cups and spoons, and food replicas (Life/Form; Nasco, Fort Atkinson, WI). The Food Processor SQL software (version 10.4, 2008; ESHA Research), which included Mexican foods, was used for nutrient analysis. Missing foods were added using the Mexican Food Exchange System19 or food labels.
Daily intake of high-fat dairy, high-energy refined grains, soda and sweetened beverages, added sugars, low-fat dairy, and fruits/vegetables was recorded as the number of servings using the Mexican Food Exchange System. In addition, cardioprotective dietary goals20,21 were established on a daily basis as follows: (a) fruits/vegetables (≥5 servings); (b) high-fat dairy (0 servings); (c) low-fat dairy (≥1 servings); (d) added sugars and sweetened beverages (<100 kcal); and (e) high-energy refined grains (0 servings).
MetS components
Adequate metabolic status was considered when women achieved optimal blood pressure (systolic blood pressure <130 mm Hg, diastolic blood pressure <85 mm Hg), optimal blood lipid concentrations (total cholesterol <200 mg/dL, HDL cholesterol >50 mg/dL, LDL cholesterol <130 mg/dL, TG <150 mg/dL), normal fasting glucose (<100 mg/dL), and/or adequate waist circumference (<88 cm; Adult Treatment Panel III). Methods for analyzing each metabolic marker have been described elsewhere.16
Statistical analysis
Analysis included data from all women in the study. Last-observation-carried-forward imputation data were assumed when women were lost to follow-up. Dietary data were analyzed at baseline (T0), 2 months (T1), 4 months (T2), and 6 months (T3). Except for baseline, two multiple-pass 24-hour recalls were averaged for each observation period.
Descriptive statistics and frequencies were performed for all variables. Mean differences were analyzed using Student’s t test or Mann-Whitney U test to assess correct randomization and to compare changes in food intake (T3 − T0). χ2 test and Fisher’s exact test were used to evaluate the association between the number of women who achieved dietary goals and the number of women who had adequate metabolic status (both independent observations). McNemar’s test was used to evaluate change in the number of women who met dietary goals from baseline to the end of the study. Repeated-measures analysis of variance was performed to assess food intake changes throughout the intervention. Multiple logistic regression models were used to evaluate the association between change in food intake and each metabolic goal, adjusting for confounding factors (energy intake, use of medication, study group, and/or baseline value).
P ≤ 0.05 was considered statistically significant. Statistics were performed with the Statistical Package for the Social Sciences software (version 17.0, 2008; SPSS Inc).
RESULTS
We approached 528 women; 357 women did not meet the inclusion criteria or were excluded, and 53 women refused to participate. A total of 118 women met our eligibility criteria and agreed to participate; 63 women were randomly assigned to group 1, and 55 women were randomly assigned to group 2 (Fig.).
FIG.
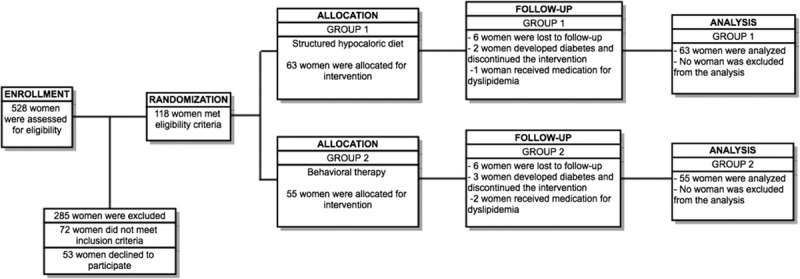
Flow chart.
As previously reported, the mean (SD) age of our population was 53.81 (6.43) years (range, 40-75 y). At baseline, the most frequent metabolic alteration was increased waist circumference (97.4% of women). The second most frequent alteration was increased HDL cholesterol (86.4%), followed by increased TG (82.1%) and total cholesterol (78.6%). The least altered MetS component was systolic blood pressure (46.4%). There were no baseline differences in metabolic, clinical, or dietary data between groups.16
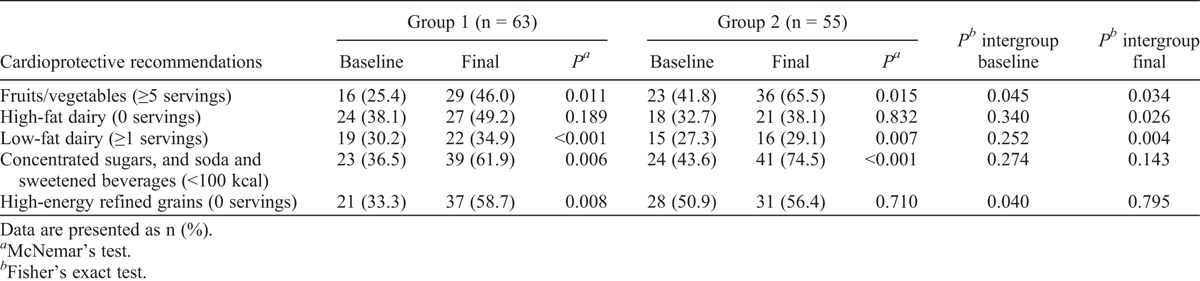
At the beginning of the study, 75% of all women consumed 3 or fewer servings of fruits, 2.5 or fewer servings of vegetables, and 1 or fewer servings of low-fat dairy daily. Both groups started with similar food serving intake, except for vegetables (P = 0.044; Table 1). The proportions of women who met established cardioprotective dietary goals at baseline were as follows: fruits/vegetables, 33.1%; high-fat dairy, 35.5%; low-fat dairy, 41.5%; added sugars and sweetened beverages, 39.8%; high-energy refined grains, 41.5%. No differences were observed between groups, except for fruits/vegetables and high-energy refined grains (Table 2).
TABLE 1.
Food servings eaten at the beginning of, during, and at the end of the study
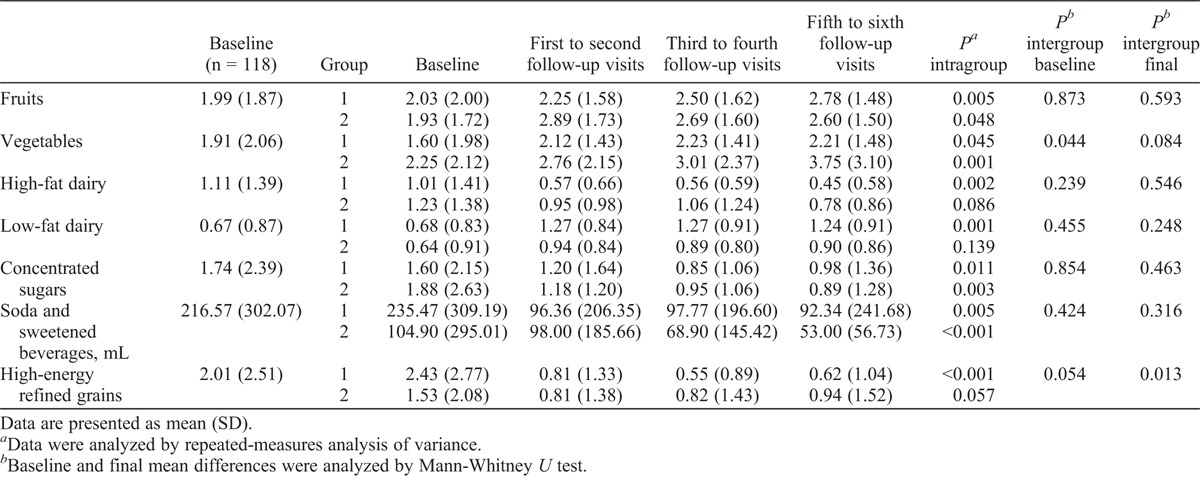
TABLE 2.
Women meeting cardioprotective recommendations before and after intervention
Throughout the study, women in both groups significantly decreased energy intake, total fat intake, saturated fat intake, and added sugars intake (data not shown).16 Women in group 1 increased their intake of fruits (P = 0.005) and low-fat dairy (P = 0.001), and decreased their intake of high-fat dairy (P = 0.002), high-energy refined grains (P < 0.001), added sugars (P = 0.011), and soda and sweetened beverages (P = 0.005). Women in group 2 increased their intake of vegetables (P = 0.001) and decreased their intake of added sugars (P = 0.003) and soda and sweetened beverages (P < 0.001). There were no between-group differences in change in food serving intake after 6 months of intervention, except for a greater decrease in high-energy refined grains observed in group 1 (P = 0.013; Table 1).
At the end of the intervention, there was a significant increase in the number of women who met the following dietary goals: five or more servings of fruits/vegetables, one or more servings of low-fat dairy, and less than 100 kcal from added sugars and soda and sweetened beverages in both groups. In group 1, a higher proportion of women eliminated intake of high-energy refined grains. Some differences were observed between groups. In group 1, more women met the recommended intake of high-fat dairy and low-fat dairy; in group 2, more women achieved the five-a-day recommendation for fruits/vegetables (Table 2).
In group 1, significant positive correlations were observed between intake of high-energy refined grains and waist circumference (r = 0.324; P = 0.010), high-fat dairy intake and HDL cholesterol (r = 0.255; P = 0.044), and low-fat dairy intake and fasting glucose (r = 0.279; P = 0.031). Women who ate one or more servings of low-fat dairy had lower mean (SD) diastolic blood pressure (≥1 serving vs <1 serving: −4.6735 [10.18] vs 6.8571 [9.58] mm Hg; P = 0.012) and higher mean (SD) HDL cholesterol (≥1 serving vs <1 serving: +1.6178 [8.41] vs −7.9071 [5.99] mg/dL; P = 0.001). In this same group, women who did not eat any high-energy refined grains had greater probability of having normal fasting glucose (relative risk, 1.514; 95% CI, 0.989-2.316; P = 0.035).
In group 2, significant correlations were observed between intake of high-energy refined grains and body weight (r = 0.266; P = 0.049), intake of low-fat dairy and systolic blood pressure (r = 0.325; P = 0.017), and intake of high-fat dairy and total cholesterol (r = 0.349; P = 0.009), LDL cholesterol (r = 0.274; P = 0.043), and TG (r = 0.307; P = 0.023). Women who did not eat any high-fat dairy had a greater probability of having a fasting glucose level lower than100 mg/dL (relative risk, 1.915; 95% CI, 1.123-3.266; P = 0.026); those who ate five or more servings of fruits/vegetables had greater probability of having altered LDL cholesterol, although this last odds was not statistically significant (relative risk, 1.583; 95% CI, 0.951-2.637; P = 0.040).
Multivariate analysis
In all women, exclusion of high-fat dairy from the diet decreases by 60% the probability of having impaired fasting glucose (>100 mg/dL; relative risk, 0.40; 95% CI, 0.181-0.906; P = 0.028). The model was adjusted by energy intake and study group.
DISCUSSION
This study presents new evidence showing that two different nutrition interventions promote positive dietary changes that modify MetS components in postmenopausal women. Overall, these two nutrition interventions were effective in achieving the recommended intakes of fruits/vegetables, low-fat dairy, added sugars, soda and sweetened beverages, and/or high-energy refined grains. The observed improvements in food intake throughout the intervention seem to have positive effects on metabolic components such as glucose, blood pressure, and HDL cholesterol.
Our findings on fruits/vegetables intake and lipid profile may seem contradictory. Women in group 2, who ate at least five servings of fruits/vegetables daily, were more likely to have altered LDL cholesterol, although this probability did not reach statistical significance. In Mexico, intake of high-glycemic-index fruits is very frequent, and this factor could influence the effect on LDL cholesterol. Some evidence has shown an inverse association between dietary glycemic index/glycemic load and total cholesterol and LDL cholesterol22,23; this effect has been reported in Hispanic postmenopausal women.24 Another factor that may affect this association is the fact that we quantified total fruits/vegetables intake without specifying type or preparation. In Mexico, it is common to eat fruits with yogurt, honey, or syrups; thus, the addition of other foods, such as high-fat dairy and added sugars, may be exerting a negative effect on LDL cholesterol. On the other hand, some studies have reported a lack of effect of increased fruits/vegetables intake on lipid profile in the studied population.25
We also found a higher probability of achieving normal fasting glucose at 6 months with elimination of high-energy refined grains from the diet (not statistically significant); the intake of these types of grains also correlated positively with waist circumference. Refined grains tend to be high in energy and low in nutrient density (fiber, magnesium, vitamin D, folic acid, and potassium) and may be associated with insulin resistance and excess weight.26 Fiber may act in a protective manner by slowing absorption and digestion of carbohydrates, leading to a reduced demand for insulin and lowering glucose responses.27 In previous studies, high intake of refined grains has been associated with insulin resistance, higher waist circumference, and various components of MetS, leading to higher risk of T2DM and cardiovascular disease.26,28
We observed that avoiding consumption of high-fat dairy decreases by 60% the risk of having impaired fasting glucose, after adjusting for energy intake and study group. This finding is in line with the fact that promoting the exchange of high-fat dairy to low-fat dairy may be a promising strategy for preventing and/or controlling T2DM. Overall, there is evidence for the beneficial effect of dairy intake on glucose homeostasis. Observational studies have found that eating higher amounts of dairy products decrease the risk of T2DM by 8% to 14%.29,30 In other clinical trials, a high-dairy diet (≥3 servings) resulted in improved insulin sensitivity, lower plasma insulin, decrease in total body fat, decrease in waist circumference, decrease in trunk fat, and increase in lean mass.30 Dairy products are good sources of vitamins (A, D, K2, B12, and riboflavin) and minerals (calcium, magnesium, and potassium), which may play a role in insulin sensitivity and/or resistance. The protective effect of dairy products could also be explained by their whey content, which may stimulate insulin secretion.29,31,32 Another link could be that, in addition to medium-chain saturated fatty acids, dairy fat contains bioactive lipids, including conjugated linoleic acid, that have the potential to improve insulin resistance.33-35
Another finding was the positive correlation between high-fat dairy and blood lipids. Whole-fat dairy products are a major source of saturated fat, which has been associated with higher risk of MetS and cardiovascular disease, mainly by increasing total cholesterol and LDL cholesterol in blood. Although some studies have found higher concentrations of total cholesterol and LDL cholesterol with a high-fat dairy diet versus a low-fat dairy diet,36 the latest evidence has failed to demonstrate this link.30,37,38 Further research is needed to clarify the role of different bioactive substances in dairy products and their interactions with other macronutrients in the diet.36
The BT intervention (group 2) was designed to promote gradual behavioral change and was conducted according to individual women’s baseline food behaviors and their motivation to commit to specific diet and lifestyle goals. BT is an excellent approach that may promote healthy dietary behaviors, similar to a stricter, less individualized diet intervention. Increasing evidence highlights the importance of including behavioral and motivational strategies to improve adherence because the latter remains an issue of concern when treatment involves diet and/or exercise.39 Women in the BT group were able to meet similar dietary goals (fruits/vegetables, low-fat dairy, and sugars) as women following the structured hypocaloric diet. Considering these results, the BT intervention seems to be an excellent approach to promoting cardioprotective dietary behaviors.
The main limitation of this study is the small sample size. The primary outcome of the clinical trial was the effectiveness of two different dietary strategies on the prevalence of MetS, and not the achievement of specific dietary changes that influence MetS components. Therefore, the sample size in each group and the low event rates may partially explain some inconsistent findings from this study and the difficulty of establishing strong associations. The observed associations from bivariate analysis may be confounded, but the study did not have enough power to conduct multivariate analysis. In addition, we could not perform cluster or factor analysis to evaluate dietary patterns. This methodology would have been ideal because we do not eat isolated nutrients but a variety of foods that are consumed in many complex combinations and may synergically interact to reduce MetS risk. We analyzed foods as separate entities and how they can affect some biochemical makers, and this relationship may be confounded by other factors.
On the other hand, our dietary data were obtained by two 24-hour recalls. Although a well-trained dietitian uses food replicas, kitchen utensils, and other methods to help reduce bias in serving estimation, this method relies mainly on the participant’s memory. The multiple-pass methodology has been shown to improve the accuracy of recalls.40 Finally, underreporting may be an issue. When nutrition education is given within a period, it is probable that the individual may be reporting what she learned rather than what she ate.41 Another limitation is the lack of long-term data. It would have been interesting to evaluate whether the dietary goals achieved could be maintained for a longer period and whether the BT intervention would promote better sustainment of the observed behaviors.
The different associations between dietary and metabolic changes observed in both study groups should be taken into consideration when designing nutrition interventions. Nutrition care at clinic level should be flexible, with individualized dietary recommendations based on metabolic risk factors.
CONCLUSIONS
A structured hypocaloric diet and a BT intervention promote achievement of cardioprotective dietary goals for fruits/vegetables, sugars, soda and sweetened beverages, low-fat dairy, and high-energy refined grains, which result in improvement of some MetS components.
Elimination of high-fat dairy from the diet decreases the risk of impaired fasting glucose, independent of total energy intake and the nutrition intervention.
Dietary strategies should be flexible and individualized based on metabolic risk factors. Empowerment to achieve self-confidence and to develop motivation is essential for promoting healthy food choices and behavioral change.
Acknowledgments
We would like to thank Dr. Karime Haua, Dr. Felipe Vadillo, LN Ana Licea, LN Lorena Pantoja, LN Marien Garza, Química Ma. Antonia Hernández Miranda, Dr. Guillermo Ortiz, Dr. Roberto Silvestri, and Dr. Rafael Aguilera for their support.
Footnotes
Funding/support: None.
Financial disclosure/conflicts of interest: None reported.
REFERENCES
- 1. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 2008; 28: 629- 636. [DOI] [PubMed] [Google Scholar]
- 2. Gutierrez JP, Rivera-Dommarco J, Shamah-Levy T, et al. Encuesta nacional de salud y nutrición 2012. Resultados Nacionales. Cuernavaca, México: Instituto Nacional de Salud Pública; 2012. [Google Scholar]
- 3. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 2003; 88: 2404- 2411. [DOI] [PubMed] [Google Scholar]
- 4. Fappa E, Yannakoulia M, Pitsavos C, et al. Lifestyle intervention in the management of metabolic syndrome: could we improve adherence issues? Nutrition 2008; 24: 286- 291. [DOI] [PubMed] [Google Scholar]
- 5. Cho YA, Kim J, Cho ER, Shin A. Dietary patterns and the prevalence of metabolic syndrome in Korean women. Nutr Metab Cardiovasc Dis 2011; 21: 893- 900. [DOI] [PubMed] [Google Scholar]
- 6. Voeghtly LM, Neatrour DM, Decewicz DJ, et al. Cardiometabolic risk reduction in an intensive cardiovascular health program. Nutr Metab Cardiovasc Dis 2013; 23: 662- 669. [DOI] [PubMed] [Google Scholar]
- 7. Amini M, Esmaillzadeh A, Shafaeizadeh S, Behrooz J, Zare M. Relationship between major dietary patterns and metabolic syndrome among individuals with impaired glucose tolerance. Nutrition 2010; 26: 986- 992. [DOI] [PubMed] [Google Scholar]
- 8. Ahluwalia N, Andreeva VA, Kesse-Guyot E, Hercberg S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab 2013; 39: 99- 110. [DOI] [PubMed] [Google Scholar]
- 9. Abete I, Goyenechea E, Zulet MA, Martinez JA. Obesity and metabolic syndrome: potential benefit from specific nutritional components. Nutr Metab Cardiovasc Dis 2011; 21 (suppl 2): B1- B15. [DOI] [PubMed] [Google Scholar]
- 10. Esmaillzadeh A, Kimiagar M, Mehrabi Y, et al. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr 2007; 85: 910- 918. [DOI] [PubMed] [Google Scholar]
- 11. Panagiotakos DB, Pitsavos C, Skoumas Y, Stefanadis C. The association between food patterns and the metabolic syndrome using principal components analysis: the ATTICA Study. J Am Diet Assoc 2007; 107: 979- 987, quiz 997. [DOI] [PubMed] [Google Scholar]
- 12. Hong S, Song Y, Lee KH, et al. A fruit and dairy dietary pattern is associated with a reduced risk of metabolic syndrome. Metabolism 2012; 61: 883- 890. [DOI] [PubMed] [Google Scholar]
- 13. Kastorini CM, Milionis HJ, Esposito K, et al. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol 2011; 57: 1299- 1313. [DOI] [PubMed] [Google Scholar]
- 14. Shirani F, Salehi-Abargouei A, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition 2013; 29: 939- 947. [DOI] [PubMed] [Google Scholar]
- 15. Bertoia ML, Triche EW, Michaud DS, et al. Mediterranean and Dietary Approaches to Stop Hypertension dietary patterns and risk of sudden cardiac death in postmenopausal women. Am J Clin Nutr 2014; 99: 344- 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perichart-Perera O, Balas-Nakash M, Munoz-Manrique C, et al. Structured hypocaloric diet is more effective than behavioral therapy in reducing metabolic syndrome in Mexican postmenopausal women: a randomized controlled trial. Menopause 2014; 21: 711- 720. [DOI] [PubMed] [Google Scholar]
- 17.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143- 3421. [PubMed] [Google Scholar]
- 18. Jonnalagadda SS, Mitchell DC, Smiciklas-Wright H, et al. Accuracy of energy intake data estimated by a multiple-pass, 24-hour dietary recall technique. J Am Diet Assoc 2000; 100: 303- 308, quiz 309-311. [DOI] [PubMed] [Google Scholar]
- 19. Lizaur ABP, Laborde LM, González BP. Sistema mexicano de alimentos equivalentes. Mexico: Fomento de Nutrición y Salud; 2008. [Google Scholar]
- 20. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336: 1117- 1124. [DOI] [PubMed] [Google Scholar]
- 21.American Heart Association Nutrition Committee, Lichtenstein AH, Appel LJ, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006; 114: 82- 96. [DOI] [PubMed] [Google Scholar]
- 22. Retterstol K, Hennig CB, Iversen PO. Improved plasma lipids and body weight in overweight/obese patients with type III hyperlipoproteinemia after 4 weeks on a low glycemic diet. Clin Nutr 2009; 28: 213- 215. [DOI] [PubMed] [Google Scholar]
- 23. Levitan EB, Cook NR, Stampfer MJ, et al. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism 2008; 57: 437- 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shikany JM, Tinker LF, Neuhouser ML, et al. Association of glycemic load with cardiovascular disease risk factors: the Women’s Health Initiative Observational Study. Nutrition 2010; 26: 641- 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Obarzanek E, Sacks FM, Vollmer WM, et al. Effects on blood lipids of a blood pressure–lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr 2001; 74: 80- 89. [DOI] [PubMed] [Google Scholar]
- 26. Radhika G, Van Dam RM, Sudha V, Ganesan A, Mohan V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57). Metabolism 2009; 58: 675- 681. [DOI] [PubMed] [Google Scholar]
- 27. Parker ED, Liu S, Van Horn L, et al. The association of whole grain consumption with incident type 2 diabetes: the Women’s Health Initiative Observational Study. Ann Epidemiol 2013; 23: 321- 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis 2008; 18: 283- 290. [DOI] [PubMed] [Google Scholar]
- 29. Salas-Salvado J, Martinez-Gonzalez MA, Bullo M, Ros E. The role of diet in the prevention of type 2 diabetes. Nutr Metab Cardiovasc Dis 2011; 21 (suppl 2): B32- B48. [DOI] [PubMed] [Google Scholar]
- 30. Da Silva MS, Rudkowska I. Dairy products on metabolic health: current research and clinical implications. Maturitas 2014; 77: 221- 228. [DOI] [PubMed] [Google Scholar]
- 31. Struijk EA, Heraclides A, Witte DR, et al. Dairy product intake in relation to glucose regulation indices and risk of type 2 diabetes. Nutr Metab Cardiovasc Dis 2013; 23: 822- 828. [DOI] [PubMed] [Google Scholar]
- 32. Faghih S, Abadi AR, Hedayati M, Kimiagar SM. Comparison of the effects of cows’ milk, fortified soy milk, and calcium supplement on weight and fat loss in premenopausal overweight and obese women. Nutr Metab Cardiovasc Dis 2011; 21: 499- 503. [DOI] [PubMed] [Google Scholar]
- 33. McGregor RA, Poppitt SD. Milk protein for improved metabolic health: a review of the evidence. Nutr Metab (Lond) 2013; 10: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han JR, Deng B, Sun J, et al. Effects of dietary medium-chain triglyceride on weight loss and insulin sensitivity in a group of moderately overweight free-living type 2 diabetic Chinese subjects. Metabolism 2007; 56: 985- 991. [DOI] [PubMed] [Google Scholar]
- 35. St-Onge MP, Bosarge A. Weight-loss diet that includes consumption of medium-chain triacylglycerol oil leads to a greater rate of weight and fat mass loss than does olive oil. Am J Clin Nutr 2008; 87: 621- 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huth PJ, Park KM. Influence of dairy product and milk fat consumption on cardiovascular disease risk: a review of the evidence. Adv Nutr 2012; 3: 266- 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prentice AM. Dairy products in global public health. Am J Clin Nutr 2014; 99 (suppl 5): 1212S- 1216S. [DOI] [PubMed] [Google Scholar]
- 38. Kratz M, Baars T, Guyenet S. The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. Eur J Nutr 2013; 52: 1- 24. [DOI] [PubMed] [Google Scholar]
- 39. Sievenpiper JL, Dworatzek PD. Food and dietary pattern–based recommendations: an emerging approach to clinical practice guidelines for nutrition therapy in diabetes. Can J Diabetes 2013; 37: 51- 57. [DOI] [PubMed] [Google Scholar]
- 40. Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc 2004; 104: 595- 603. [DOI] [PubMed] [Google Scholar]
- 41. Abbot JM, Thomson CA, Ranger-Moore J, et al. Psychosocial and behavioral profile and predictors of self-reported energy underreporting in obese middle-aged women. J Am Diet Assoc 2008; 108: 114- 119. [DOI] [PubMed] [Google Scholar]


