Abstract
Background:
Snoring is a common symptom of obstructive sleep apnea syndrome (OSA) and has recently been considered for diagnosis of OSA.
Objectives:
The goal of the current study was to systematically determine the accuracy of acoustic analysis of snoring in the diagnosis of OSA using a meta-analysis.
Methods:
PubMed, Cochrane Library database, and EMBASE were searched up to July 15, 2014. A systematic review and meta-analysis of sensitivity, specificity, and other measures of accuracy of acoustic analysis of snoring in the diagnosis of OSA were conducted. The median of apneahypopnea index threshold was 10 events/h, range: 5–15 or 10–15 if aforementioned suggestion is adopted.
Results:
A total of seven studies with 273 patients were included in the meta-analysis. The pooled estimates were as follows: sensitivity, 88% (95% confidence interval [CI]: 82–93%); specificity, 81% (95% CI: 72–88%); positive likelihood ratio (PLR), 4.44 (95% CI: 2.39–8.27); negative likelihood ratio (NLR), 0.15 (95% CI: 0.10–0.24); and diagnostic odds ratio (DOR), 32.18 (95% CI: 13.96–74.81). χ2 values of sensitivity, specificity, PLR, NLR, and DOR were 2.37, 10.39, 12.57, 3.79, and 6.91 respectively (All p > 0.05). The area under the summary receiver operating characteristic curve was 0.93. Sensitivity analysis demonstrated that the pooled estimates were stable and reliable. The results of publication bias were not significant (p = 0.30).
Conclusions:
Acoustic analysis of snoring is a relatively accurate but not a strong method for diagnosing OSA. There is an urgent need for rigorous studies involving large samples and single snore event tests with an efficacy criterion that reflects the particular features of snoring acoustics for OSA diagnosis.
Citation:
Jin H, Lee LA, Song L, Li Y, Peng J, Zhong N, Li HY, Zhang X. Acoustic analysis of snoring in the diagnosis of obstructive sleep apnea syndrome: a call for more rigorous studies. J Clin Sleep Med 2015;11(7):765–771.
Keywords: snoring, obstructive sleep apnea syndrome, acoustic analysis, review, meta-analysis
Obstructive sleep apnea (OSA) syndrome is a serious sleep disorder characterized by the repeated closure of the upper airway during sleep, among adults 30–70 y of age, approximately 13% of men and 6% of women have moderate to severe OSA, 14% of men and 5% of women have an apnea-hypopnea index (AHI) ≥ 5 plus symptoms of daytime sleepiness.1 It is also being recognized as an independent risk factor for several clinical consequences, including daytime sleepiness,2 systemic hypertension,3 increased risk of cardiovascular and cerebrovascular disease,4–6 traffic accidents,7–10 and impaired quality of life.11
Polysomnography (PSG) is currently the gold standard of OSA diagnosis.12,13 Unfortunately, PSG requires a full-night hospital stay in a specifically equipped sleep suite, connected to more than 15 channels of measurements requiring physical contact with sensors.14 PSG is inconvenient, expensive, and not suitable for mass screening. The limited number of PSG facilities around the world has long waiting lists, rendering it impossible to test all the patients in need of such assessment. Approximately 80–90% of patients with OSA are believed to be undiagnosed.15 With advances in technology and the development of portable monitors, home testing for sleep related breathing disorders is now feasible and circumvents many of the limitations of an attended in-laboratory polysomnogram. Although the portable monitors may expedite the diagnosis of OSA for many, it is essential that health care professionals using these methods recognize several inherent limitations.16 There is an enormous need for a simplified screening instrument capable of convenient and reliable diagnosis of OSA.
Snoring is one of the common and earliest symptoms of OSA. Snoring has long been viewed as a potential indicator for monitoring OSA. It has a unique advantage over other physiological signals, in that it can be acquired conveniently with only one to two low-cost noncontact or contact microphones. Importantly, the test does not affect the patient's sleep quality. Interest in the acoustic characteristics of snores began almost 2 decades ago. Recently several papers have proposed OSA detection systems17 and AHI estimation based on whole-night audio recording of snoring18,19 For devices utilizing acoustic signals, the data are insufficient to determine whether the use of acoustic signals with other signals as a substitute for airflow is adequate to diagnose OSA.20
The aim of this review was to systematically determine the sensitivity and specificity of acoustic analysis of snoring in the diagnosis of OSA. In this paper, we (1) discuss the consistencies and differences across the existing research studies and (2) identify opportunities for methodological improvements.
METHODS
Search Strategy
This review was performed according to a protocol designed a priori and recommended for systematic reviews and meta-analyses.21–23 We searched PubMed, Cochrane Library database, and EMBASE electronically up to July 15, 2014, utilizing combinations of the relevant medical subject heading terms, key words, and word variants for ‘obstructive sleep apnea syndrome’, ‘snoring analysis’, ‘acoustic analysis’, and ‘diagnosis’. In addition, we reviewed the reference lists from all relevant articles to identify additional studies. All searches were conducted independently by 2 reviewers (H.J. & L.S.). The results were compared, and any questions or discrepancies were resolved through iteration and consensus.
Inclusion Criteria for Included Studies
A study was included if it met the following inclusion criteria: (1) the study was written in English; (2) full text literature was available; (3) AHI assessment was based mainly on acoustic analysis of snoring; (4) subjects underwent PSG monitoring; (5) the study reported the sensitivity and specificity of the acoustic analysis; (6) the study clearly stated the number of true positive (TP), false positive (FP), false negative (FN), and true negative (TN) results in the diagnosis of OSA, or they could be calculated from the data; (7) the study was performed on adults (age 18 y or older); and (8) snore information was collected using microphones. We excluded a study from the analysis if it did not meet the above criteria or the experimental conditions were not clear.
Data Extraction and Quality Assessment
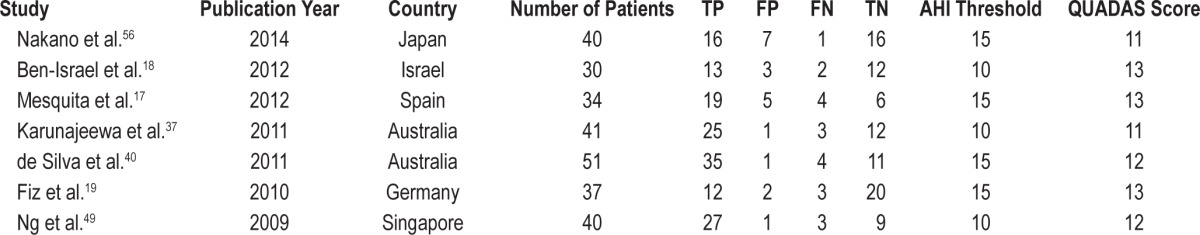
Data extraction was performed independently by two reviewers (H.J. & L.S.). The reviewers were blinded to publication details, and disagreements were resolved by consensus. Data retrieved from the reports included first author, country, publication year, participant characteristics, sensitivity and specificity data, diagnostic cutoff values, number of patients, and study design. Table 1 demonstrates a summary of each study, including the number of TP, FP, FN, and TN. We did not contact the authors for further details. The methodological quality of each study was assessed by a quality assessment for studies of diagnostic accuracy (QUADAS) that is an evidence-based quality assessment tool for use in systematic reviews of diagnostic accuracy studies (maximum score, 13).24 When a criterion was fulfilled, a score of 1 was given; 0 if a criterion was unclear; and −1 if a criterion was not achieved.
Table 1.
Characteristics of the eight studies finally selected.
Ethical Considerations
This study was approved by the Ethics Committee of First Affiliated Hospital, Guangzhu Medical University (Guangzhou, Guangdong, China).
Statistical Analysis
We used standard methods recommended for meta-analyses of diagnostic test evaluations.25 Analyses were performed using two professional statistical software programs (Meta-DiSc 1.4 for Windows, XI Cochrane Colloquium, Barcelona, Spain; and STATA version 12.0, STATA Corporation, TX, USA). Heterogeneity in meta-analysis refers to the degree of variability in results across studies. We used the Q statistic of χ2 test and inconsistency index (I2) to estimate the heterogeneity of individual studies contributing to the pooled estimate. For the χ2 test, significance was set at p < 0.05; for I2 statistics, significance was set at p > 0.05.26 Because of a lack of standardization, different thresholds may have been used in the included studies to define a positive test result. To detect threshold effects, the relationship between sensitivity and 1-specificity was evaluated by using Spearman correlation coefficient. A strong positive correlation would suggest threshold effect.27
The following indices of diagnostic accuracy were calculated by using Meta-DiSc 1.4 for Windows and analyzed for each study: sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). The pooled sensitivity, specificity, and other related indices across studies were calculated using both Mantel–Haenszel method (fixed effects model) and DerSimonian–Laird method (random effects model). In addition, diagnostic threshold variation among studies was assessed by using a summary receiver operating characteristic (SROC) curve. The SROC curve (and the area under the curve [AUC]) represents the overall performance of the test and depicts the tradeoff between sensitivity and specificity. In order to evaluate the statistical outcome validity, we detected the pooled outcome by sensitivity analysis. We used a random-effects model to reanalyze the data as an alternative to the fixed effect model. Because publication bias is of concern for meta-analysis of diagnostic studies, we tested for the potential presence of this bias using Deeks funnel plots. Publication bias was assessed visually by using a scatter plot of the inverse of the square root of the effective sample size (1/root [effective sample size]) versus the DOR, which should have a symmetrical funnel shape when publication bias is absent.28
RESULTS
Study Selection Process
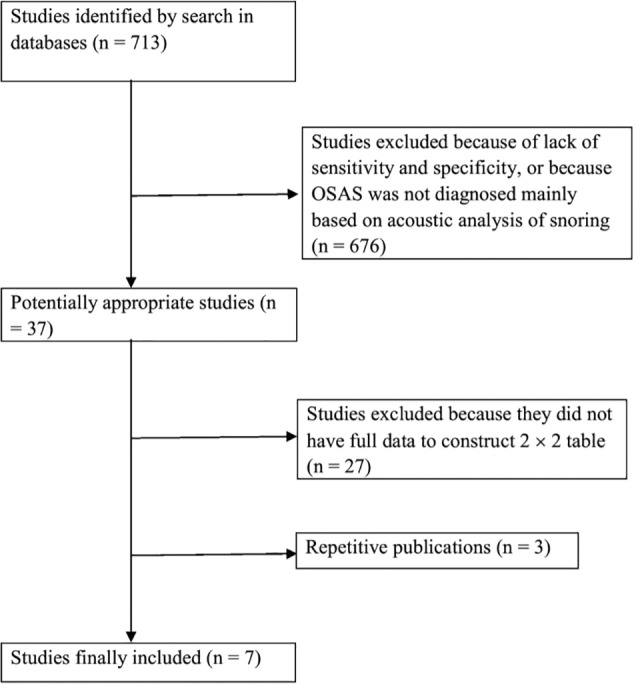
The primary search retrieved 713 studies. At review, 676 studies were excluded after reading the abstract and an additional 27 studies were excluded after reading the full text because they did not meet the inclusion criteria. In addition, three studies were excluded because of repetitive publication. We finally included seven studies. Figure 1 shows a flowchart of the study selection process.
Figure 1. Flowchart of the study selection process.
The characteristics of the seven studies finally selected are shown in Table 1. The publication years spanned from 2009 to 2014. The number of patients enrolled in single studies ranged from 30 to 51 patients. The total number of patients was 273. The selected AHI threshold value was 10 or 15 times per hour. All studies had a high QUADAS score (≥ 10).
Threshold Effect
Computation of Spearman correlation coefficient between log of sensitivity and log of [1- specificity] of acoustic analysis of snoring was 0.00 (p = 1.00), indicating no statistically significant threshold effect.
Diagnostic Accuracy
Figure 2 shows the forest plot of sensitivity and specificity for acoustic analysis of snoring in the diagnosis of OSA for the seven studies. The sensitivity ranged from 0.80 to 0.94 (pooled: 0.88; 95% CI: 0.82–0.93), and the specificity ranged from 0.70 to 0.92 (pooled: 0.81; 95% CI: 0.72–0.88). We also found that pooled PLR was 4.44 (95% CI: 2.39–8.27), NLR was 0.15 (95% CI: 0.10–0.24), and DOR was 32.18 (95% CI: 13.96–74.18). χ2 values of sensitivity, specificity, PLR, NLR, and DOR were 2.37 (p = 0.88), 10.39 (p = 0.10), 12.57 (p = 0.05), 3.79 (p = 0.71), and 6.91 (p = 0.33), respectively. I2 less than 50% in all parameters except pooled Positive LR (52.3%), indicating no signifi-cant heterogeneity between studies.
Figure 2. Forest plots of sensitivity and specificity estimates for acoustic analysis of snoring in the diagnosis of obstructive sleep apnea syndrome (OSAS) in the seven studies selected.
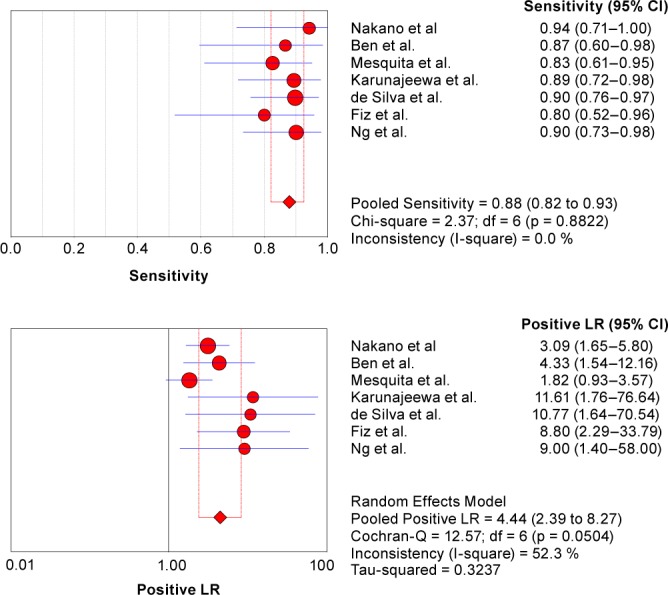
The point estimates of sensitivity and specificity from each study are shown as solid circles and the size of each study is indicated by the size of the solid circle. Error bars are 95% confidence intervals (CI). LR, likelihood ratio.
A SROC curve for acoustic analysis of snoring showing TP rates versus FP rates from individual studies is shown in Figure 3. As a global measure of test efficacy, we used the Q-value (the intersection point of the SROC curve with a diagonal line from the left upper corner to the right corner of the ROC space), which corresponds to the highest common value of sensitivity and specificity for the test. This point does not indicate the only or even the best combination of sensitivity and specificity for a particular clinical setting but represents an overall measure of the discriminatory power of a test. Our data showed that the SROC curve was positioned near the desirable upper left corner of the SROC curve. The maximum joint sensitivity and specificity (i.e., the Q-value) was 0.87, and the AUC was 0.93, indicating a high level of overall accuracy.
Figure 3. Summary receiver operating characteristic (SROC) curves for acoustic analysis of snoring in the diagnosis of obstructive sleep apnea.
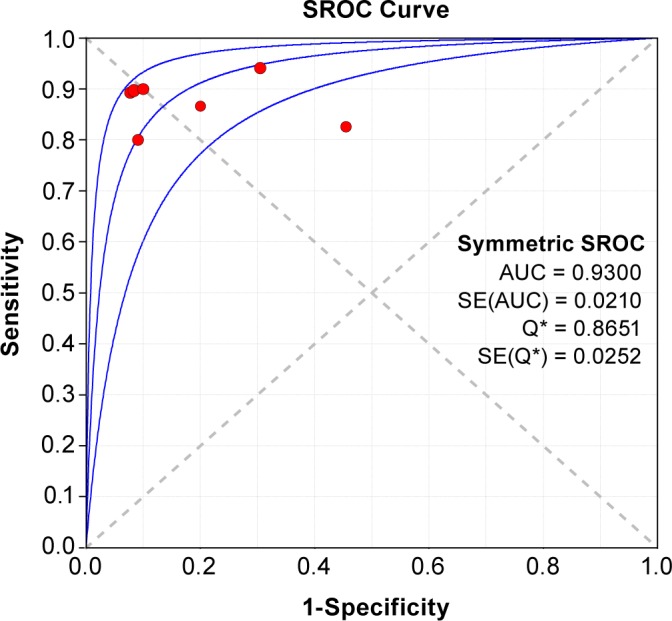
Each circle represents each of the seven studies included in the meta-analysis. The size of each study is indicated by the size of the solid circle. The regression SROC curve summarizes the overall diagnostic accuracy. AUC, area under the curve; SE, sleep efficiency.
Although the SROC curve and DOR are complicated as to their interpretation and use in clinical practice, the likelihood ratios (PLR and NLR) are more clinically meaningful for the measurement of diagnostic accuracy.29 For these two ratios, the diagnostic value for OSA is considered high when PLR > 10 and NLR < 0.1, moderate when PLR = 5.0–10.0 and NLR = 0.1–0.2, and low when PLR < 5.0 and NLR = 0.2–0.5.30
Sensitivity Analysis and Publication Bias
As an alternative to the random effect model, we used a fixed-effect model. However, the new analysis produced results similar to those obtained by the fixed- effect model. After excluding suspicious studies, the results were still similar to the original results. Although the funnel plots for publication bias showed some asymmetry due to the limited number of studies (Figure 4), Deeks test showed no statistical significance (p = 0.30). These results indicated no potential for publication bias.
Figure 4. Funnel graph for the assessment of potential publication bias in the 7 studies selected.
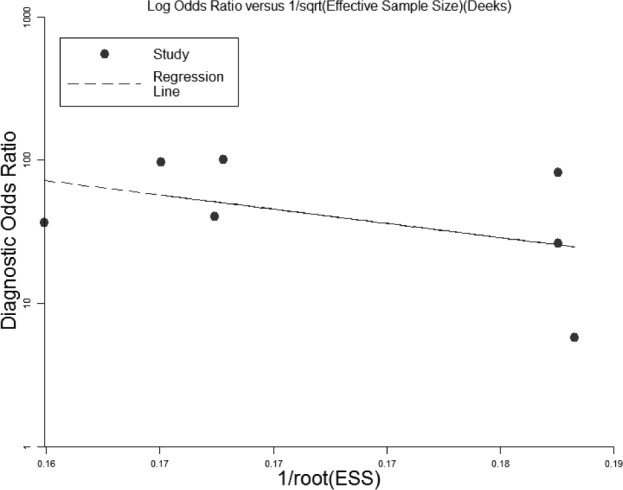
The funnel graph plots the diagnostic odds ratio against the 1/root (effective sample size). The dotted line is the regression line. The result of the test for publication bias was not statistically significant (p = 0.30). ESS, effective sample size.
DISCUSSION
This is the first study to investigate the accuracy of acoustic analysis of snoring in the diagnosis of OSA using a critical literature review and meta-analysis. The current meta-analysis of seven high-quality studies indicates that acoustic analysis of snoring is a relatively highly accurate diagnostic tool for OAS, with the pooled sensitivity being 0.89, specificity 0.84, AUC 0.94, and DOR 48.06. The PLR value is 4.44 and the NLR was 0.15, the results of likelihood ratios suggest that acoustic analysis of snoring currently is inadequate as a robust method for diagnosing OSA.
The weakness of clinical studies that we reviewed prompted the following summary and suggestions.
First, some studies have been showing that snore-related sound (SRS) classification is a first step in the detection of snoring and OSA.31–37 Duckitt et al.38 recorded sound with an ambient microphone from six subjects that was segmented into snoring episodes, breathing, duvet noise, and silence periods using hidden Markov models and spectral-based features. Cavusoglu et al.31 classified snore types with an accuracy ranging from 86.8% to 97.3% using a linear regression fed by sub-band spectral energy distributions processed by principal component analysis. Yadollahi and Moussavi35 investigated the effect of body and neck position on the classification results. The overall accuracies were found to be 95.7% and 93.2% for tracheal and ambient recordings, respectively, regardless of the neck position. Karunajeewa et al.37 used three noise reduction techniques and compared their performance with an overall classification accuracy of 90.74%; however, their accuracy was up to 96.78% with noise reduction techniques and a proper choice of features. However, only some studies took concerted effort to explore the effect of the signal-to-noise ratios on the methods proposed.33,39,40 Noise reduction together with proper choice of parameters derived from SRS analysis could help develop an accurate and possibly automated diagnostic tool.
Second, it is important to note that snoring does not have a fixed and constant occurrence,41 and not all snoring episodes are due to the same mechanisms during sleep.42 Some studies have already reported significant differences between postapneic snores (snores that are produced immediately after an apnea) and all other snores.43–45 Lee et al.46 reported the existence of monosyllabic low-frequency snore, duplex low- and mid-frequency snore, duplex low- and high-frequency snore, and triplex low-, mid-, and high-frequency snore in OSA. It is possible that some events were overscored and some were underscored with a reasonable overnight average, and thus, only a subset of snores may have utility for indicating OSA.
Third, the acoustic analysis of snoring has demonstrated good ability to discriminate between simple snorers and OSA patients.47–51 Primary snoring was defined as snoring without further respiratory events such as apneas or hypopneas.52 Sola-Soler et al.53 mentioned there were normal snores in patients with OSA. Our recent study showed that 87.32%, 78.38%, and 39.55% of all snores were primary snores in patients with mild, moderate, and severe OSA, respectively.54 The acoustic analysis of snoring could discriminate between “simple snorers” and patients with OSA, but it was difficult to estimate obstructive AHI accuracy without distinguishing the special characteristic of snore sound in OSA; this may be the most critical reason that acoustic analysis of snoring is inadequate as a robust method for diagnosing OSA.
Fourth, different studies have chosen different snoring parameters for acoustic analysis, such as pitch (sensitivity and specificity were 89.3% and 92.3%),36 formant frequencies (sensitivity and specificity were 0.88% and 0.82%)47 or peak frequencies (sensitivity and specificity were 97.7% and 85%),48 sound intensity (sensitivity and specificity were 87.0% and 100%)55 or power spectrum (sensitivity and specificity were 80.0% and 90.9%,20 and 94.1% and 78.9%,55 respectively), and mel-frequency cepstrum stability, pitch density, running variance, apnea phase ratio, and interevent silence (sensitivity and specificity were 87% and 80%).17 The aforementioned findings indicate that many snoring parameters have relatively good diagnostic capacity. Further investigation is needed to understand which parameters or combinations of parameters are best.
Fifth, OSA was defined as mild, moderate, or severe depending on whether the AHI was between 5 and 15, 15 and 30, or greater than 30, respectively. The major challenge for the interpretation of the data presented is that the primary AHI cutoff across studies was highly variable. Mesquita et al.,17 de Silva et al.,40 Fiz et al.,19 and Nakano et al.56 report AHI cutoffs of 15 events/h. The other studies selected an AHI threshold value of 5 or 10 for distinguishing between simple snorers and patients with OSA. Already, using current technology, the data suggest that severe sleep apnea might be ruled out in a proportion of individuals, or sleep apnea ruled in for many individuals, based on snoring estimates of AHI. For example, according to Nakano et al.,56 every individual with an estimated AHI of > 30 had moderate to severe OSA and should be expediently assessed for OSA and treated (regardless of symptoms, according to current recommendations). Likewise, every individual who had an estimated AHI < 15 did not have severe OSA and may not require prioritized assessment and intervention (unless symptoms of sleepiness/fatigue and cardiovascular comorbidities are present, or operates heavy machinery, etc). Sensitivity/specificity analysis using a single cutoff does not give credit for this potential utility, as the dependent variable is dichotomized.
Sixth, the samples were small in those seven publications, and the limited number of patients may have influenced the outcomes. Moreover, little attention has been placed on sex, age, or obesity, factors that may require adjustment of the thresholds used for each technology. At minimum, some mention that research of the utility of the approaches within sex, age and BMI subgroups will be important to study in the future. Furthermore, a very limited number of papers published in languages other than English were not included in this meta-analysis, which may be a potential source of bias. Based on those limitations, a definitive conclusion about the accuracy of diagnosis of acoustic analysis of snoring should not be drawn. Nevertheless, the studies included in our analysis are of high-quality research, and therefore may provide a considerable power, although further studies on a large scale are needed to confirm the diagnostic value of acoustic analysis of snoring for OSA.
In conclusion, acoustic analysis of snoring is a relatively accurate but not a strong method for diagnosing OSA. Appropriate methods for unequivocal classification of snoring and special characteristic of snore sound in OSA are to be elaborated. Although the acoustics of snoring, as a diagnostic tool, is from a burgeoning stage to growing maturity, there is an urgent need for a rigorous, large sample size, and a single snore event test with an efficacy criterion that reflects the particular features of snores to diagnose OSA, and that this information can ultimately be of benefit to public health.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by Guangdong province science and technology plan (2013B060100005) and China National Natural Science Foundation (81271066). The authors have indicated no financial conflicts of interest. This work was performed at First Affiliated Hospital, Guangzhou Medical University, Guangzhou, Guangdong 510120, China.
ACKNOWLEDGMENTS
The authors are grateful to Prof. Guangqiao Zeng for his critical reading of the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ANFIS
adaptive network fuzzy inference system
- AUC
area under the curve
- DOR
diagnostic odds ratio
- ESS
effective sample size
- FN
false negative
- FP
false positive
- I2
inconsistency index
- NLR
negative likelihood ratio
- OSA
obstructive sleep apnea syndrome
- PLR
positive likelihood ratio
- PSG
polysomnography
- QUADAS
quality assessment for studies of diagnostic accuracy
- SROC
summary receiver operating characteristic
- SRS
snore-related sound
- SVMs
support vector machines
- TN
true negative
- TP
true positive.
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rakel RE. Clinical and societal consequences of obstructive sleep apnea and excessive daytime sleepiness. Postgrad Med. 2009;121:86–95. doi: 10.3810/pgm.2009.01.1957. [DOI] [PubMed] [Google Scholar]
- 3.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 4.Takama N, Kurabayashi M. Influence of untreated sleep-disordered breathing on the long-term prognosis of patients with cardiovascular disease. Am J Cardiol. 2009;103:730–4. doi: 10.1016/j.amjcard.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton GS, Meredith IT, Walker AM, Solin P. Obstructive sleep apnea leads to transient uncoupling of coronary blood flow and myocardial work in humans. Sleep. 2009;32:263–70. doi: 10.1093/sleep/32.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvin AD, Somers VK. Obstructive sleep apnea and risk of stroke: time for a trial. Nat Clin Pract Cardiovasc Med. 2009;6:90–1. doi: 10.1038/ncpcardio1418. [DOI] [PubMed] [Google Scholar]
- 7.Barb F, Perics J, Muoz A, Findley L, Ant J, Agust A. Automobile accidents in patients with sleep apnea syndrome. An epidemiological and mechanistic study. Am J Respir Crit Care Med. 1998;158:18–22. doi: 10.1164/ajrccm.158.1.9709135. [DOI] [PubMed] [Google Scholar]
- 8.Tern-Santos J, Jimnez-Gmez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents cooperative group Burgos-Santander. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 9.Shiomi T, Arita AT, Sasanabe R, et al. Falling asleep while driving and automobile accidents among patients with obstructive sleep apnea-hypopnea syndrome. Psychiatry Clin Neurosci. 2002;56:333–4. doi: 10.1046/j.1440-1819.2002.01004.x. [DOI] [PubMed] [Google Scholar]
- 10.Pizza F, Contardi S, Mondini S, Trentin L, Cirignotta F. Daytime sleepiness and driving performance in patients with obstructive sleep apnea: comparison of the MSLT, the MWT, and a simulated driving task. Sleep. 2009;32:382–91. doi: 10.1093/sleep/32.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flemons WW, Tsai W. Quality of life consequences of sleep-disordered breathing. J Allergy Clin Immunol. 1997;99:S750–6. doi: 10.1016/s0091-6749(97)70123-4. [DOI] [PubMed] [Google Scholar]
- 12.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 13.Pack AI, Gurubhagavatula I. Economic implications of the diagnosis of obstructive sleep apnea. Ann Intern Med. 1999;130:533–4. doi: 10.7326/0003-4819-130-6-199903160-00019. [DOI] [PubMed] [Google Scholar]
- 14.Ghaemmaghami H, Abeyratne UR, Hukins C. Normal probability testing of snore signals for diagnosis of obstructive sleep apnea. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:5551–4. doi: 10.1109/IEMBS.2009.5333733. [DOI] [PubMed] [Google Scholar]
- 15.Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009;10:753–8. doi: 10.1016/j.sleep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Punjabi NM, Aurora RN, Patil SP. Home sleep testing for obstructive sleep apnea: one night is enough! Chest. 2013;14:291–4. doi: 10.1378/chest.12-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesquita J, Solà-Soler J, Fiz JA, Morera J, Jané R. All night analysis of time interval between snores in subjects with sleep apnea hypopnea syndrome. Med Biol Eng Comput. 2012;50:373–81. doi: 10.1007/s11517-012-0885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Israel N, Tarasiuk A, Zigel Y. Obstructive apnea hypopnea index estimation by analysis of nocturnal snoring signals in adults. Sleep. 2012;35:1299–305C. doi: 10.5665/sleep.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiz JA, Jané R, Solà-Soler J, Abad J, García MA, Morera J. Continuous analysis and monitoring of snores and their relationship to the apneahypopnea index. Laryngoscope. 2010;120:854–62. doi: 10.1002/lary.20815. [DOI] [PubMed] [Google Scholar]
- 20.Collop NA, Tracy SL, Kapur V, et al. Obstructive sleep apnea dDevices for outof-center (OOC) testing: technology evaluation. J Clin Sleep Med. 2011;7:531–48. doi: 10.5664/JCSM.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson LK, Craig JC, Willis NS, Tovey D, Webster AC. How to write a Cochrane systematic review. Nephrology (Carlton) 2010;15:617–24. doi: 10.1111/j.1440-1797.2010.01380.x. [DOI] [PubMed] [Google Scholar]
- 22.NHS Centre for Reviews and Dissemination. York, UK: University of York; 2009. Systematic reviews: CRD's guidance for undertaking reviews in health care. [Google Scholar]
- 23.Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM Cochrane Diagnostic Test Accuracy Working Group. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149:889–97. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devillé WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–93. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323:157–62. doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaeschke R, Guyatt GH, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271:703–7. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 31.Cavusoglu M, Kamasak M, Erogul O, Ciloglu T, Serinagaoglu Y, Akcam T. An efficient method for snore/nonsnore classification of sleep sounds. Physiol Meas. 2007;28:841–53. doi: 10.1088/0967-3334/28/8/007. [DOI] [PubMed] [Google Scholar]
- 32.Herzog M, Schmidt A, Bremert T, Herzog B, Hosemann W, Kaftan H. Analysed snoring sounds correlate to obstructive sleep disordered breathing. Eur Arch Otorhinolaryngol. 2008;265:105–13. doi: 10.1007/s00405-007-0408-8. [DOI] [PubMed] [Google Scholar]
- 33.Karunajeewa AS, Abeyratne UR, Hukins C. Silence breathing snore classification from snore related sounds. Physiol Meas. 2008;29:227–43. doi: 10.1088/0967-3334/29/2/006. [DOI] [PubMed] [Google Scholar]
- 34.Lee TH, Abeyratne UR, Puvanendran K, Goh KL. Formant-structure and phase-coupling analysis of human snoring sounds for detection of obstructive sleep apnea. In: Middleton J, Jones ML, Pande GN, editors. Computer Methods in Biomechanics and Biomedical Engineering. 3rd ed. Amsterdam: Gordon & Breach; 2000. [Google Scholar]
- 35.Yadollahi A, Moussavi Z. Automatic breath and snore sounds classification from tracheal and ambient sounds recordings. Med Eng Phys. 2010;32:985–90. doi: 10.1016/j.medengphy.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Ankışhan H, Yılmaz D. Comparison of SVM and ANFIS for snore related sounds classification by using the largest Lyapunov exponent and entropy. Comput Math Methods Med. 2013;2013:238937. doi: 10.1155/2013/238937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karunajeewa AS, Abeyratne UR, Hukins C. Multi-feature snore sound analysis in obstructive sleep apnea-hypopnea syndrome. Physiol Meas. 2011;32:83–97. doi: 10.1088/0967-3334/32/1/006. [DOI] [PubMed] [Google Scholar]
- 38.Duckitt WD, Tuomi SK, Niesler TR. Automatic detection, segmentation and assessment of snoring from ambient acoustic data. Physiol Meas. 2006;27:1047–56. doi: 10.1088/0967-3334/27/10/010. [DOI] [PubMed] [Google Scholar]
- 39.Alshaer H, Levchenko A, Bradley TD, Pong S, Tseng WH, Fernie GR. A system for portable sleep apnea diagnosis using an embedded data capturing module. J Clin Monit Comput. 2013;27:303–11. doi: 10.1007/s10877-013-9435-8. [DOI] [PubMed] [Google Scholar]
- 40.de Silva S, Abeyratne UR, Hukins C. A method to screen obstructive sleep apnea using multi-variable non-intrusive measurements. Physiol Meas. 2011;32:445–65. doi: 10.1088/0967-3334/32/4/006. [DOI] [PubMed] [Google Scholar]
- 41.Becker HF, Piper AJ, Flynn WE, et al. Breathing during sleep in patients with nocturnal desaturation. Am J Respir Crit Care Med. 1999;159:112–8. doi: 10.1164/ajrccm.159.1.9803037. [DOI] [PubMed] [Google Scholar]
- 42.Pevernagie D, Aarts RM, Meyer MD. The acoustics of snoring. Sleep Med Rev. 2010;14:131–44. doi: 10.1016/j.smrv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Padilla JR, Slawinski E, Difrancesco LM, Feige RR, Remmers JE, Whitelaw WA. Characteristics of the snoring noise in patients with and without occlusive sleep apnea. Am Rev Respir Dis. 1993;147:635–44. doi: 10.1164/ajrccm/147.3.635. [DOI] [PubMed] [Google Scholar]
- 44.Fiz JA, Abad J, Jane R, et al. Acoustic analysis of snoring sound in patients with simple snoring and obstructive sleep apnea. Eur Respir J. 1996;9:2365–70. doi: 10.1183/09031936.96.09112365. [DOI] [PubMed] [Google Scholar]
- 45.Tobin MJ, Mador MJ, Guenther SM, Lodato RF, Sackner MA. Variability of resting respiratory drive and timing in healthy-subjects. J Appl Physiol. 1998;65:309–17. doi: 10.1152/jappl.1988.65.1.309. [DOI] [PubMed] [Google Scholar]
- 46.Lee LA, Yu JF, Lo YL, et al. Energy types of snoring sounds in patients with obstructive sleep apnea syndrome: a preliminary observation. PLoS One. 2012;7:e53481. doi: 10.1371/journal.pone.0053481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Brunt DL, Lichstein KL, Noe SL, Aguillard RN, Lester KW. Intensity pattern of snoring sounds as a predictor for sleep disordered breathing. Sleep. 1997;20:1151–6. doi: 10.1093/sleep/20.12.1151. [DOI] [PubMed] [Google Scholar]
- 48.Ng AK, Koh TS, Baey E, Lee TH, Abeyratne UR, Puvanendran K. Could formant frequencies of snore signals be an alternative means for the diagnosis of obstructive sleep apnea? Sleep Med. 2008;9:894–8. doi: 10.1016/j.sleep.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Ng AK, Koh TS, Abeyratne UR, Puvanendran K. Investigation of obstructive sleep apnea using nonlinear mode interactions in non-stationary snore signals. Ann Biomed Eng. 2009;37:1796–806. doi: 10.1007/s10439-009-9744-8. [DOI] [PubMed] [Google Scholar]
- 50.Hara H, Murakami N, Miyauchi Y, Yamashita H. Acoustic analysis of snoring sounds by a multidimensional voice program. Laryngoscope. 2006;116:379–81. doi: 10.1097/01.mlg.0000195378.08969.fd. [DOI] [PubMed] [Google Scholar]
- 51.Dreher A, Rader T, Patscheider M, et al. The annoyance of snoring. Eur Arch Otorhinolaryngol. 2009;266:293–6. doi: 10.1007/s00405-008-0750-5. [DOI] [PubMed] [Google Scholar]
- 52.Guilleminault C, Stoohs R, Duncan S. Snoring (I). Daytime sleepiness in regular heavy snorers. Chest. 1991;99:40–8. doi: 10.1378/chest.99.1.40. [DOI] [PubMed] [Google Scholar]
- 53.Sola-Soler J, Jane R, Fiz JA, Morera J. Spectral envelope analysis in snoring signals from simple snorers and patients with obstructive sleep apnea. Engineering in Medicine and Biology Society, 2003. Proceedings of the 25th Annual International Conference of the IEEE; 2003. pp. 2527–30. [Google Scholar]
- 54.Mei ZF, Song LJ, Jin H, Li Y, Zhang XW. Investigation of respiratory events interval between snores in obstructive sleep apnoea hypopnea syndrome. China Journal of Modern Medicine. 2013;27:71–4. [Google Scholar]
- 55.Solà-Soler J, Jané R, Fiz JA, Morera J. Automatic classification of subjects with and without sleep apnea through snoring analysis. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:6094–7. doi: 10.1109/IEMBS.2007.4353739. [DOI] [PubMed] [Google Scholar]
- 56.Nakano H, Hirayama K, Sadamitsu Y, et al. Monitoring sound to quantify snoring and sleep apnea severity using a smartphone: proof of concept. J Clin Sleep Med. 2014;10:73–8. doi: 10.5664/jcsm.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]


