Abstract
LEAPS (ligand epitope antigen presentation system) vaccines consist of a peptide containing a major histocompatibility antigen binding peptide conjugated to an immune cell binding ligand (ICBL) such as the 'J' peptide from beta-2-microglobulin. Treatment of monocytes, monocytes plus GMCSF, or monocytes plus GMCSF and IL4 with JgD (containing a peptide from gD of herpes simplex virus type 1) or JH (with a peptide from HIV p17 gag protein) was sufficient to promote their maturation into Interleukin 12 producing dendritic cells. JgD dendritic cells supported allotypic activation of T cells to produce Th1 related cytokines.
Keywords: Dendritic cell, Human, Monocyte, Vaccine, LEAPS
1.Introduction
Dendritic cells (DC) are the bridge between the innate and adaptive immune systems. Mature DCs direct the nature of an immune response by producing different cytokine environments while presenting antigen to T cells. Dendritic cells that produce IL12 promote the development of a Thl response which is characterized by the production of interferon gamma (IFNγ) [1-3]. Precursors to both human and murine DCs include monocytes and myeloid stem cells [2,4-7] which convert to DCs upon treatment with appropriate stimuli. Ex vivo, monocytes can be converted into DCs in several ways. Treatment of human monocytes or mouse bone marrow cells with GMCSF and IL4 generates a precursor that converts to an IL12 producing DC upon treatment with CD40 ligand + IFNγ or two different TLR ligands [6,8,9]. Treatment of monocytes with GMCSF and calcium ionophore can also promote the generation of DCs [10].
In this study we treated human GMCSF (G monocytes), GMCSF plus IL4 pulsed (G4 monocytes), or untreated monocytes with immunogens developed by the LEAPS technology. The ligand antigen epitope presentation system (LEAPS) converts small peptides into immunogens by chemical conjugation to an immune cell binding ligand (ICBL) such as J ((DLLKNGERIEKVE), amino acid 38-50 from the beta-2-microglobulin) [11]. The JgD [12] and JH [13] heteroconjugate peptide immunogens consist of a peptide from the N-terminus of HSV-1 glycoprotein D (SLKMADPNRFRGKDLP, amino acid 8-23) or the HGP-30 (H) peptide from the pl7 HIV gag protein (YSVHQRIDVKDTKEALEKIEEEQNKSKKKA (aa 85-115)) conjugated to the J-ICBL through a triglycine linker. We show that these LEAPS immunogens can promote the maturation of monocytes into IL12-producing DCs.
2. Materials and methods
2.1. Human monocyte preparation and purification
Monocytes (>95% pure) were collected by leukapheresis (Baxter CS 3000) (Apheresis unit, Cleveland Clinic Foundation), followed by elutriation (Beckman Elutriator), washed and frozen [10]. After thawing, cells were plated at 3 × 106 cells/ml in monocyte-macrophage serum free medium (Life Technologies, Gaithersburg, MD) with or without 50ng/ml recombinant human GMCSF (Immunex, Seattle, WA) (GM-monocytes) or GMCSF+ 500U/ml IL4 (Schering-Plough, Bloomfield, NJ) (GM-4 monocytes) for 24 h at 37°c. After 24 h, the cells were treated with 14.5 µmolof JgD, JH, J, gD, or H peptides or HBSS.
2.2. J LEAPSs=tm immunogens
Peptide immunogens synthesized by UCB (Atlanta, GA) and supplied by Cel-Sci (Vienna, VA) were dissolved in Hanks Balanced Salt Solution (HBSS) to produce a stocksolution with a concentration of 2 mM adjusted to neutral pH. Each of the vaccine solutions (100 µl) was tested by a Limulus Amoebocyte Lysate assay as per manufacturer’s instructions (Cambrex Biosciences Walkersville, MD) and shown to be endotoxin free.
2.3. Cytokine arrays
Medium from peptide treated and untreated cells were obtained after 3 days and assayed for the presence of 42 different cytokine and chemokine proteins using RayBio® Human Cytokine Antibody Array 3 membranes (RayBiotech, Inc., Norcross, GA). Cytokines were detected by chemiluminescence, and the results captured on X ray film were analyzed by densitometry (Total Lab Array Analysis, Nonlinear Dynamics). In Figs. 2 and 3 array results were quantitated by densitometry, and normalized to the summation values for each array to allow for comparative analysis of JgD or JH treated to untreated dendritic cell array results. These values were then compared to the values obtained for untreated supernatants and results presented as a fold change. Statistical analysis for significant differences for each comparison was performed by equating p values via ANOVA analysis. In Table 1 densitometric results for each cytokine were divided by the results for EGF (which should not be affected by treatment) to allow comparison of results between array samples.
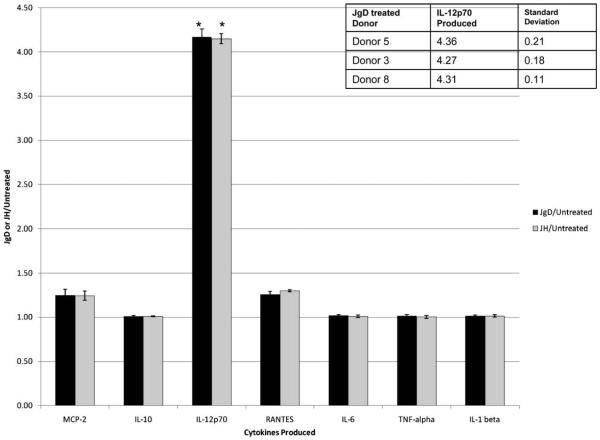
Fig. 2.
Survey of cytokine production following JgD or JH treatment. Human blood derived monocytes were treated with JgD or JH in two separate experiments. Spent media were collected three days post treatment, and evaluated by protein array (RayBio® Human Cytokine Antibody Array 3). Array results were quantitated by densitometry, and normalized to the summation values for each array to allow for comparative analysis of JgD or JH treated to untreated dendritic cell array results. The data shown are the mean scores for the fold increase or decrease to the untreated control for each of the 42 cytokines on the replicated arrays. The error bars represent the standard deviation between trials. Inset, human monocytes from different donors produced similar amounts of IL12p70 after being treated with JgD. Spent media was obtained from monocytes from donors 3, 5, and 8 after incubation with JgD in separate and repeated experiments, and analyzed, as discussed above. (*) Significant change in cytokine production from untreated cells.
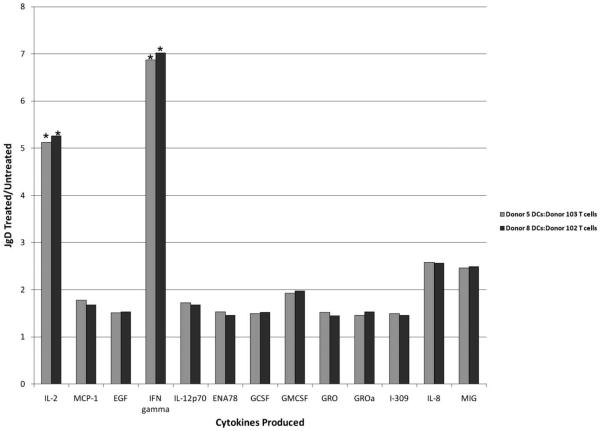
Fig. 3.
JgD treated human monocytes activate allogeneic T cells to produce IFNα and IL2. Monocytes and T cells were obtained after elutriation of the human apheresis product. CD4+ T cells were further purified with T cell isolation columns. Monocytes harvested 24 h after treatment with JgD or HBSS were added to T cell cultures at a 1 DC: 10 T cell ratio. Spent media were collected six days after co-culture, and assayed by protein array as described above. (*) Significant change in cytokine production from untreated cells.
Table 1.
Cytokine response to JgD of monocytes, GMCSF treated monocytes and iDCs.
| Cytokine | Monocyte | GMCSF | GMCSF + IL4 |
|---|---|---|---|
| IL12p70 | 3.09a | 3.05 | 3.58 |
| MCP-1b | 2.54 | 2.45 | 0.80 |
| MCP-2 | 2.45 | 2.40 | 1.84 |
| RANTES | 1.61 | 1.51 | 1.57 |
| PDGF-BB | 1.38 | 1.44 | 1.25 |
| MIP-1 delta | 1.15 | 1.10 | 1.64 |
| ENA-78 | 0.77 | 0.76 | 0.74 |
| MCSF | 0.68 | 0.60 | 1.49 |
| MDC | 0.68 | 0.67 | 1.89 |
| MIG | 0.37 | 0.37 | 0.39 |
| Angiogenin | 0.60 | 0.59 | 1.00 |
| Oncostatin | 0.52 | 0.50 | 1.17 |
| TARC | 0.22 | 0.24 | 0.47 |
| VEGF | 0.15 | 0.21 | 1.49 |
| GCSF | 0.00 | 0.00 | 1.22 |
| IL1α | 0.00 | 0.00 | 0.36 |
| IL10 | 0.00 | 0.00 | 0.34 |
| TNFα | 0.00 | 0.00 | 0.27 |
| IL1β | 0.00 | 0.00 | 0.23 |
Densitometric values were normalized to EGF for standardization between cytokine protein arrays.
MCP-1 and -2, monocyte chemoattractant proteins; RANTES, regulated upon activation normal T cell express sequence; PDGF-BB, platelet derived growth factor; MIP-1 delta, macrophage inflammatory protein-1 delta; ENA-78, epithelial neutrophil activating peptide 78; MCSF, macrophage colony-stimulating factor; MDC, macrophage derived chemokine; MIG, monokine-induced by interferon gamma; TARC, thymus and activation regulated chemokine; VEGF, vascular endothelial growth factor; GCSF, granulocyte colony-stimulating factor; EGF, epidermal growth factor; GMCSF, granulocyte macrophage colony-stimulating factor.
2.4. Flow cytometry analysis
Untreated and immunogen treated monocytes were labeled with PE-anti-DR or PE-anti-CD86. At least 5 × 105 cells were analyzed by flow cytometry (FACS Calibur; Cell Quest Pro software) (BD Biosciences San Jose, CA).
2.5. Allogeneic mixed leukocyte cultures
Monocytes harvested 24 h after treatment with JgD or HBSS were co-cultured with CD4 T cells, obtained as a byproduct of elu-triation and purified by negative selection (T cell isolation columns; R&D, Minneapolis, MN) (1 × 106 cells), at a monocyte: T cell ratio of 1:10 for 6 days at 37°C in RPMI 1640 medium supplemented with 5% human AB serum (Cambrex, East Rutherford, NJ). Culture supernatants were collected and assayed via RayBio® Human Cytokine Antibody Array 3 for cytokine production.
3. Results
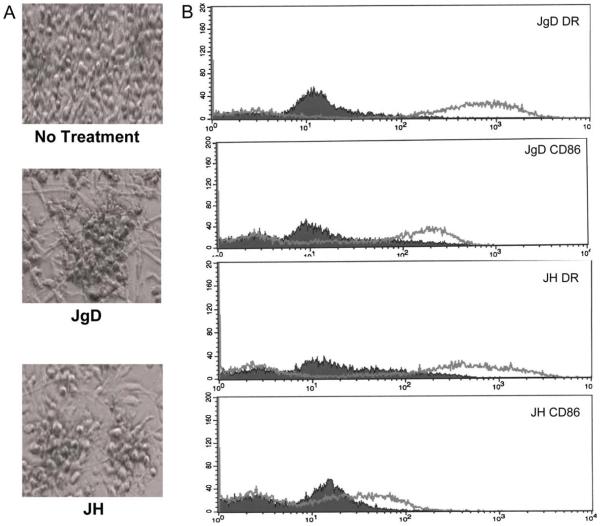
Based on previous work with mouse bone marrow cells, we hypothesized that JgD and JH will promote the maturation of human dendritic cell precursors into IL12 producing DCs that elicit Th1 related cytokine production. In the first step towards proving this hypothesis, precursor DCs, obtained by treating purified monocytes with GMCSF and IL4 [14,15], were incubated with JgD or JH. As shown in Fig. 1A, monocytes changed from individual and round cells to clumped cells with dendritic extensions after treatment with either JgD or JH. The immunophenotype of the cells (Fig. 1B) also changed with an upregulation of CD86 and HLA-DR expression within 72 h of treatment. Similar results were obtained for DC precursors treated with JH. The morphology, behavior, and increased expression of CD86 and HLA-DR are consistent with maturation of the DC precursors to mature DCs [7].
Fig. 1.
Treatment with JgD or JH promotes maturation of human monocytes into dendritic cells. Monocytes obtained by leukapheresis of blood and purified by elutriation were cultured in serum free media + GMCSF and IL4 for 24 h. The cells were then treated with 14.5 µmol of JgD or JH and incubated for 3 days at 37°C. (A) Microscopic photographs of human monocytes show the phenotypic changes after treatment including dendrite formation and clustering of the cells. (B) Cells shown were fixed, stained with PE-anti-CD86 or PE-anti-DR and analyzed by flow cytometry.
Different types of DCs are characterized by the cytokines that they produce and the subsequent T cell responses that they mediate [3,14,16,17]. A survey of cytokine production was performed by protein array to determine the nature of the DC that was produced upon treatment of the DC precursors with JgD or JH. The protein array analysis is a very sensitive assay for the presence of multiple cytokines giving an output similar to a western blot. Densitometric values of spots indicate the amount of cytokine present in the spent medium of cells from treated or untreated cells. The normalized ratio of values for the cytokines in spent medium from treated or untreated cells provides a semi-quantitative analysis of the cytokine spectrum produced by the cells. Treatment with either the unconjugated H or gD epitopes or the J-ICBL caused no significant production of cytokines. Fig. 2 shows those cytokines whose production was enhanced after a 72 h treatment with JgD or JH. The amount of IL12p70 was significantly increased by >4-fold following either treatment compared to control (Per ANOVA, p values = 2.03 × 10−5 (JgD) and 3.31 × 10−5 (JH) when compared to normalized untreated IL12p70 values) with a visible change in the levels of MCP-2 and RANTES. These results were reproduced for three different individuals and were similar following treatment with either JgD or JH. In each case, IL12p70 production was enhanced following treatment with either JgD or JH but production of IL1, TNFα and IL6 was the same as untreated cells Production of IL12p70 without concomitant enhancement of these proinflammatory cytokines is a different outcome than obtained with treatment by two TLR ligands, such as LPS and CpG [6,18].
Previous studies with mouse bone marrow cells showed that JgD or JH treatment was sufficient to convert DC precursor cells into IL12p70 producing DCs without a need for the addition of other cytokines or TLR ligands [19]. Similarly, JgD treatment of human monocytes was sufficient to promote the maturation of these cells into DCs that produce IL12p70. Table 1 shows the cytokine protein array ratios for monocytes, monocytes treated with GMCSF (G-monocytes), or as for the previous experiments, DC precursors generated with GMCSF plus IL4 (GM-4 monocytes). The levels of IL12p70, RANTES, MCP-1, and MCP-2 produced by monocytes after 24 h treatment with only JgD was most similar to cells pretreated for 24 h with GMCSF and then JgD. The GM-4 monocytes also produced elevated levels of IL12p70 and MCP-2 but the trends for some other cytokines and chemokines differed from that of JgD treated monocytes or GM-monocytes.
Ultimately, a DC1 cell must be able to activate T cells and promote IFNγ and IL2 production in order to mediate a Th1 immune response. The ability of JgD treated monocytes to support allotypic activation of T cells was tested [1]. For the experiment depicted in Fig. 3, monocytes from two separate donors were treated with JgD or medium for 24 h prior to addition of T cells from other donors, and after 6 days spent medium was analyzed for cytokine production. Significantly large differences in the Th1 cytokines, IFNγand IL2, were present in the spent medium from T cells mixed with JgD treated monocytes compared to those mixed with untreated monocytes. The same results were obtained with monocytes and T cells from another set of donors. No changes in cytokine production followed JgD treatment of a T cell-containing lymphocyte pool purified by elutriation and cell sorting (based on light scatter parameters) (data not shown). These results demonstrate that the JgD acts on monocytes to promote their maturation into DCs capable of promoting a Th1-like cytokine response by T cells.
4. Discussion
Conjugation of an antigenic peptide to the J-ICBL appears to create an immunogen that can activate and promote the maturation of dendritic cell precursors into DCs which produce IL12p70. Treatment of mouse bone marrow cells with JgD or JH, but not the unconjugated J-ICBL or gD or H peptides, promoted the maturation of DC precursors from bone marrow into IL12p70 producing DCs. The cells generated by treatment with JgD could present antigen to immune T cells to generate a booster-like enhancement of IFNγ production [19]. In a similar manner, monocytes, GM-monocytes and GM-4 monocytes treated with either JgD or JH, produced cells which phenotypically resemble DCs and produce IL12p70, whereas GM-4 monocytes treated with the unconjugated gD, H or J peptides did not.
Although very similar, the cytokine/chemokine profile produced by JgD treated GM-4 monocytes differed from that of JgD treated monocytes or monocytes treated with GMCSF. Interestingly, JgD treatment of monocytes or GM-monocytes did not generate the proinflammatory cytokines TNFα, IL1 or IL6 but very small amounts of these cytokines were produced after JgD treatment of GM-4 monocytes. This suggests that the type of DC generated by JgD treatment depends upon the nature of the starting cell. The IL4 treated monocyte behaves differently [1] and differentiates into a different IL12-producing DC after JgD treatment than monocytes or GM-monocytes.
The DCs generated by JgD treatment were sufficient to promote Th1-like cytokine responses upon allotypic interactions with T cells. Although this does not demonstrate antigen specificity, it does demonstrate that sufficient amounts of IL12p70 are generated by the JgD-DCs to steer the cytokine response of the T cells with which they interact towards a Th1 response, which is characterized by the production of IFNγ and IL2.
Addition of JgD to monocytes was sufficient to convert the cells into a unique type of IL12-producing DC. Unlike DCs generated with multiple TLR ligands, these cells did not produce increased amounts of IL1, TNFα, or IL6. The mechanism of induction promoted by JgD or JH is not known but is likely to be the result of cross-linking of the receptor for the J-ICBL to MHC I molecules through the linked epitope within the heteroconjugate peptide. This complex is likely to remain on the cell surface for long periods since mouse DCs bearing JgD could be washed free of unbound peptide after 24 h incubation and still provide an antigenic boost to immune splenic T cells [19]. In conclusion, the J-LEAPS immunogens, exemplified by JgD and JH, are sufficient to convert monocytes to a unique form of DC that produces IL12 but not acute phase cytokines and is sufficient to activate Th1 responses.
Acknowledgements
GKK was supported in part by NIH/NCRR Cleveland CTSA UL 1RR024989 and by R01 CA100163.
References
- [1].Roses R, Xu S, Xu M, Koldovsky U, Koski G, Czerniecki B. Differential production of IL-23 and IL-12 by myeloid-derived dendritic cells in response to TLR agonists. Journal of Immunology. 2008;181:5120–5127. doi: 10.4049/jimmunol.181.7.5120. [DOI] [PubMed] [Google Scholar]
- [2].Czerniecki B, Koski G, Koldovsky U, Xu S, Cohen P, Mick R, Nisenbaum H, Pasha T, Xu M, Fox K, Weinstein S, Orel S, Vonderheide R, Coukas G, DeMichele A, Araujo L, Spitz F, Rosen M, Levine B, June C, Zhang P. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Research. 2007;67(4):1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- [3].Kapsenberg M, Hilkens U, Wierenga A, Kalinski P. The paradigm of type 1 and type 2 antigen presenting cells. Implications for atopic allergy. Clinical and Experimental Allergy. 1999;29(2):33–36. [PubMed] [Google Scholar]
- [4].Shimizu K, Kuriyama H, Kjaergaard J, Lee W, Tanaka H, Shu S. Comparative analysis of antigen loading strategies of dendritic cells for tumor immunotherapy. Journal Immunotherapy. 2004;27:265–272. doi: 10.1097/00002371-200407000-00002. [DOI] [PubMed] [Google Scholar]
- [5].Inaba K, Inaba M, Romani N, Ava H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with a granulocyte macrophage stimulating factor. The Journal of Experimental Medicine. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Snijders A, Kaliniski P, Hilkens CM, Kaspenberg ML. High level IL-12 production by human DCs requires 2 signals. International Immunology. 1998;10(11):1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- [7].Koski G, Lyudmila A, Rice N. Rapid lipopolysaccharide-induced differentiation of CD14+ monocytes into CD83+ dendritic cells is modulated under serum-free conditions by exogenously added IFN-gamma and endogenously produced IL-10. European Journal of Immunology. 2001;31:3773–3781. doi: 10.1002/1521-4141(200112)31:12<3773::aid-immu3773>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- [8].Mosca P, Hobeika A, Clay T, Nair S, Thomas E, Morse M, Lyerly H. A subset of human monocyte-derived dendritic cells express high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 2000;96:3499–3504. [PubMed] [Google Scholar]
- [9].Hocherein H, Keeffe M, Luft T, Vandenbeele S, Grumont R, Maraskovsky E, Shortman K. Interleukin-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. The Journal of Experimental Medicine. 2000;192(6):823–833. doi: 10.1084/jem.192.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Czernicki B, Carter C, Rivoltini L, Koski G, Kim H, Weng D, Roros J, Hijazi Y, Xu S, Rosenberg S, Cohen P. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. Journal of Immunology. 1997;159:3823–3837. [PubMed] [Google Scholar]
- [11].Zimmerman DH, Rosenthal KS. The L.E.A.P.S. approach to vaccine development. Frontiers of Bioscience. 2005;10:790–798. doi: 10.2741/1572. [DOI] [PubMed] [Google Scholar]
- [12].Goel N, Rong Q, Zimmerman D, Rosenthal KS. A L.E.A.P.S. heteroconjugate vaccine containing a T cell epitope from HSV-1 glycoprotein D elicits Th1 responses and protection. Vaccine. 2003;21:4410–4420. doi: 10.1016/s0264-410x(03)00429-8. [DOI] [PubMed] [Google Scholar]
- [13].Zimmerman DH, Lloyd JP, Heisey D, Winship MD, Siwek M, Taylor E, Sarin PS. Induction of cross clade reactive antibodies in mice conjugates of HGP-30 peptide analog of HIV-1 (SF2 p17) and peptide segments of human beta-2-microglobulin or MHC II beta chain. Vaccine. 2001;19:4750–4759. doi: 10.1016/s0264-410x(01)00247-x. [DOI] [PubMed] [Google Scholar]
- [14].Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lazavecchia A. Selected toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature Immunology. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shuwen X, Koski G, Faries M, Bedrosian I, Mick R, Maeurer M, Cheever M, Cohen P, Czerniecki B. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. Journal of Immunology. 2003;171:2251–2261. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- [16].Banchereau J, Steinman R. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- [17].Schnurr M, Then F, Galambos P, Scholz C, Siegmund B, Endres S, Eigler A. Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. Journal of Immunology. 2000;165:4704–4709. doi: 10.4049/jimmunol.165.8.4704. [DOI] [PubMed] [Google Scholar]
- [18].Zheng R, Cohen PA, Paustian C, Johnson TD, Lee WT, Shu S, Koski GK. Paired TLR agonists enhance vaccine therapy through induction of IL-12. Cancer Research. 2008;68(11):4045–4049. doi: 10.1158/0008-5472.CAN-07-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Taylor P, Koski G, Cohen P, Moore FBG, Zimmerman DH, Rosenthal KS. J-L.E.A.P.S.™ vaccines initiate murine Th1 responses by activating dendritic cells, submitted for publication. doi: 10.1016/j.vaccine.2010.06.043. [DOI] [PubMed] [Google Scholar]