Abstract
Vitamin D metabolites are widely studied for their roles in bone health, immune functions and other potential physiologic roles in humans. However, the optimal blood levels of vitamin D metabolites are still unclear. Various methods for measuring vitamin D metabolites have been used and recently liquid chromatography tandem mass spectroscopy (LC-MS/MS) has been adopted as the gold standard for vitamin D metabolite measurement. Here we report the use of LC-MS/MS to measure 25-hydroxyvitamin D (25(OH)D2&3), and 1,25-dihydroxyvitamin D (1,25(OH)2D2&3), in three laboratory nonhuman primate species: common marmoset (Callithrix jacchus), rhesus macaque (Macaca mulatta), and cynomolgus macaque (Macaca fascicularis), and compare them to humans using the same technique. The nonhuman primates showed blood levels for 25(OH)D3 and 1,25(OH)2D3 significantly higher than human values with marmosets having the highest levels. Marmoset samples showed significantly more variability among individuals than those from macaques for both metabolites, but all three nonhuman primate species exhibited large variation within species for both 25(OH)D2&3 and 1,25(OH)2D2&3. Marmoset females had significantly lower values than the males for 25(OH)D3, while rhesus males showed a significant decrease in 25(OH)D3 with age. The most striking finding is the variation within species for vitamin D levels even in laboratory primates that have a controlled diet, UV exposure, and in some cases, genetic constraints. Similar variation in 25(OH)D responses to a fixed dose of oral vitamin D supplementation has been reported in humans. We suggest that these species can provide primate models for examining the factors influencing variation in the levels of vitamin D necessary for human and nonhuman primate health.
Keywords: marmoset; rhesus; cynomolgus; 25 hydroxyvitamin D; 1,25 dihydroxyvitamin D
INTRODUCTION
Vitamin D has multiple physiologic roles and affects multiple systems, organs, hormones and cells [Bikle, 2010]. Vitamin D comes in two forms: cholecalciferol is Vitamin D3 and erogcalciferol is Vitamin D2. Vitamin D3 is synthesized in the skin of vertebrates during exposure to UVB rays from the sun and is converted to 25 hydroxyvitamin D3 in the liver. It is the most common form found in primates and although the skin is the major route it is also found in fish, eggs, meat, dairy and poultry where it is absorbed in the intestinal tract. Vitamin D2 is found in fungi and some plants and is a lesser form than vitamin D3. It also is converted to 25 hydroxyvitamin D2 in the liver. Metabolite measurements have largely focused on circulating 25 hydroxyvitamin D2&3 (25[OH]D2&3) which is widely recognized as the measurement to define an individual’s vitamin D status [Ross et al. 2011] and the bioactive form 1,25 dihydroxyvitamin D2&3 (1,25[OH]2D2&3) which is a metabolite of 25[OH]D2&3. Measurement of these metabolites has dramatically increased due to implications of a vitamin D role in cancer prevention, neural function, immune modulation and effects on inflammation [Holick 2007].
How to define optimal vitamin D status is controversial and expert group recommendations are not congruent [Ross et al 2011; Holick et al. 2011]. Meta- and even mega-analyses, have failed to clarify optimal 25(OH)D levels [Hilger et al. 2014]. As an example, a report of 195 studies from 44 countries found no significant age or sex-related differences in 25(OH)D although there have been reports of significantly lower levels in aged people and may predict mortality [Lee et al., 2013]. Importantly, virtually all studies find substantial heterogeneity in 25(OH)D levels even in populations within limited geographical regions [Hilger et al. 2014]. Moreover, major between-individual variability exists in 25(OH)D response to oral vitamin D supplementation [Binkley et al. 2011; Aloia et al. 2008]. The factors causing this variability are not adequately defined.
Nonhuman primates are excellent translational models [Schnatz et al. 2012] that, given their similar physiology, may enhance understanding of human vitamin D metabolism. However, they have not been widely investigated for the study of vitamin D measurement and actions. Indeed, only a relatively small number of studies (using different measurement methods) have reported vitamin D metabolite levels in marmosets (Callithrix jacchus), rhesus (Macaca mulatta), and cynomolgus macaques (Macaca fascicularis). Reported values of 25(OH)D2&3 and 1,25(OH)2D2&3 for nonhuman primates generally are higher than human values [Shinki et al. 1983]. Most New World primate levels reported are 2–10 fold higher than Old World monkeys (Adams et al., 1985; but see Crissey et a., for lower values in Callimico goeldii) probably due to high expression of intracellular vitamin D binding proteins and concurrent high baseline levels of 1,25(OH)2D3. Additionally New World monkeys cannot utilize vitamin D2 and rely solely on vitamin D3 [Marx et al. 1989].
Various methods exist to measure vitamin D metabolites including RIA (radioimmunoassay), ELISA (enzyme linked immunosorbent assay), HPLC-UV (high pressure liquid chromatography - ultraviolet detection) and LC/MS/MS (liquid chromatography - tandem mass spectrometry) [Binkley et al. 2009]. ELISA and RIA methods do not separately measure the two forms (D2 and D3) without prior extensive separation extractions. Automated immunoassays are the most common methodology in routine human clinical use. Multiple comparisons of the various methodologies with LC/MS/MS have been performed [Koivula et al. 2013]; as a generalization, there is reasonable agreement between methods, but greater variability with immune based approaches. International efforts are ongoing to standardize vitamin D metabolite measurements; this essential effort is being facilitated by the availability of standard reference materials from the National Institute of Standards and Technology (NIST) [Binkley and Sempos 2014; Sempos et al. 2012].
Vitamin D metabolite measurement in research and some clinical laboratories has largely shifted to LC-MS/MS methodology which allows simultaneous measurement of vitamin D2 and D3 metabolites and is capable of detecting the low (pg/mL) levels of 1,25(OH)2D2&3 [de la Hunty et al. 2010]. Mass spectrometry methods, while very sensitive, require derivatization to measure these low 1,25(OH)2D2&3 levels [Netzel et al. 2011; Hedman et al. 2014]. We recently reported on a LC-MS/MS method for 25(OH)D2&3 used in our laboratory that requires a low sample volume of 50 µl with a 5 minute run time [Hedman et al. 2014]. Sample volume and method sensitivity are important when measuring vitamin D metabolites in nonhuman primates due to lower blood volume available for analysis.
Despite the advantages of LC-MS/MS, to our knowledge there is only one lab that has measured vitamin D metabolites using this methodology in nonhuman primates, [Schnatz et al. 2012] and that study evaluated only one species, the cynomolgus monkey. Here we present LC-MS/MS vitamin D metabolite data from three nonhuman primate research species: the rhesus monkey, the cynomolgus monkey, and the common marmoset, and compare human values obtained using the same methodology. We performed this study to: 1) establish normal values for these species under laboratory conditions using an LC-MS/MS methodology that is traceable to the NIST standards; 2) provide a comparison with human data, 3) use the state-of-the-art methodology and the same methods for all species, and 4) to evaluate the three nonhuman primates as models for disease related research.
METHODS
Subjects
Serum samples were collected as a one-time blood draw from representative monkeys of each species at the Wisconsin National Primate Research Center (WNPRC). We used 25 adult monkeys in good health from each species. All marmosets and rhesus macaques used in this study were born at the Wisconsin National Primate Research Center (WNPRC). The cynomolgus monkeys were originally from an outdoor, provisioned colony on the island of Mauritius where the very small founding population has reduced genetic differences among individuals [Burwitz et al., 2009], but all had lived at the WNPRC for 14 months prior to use. The cynomolgus monkeys (mean ± SD = 6.8 ± 0.8 years; range of 6.7 to 8 years) were all males, while the rhesus and marmoset monkeys were both male and female adults. The rhesus group included 18 males and 7 females (mean ± SD = 7.83 ± 4.86 years; range of 3 to 25 years). The marmoset group consisted of 15 males and 10 females (mean ± SD 6.2 ± 1.79 years; range of 3 to 10 years). None of the rhesus females were pregnant at the time of the blood sampling, as this would alter their vitamin D metabolite concentration [Novakovic et al., 2009; Zhang et al., 2014]. However, half of the marmoset females were in very early pregnancy, prior to receiving estrumate (prostaglandin F2a analogue to end the luteal phase; Mobay Corp., Shawnee KS) to terminate the pregnancy. Mean levels of 25(OH)D3 were not different between females who were pregnant (256.4 ± 64.43, n=5) compared to non-pregnant females (289.32 ± 101.41, n=5; P = 0.79, t = 0.27, df = 8) and mean levels of 1,25(OH)2D3 were not significantly different between females who were pregnant (864.0 ± 135.6, n=5) compared to non-pregnant females (755.8 ± 148.46, n=5; P = 0.79, t = 0.27, df = 8) so it is unlikely that the early pregnancies contributed to the relationship of the vitamin D metabolites. All blood-sampling procedures reported in this study were in a protocol reviewed and approved by the University of Wisconsin Graduate School Animal Care and Use Committee (ACUC). The research adhered to the American Society of Primatologists Principles for the Ethical Treatment of Non Human Primates.
All monkeys were housed indoors with controlled lighting conditions 12:12 hr light:dark) and temperature and humidity. Light was provided by fluorescent lamps (GE F32T8) that emit UV light in the spectrum 400–100 nm) with resin coating that attenuates UV transmission in the range 380–180 nm. The plastic lamp covers further attenuate UV transmission.
Diet and vitamin D supplements
Both macaque species received the same diet produced by Harlan (#2050, Teklad Global 20% Protein Primate Diet, Madison, WI). The diet contained 1% calcium and vitamin D3 as 8,000 IU/kg or 200 ng/g cholecalciferol. The amount of diet provided to individual macaques was based on estimated energy needs (i.e., body condition scores, age and appetite) and ranged from approximately 65 to 170 grams/day. The diet was supplemented with fresh fruits and vegetables daily.
The marmosets were fed Mazuri Callitrichid High Fiber Diet (Mazuri 5MI6, Land O’Lakes, Arden Hills, MN) once per day in an amount that would provide on average 20 grams per animal in the group, as well as browse material daily (fruit, mealworms, vegetables). The marmoset diet contained 1.1% calcium and Vitamin D3 as 5,690 IU/kg or 142 ng/g cholecalciferol. It was supplemented with a Vitamin D3 as a premix (TD.10936.PWD, as 8 g:992 g maltodextrin) to bring the total concentration of D3 in the diet to 13,000 IU/kg or approximately 26 IU per animal per day.
Vitamin D metabolite analyses
For between species comparisons, the samples from the three nonhuman primates were processed simultaneously and along with human samples and analyzed identically for comparison of the vitamin D metabolites. We used 20 human serum samples obtained from a reference laboratory (ARUP Laboratories, Salt Lake City, Utah, USA; http://www.aruplab.com) and analyzed for their vitamin D metabolites: 25(OH)D2&3 and 1,25(OH)2D2&3 along with the nonhuman primate samples. The ARUP samples were analyzed both by the University of Wisconsin Hospital Clinics and our Assay Services Laboratory at the WNPRC. These human samples have a known value to which we found matching results. Details have been published (Hedman et al., 2014). Acetonitrile was added to samples to be analyzed for 25(OH)D2&3 for protein precipitation according to the method developed by AB SCIEX (Foster City, CA). For the macaques and human samples, 50 µl of serum was combined with 100 µl acetonitrile containing 12 ng/ml of d6–25(OH)D3 as the deuterated internal standard. For the marmoset, 10 µl of serum plus 40 µl of distilled water was added to the acetonitrile. The sample was vortexed, sonicated for 10 minutes, then centrifuged at 14,500 rpm for 5 minutes. An 100 µl aliquot was put into a minivial for LC/MS/MS analysis. Vitamin 25(OH) D2&3 calibrators were purchased from the National Institute of Standards and Technology (NIST 972A, Gaithersburg, MD) and quality control pools were purchased from Utak Laboratories (Valencia, CA). Calibrators and pools were extracted in an identical manner as the unknowns. Liquid chromatography separated the 25(OH) D2&3 on a Phenomenex Luna column of 3 µ C8(2), 100A (50 mm × 2.0 mm); gradient: A=0.1%formic acid in ultrapure water, B=acetonitrile with 1% formic acid; program %B at 0 minutes = 50%, 2.7 to 3.7 minutes = 98%, 3.8 min=50% on a Shimadzu Prominence (Addison, Il, USA) integrated PLC interfaced with an AB SCIEX (Foster City, CA) QTRAP 5500 Quadrupole – Linear Ion Trap Mass Spectrometer equipped with an ACPI source. The lowest level of detection was 0.5 ng/ml for both 25(OH)D2&3.
Aliquots of all the samples were then tested for 1,25(OH)2D2&3 concentrations. The extraction procedure, derivatization and LC/MS/MS procedures followed our recently published method (Hedman et al., 2014). All samples from each species were analyzed at the same volume. 200 µl of serum was combined with 700 µl of deionized water and 20 µl of internal standard (50 pg, d6–1,25-(OH)2D2&3) before double extracting with a dual column SPE (solid phase extraction) using Chromabond XTR (6 ml, 1 g, Nacherey-Nagel) and silica (6 cc, 500 mg, Waters, Milford, MA). Dried samples were derivatized with Amplifex Diene (AB SCIEX) by adding 30 µl of a 1.5 mg/ml solution, vortexed, centrifuged and incubated for 30 minutes. Deionized water was then added at 30 µl and the samples were transferred into injection vials for LC injection. Samples were eluted at a flow rate of 0.25 ml/min using a binary reversed phase gradient (Channel A = 0.1% formic acid in 18 MΩ/cm water, Channel B = methanol) as follows: 0 min, 2% B; 1 min, 2% B; 17.8 min, 65% B; 18 min, 100% B; 19.8 min, 100% B; 20 min, 2% B; 30 min, 2% B. Relevant MS/MS settings were: CAD gas at 6 psig; CUR at 20 psig; GS1 at 60 psig; GS2 at 30 psig; IS at 5000 V; EP at 10 V; and TEM at 600 °C. The limit of quantitation was 15.63 pg/ml. Intra-assay coefficient of variation, CV, for the 1,25(OH)2D3 was 5.79. Since all samples were run at the same time, there was not an inter-assay CV. As part of the NIST program our mass spectrometry results for 25(OH)D2&3 values show excellent placement.
Statistical analyses
The 25(OH)D3 and 1,25(OH)2D3 data for the four species (marmoset, rhesus, cynomolgus and human), were determined to be normal by the D’Agostino & Pearson omnibus normality test. Since the vitamin D2 metabolites were either very low or nondetectable, there were no statistics performed for comparison between species. To determine if the values for the monkey species were different, we used One-Way Analysis of Variance, ANOVA. Post-hoc differences were analyzed by Tukey’s Multiple Comparison Test. Bartlett’s test for equal variances was used to determine the variability within each species for the vitamin Ds and to compare it with the other species. We used Pearson’s r to test for correlation with a two-tailed P value. Unpaired t-tests, two-tailed, were used to compare marmoset samples analyzed by two different methods.
RESULTS
Mean vitamin D metabolite concentrations were compared to other previously published methods for the D3 forms. The prior reports contrasted with our data as they only reported the D3 form, or the two forms were not separated (Table I). Our present study indicated numerically higher levels of 25(OH)D3 than all but one study. In an attempt to validate the higher values obtained by LC-MS/MS, marmoset samples in this study were compared with other marmoset samples from the WNPRC colony analyzed by RIA 25(OH)D3, (Diasorin); 1,25(OH)2D3, (WI NPRC in-house RIA). We found no significant difference between the RIA and LC-MS/MS for 25(OH)D3 (t = 0.83, df 68, P = 0.41) but did find significant differences for 1,25(OH)2D3 where the LC-MS/MS method provided higher levels (LC-MS/MS, mean ± SEM = 726 ± 85; RIA = 464 ± 64; t = 2.45, df = 18, P = 0.03). For rhesus monkeys, our 25(OH)D3 results were similar to some of the mean values previously reported, although several other studies were substantially lower. The cynomolgus monkey values were similar to the rhesus monkeys.
TABLE I.
Comparisons of mean 25-hydroxyvitamin D (25(OH)D3) and 1,25-dihydroxyvitamin D (1,25(OH)2D3) concentrations from this study with concentrations from other methods and facilities for four primate species; Common marmoset (Callithrix jacchus); rhesus monkey (Macaca mullata), cynomolgus monkey (Macaca fascicularis) and human (Homo sapiens).
| Marmoset | ||||||
| 25(OH)D3 ng/ml | 1,25(OH)2D3 pg/ml | Method | Reference | |||
| 394.81±43.5 (25) | 694.78±52.3 (25) | LC/MS/MS | this study | |||
| 94.51±30.5 (7) | 361.5±60.6 (7) | HPLC/RIA | Shinki et al., 1983 | |||
| 474±96 (17) | 481±82 (17) | HPLC/RIA | Yamaguchi et al., 1986 | |||
| 61.7±20.8 (15) | - | RIA | Teixeira et al., 2012* | |||
| 119±46.2 (7)** | - | RIA | Jarcho et al., 2013 | |||
| Rhesus | ||||||
| 154.84±5.5 (25) | 205.91±18.5 (24) | LC/MS/MS | this study | |||
| 50±5.1 (6) | 100+10 (6) | HPLC/RIA | Shinki et al., 1983 | |||
| 50±4 (5) | 95±17 (5) | HPLC/RIA | Yamaguchi et al., 1986 | |||
| 198.36±62.25 (20) | 169.62±34.6 (20) | HPLC/RIA | Vieth et al., 1987 | |||
| 114.8±8.6 (11) | - | RIA | Colman et al., 1999 | |||
| 10–30*** (44) | - | RIA | Black et al., 2001 | |||
| 144.23±24.0 (6) | - | HPLC/UV or RIA | Marx et al., 1989 | |||
| Cynomolgus | ||||||
| 164.81±6.9 (25) | 192.76±14.9 | LC/MS/MS | this study | |||
| 48.9+16.6 (74) | - | LC/MS/MS | Schnatz et al., 2012 | |||
| 96.15±16.0 (6) | - | HPLC/UV or RIA | Marx et al., 1989 | |||
| Human | ||||||
| 57.01±6.6 (14) | 55.26±6.14 (20) | LC/MS/MS | this lab | |||
| 20±2.5 (6) | 45±3.1 (6) | HPLC/RIA | Shinki et al., 1983 | |||
| 30.38±9.9 (61) | - | RIA | Orgaz-Molina et al., 2013 | |||
Numbers are mean ± standard error (N)
Callithrix pencillata, a closely related marmoset species
Based on Study 1
range, including males and females
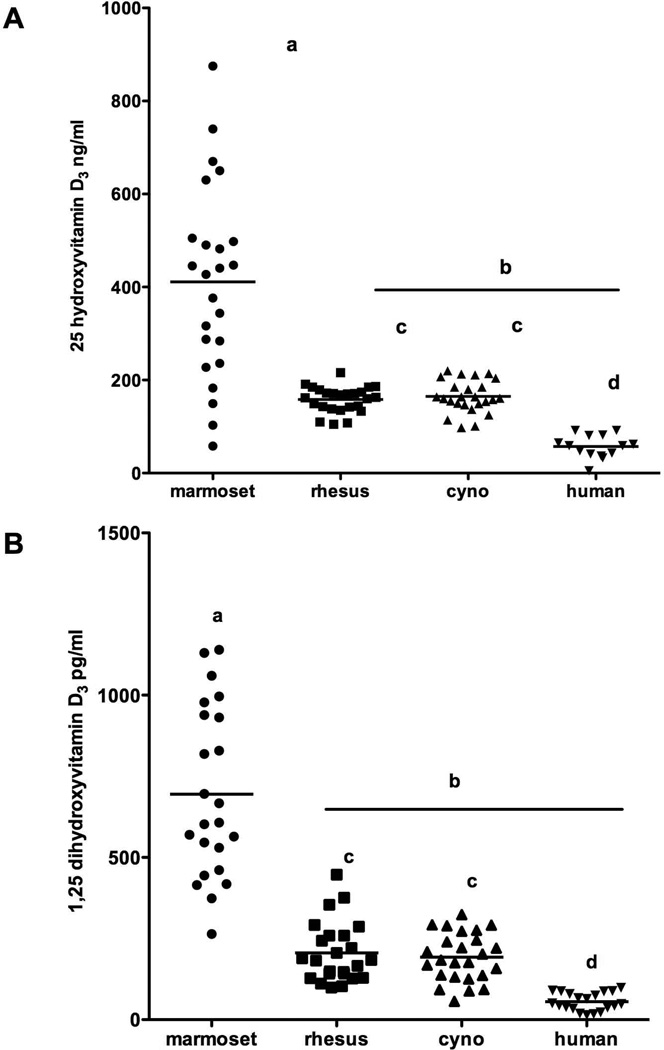
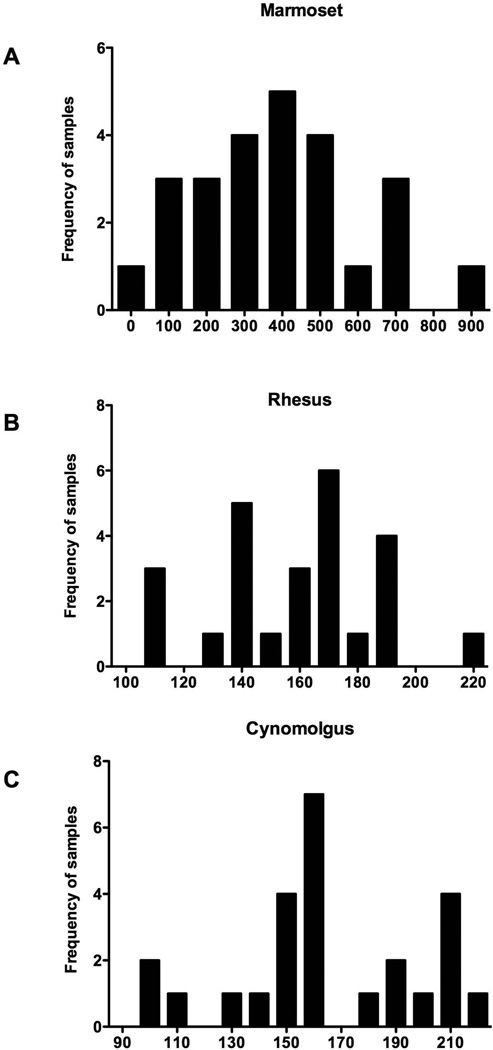
ANOVA indicated that significant differences for 25(OH)D3 were found between the values for the 4 species (F = 38, df = 3,84, P = 0.0001, Fig. 1). Marmosets were significantly higher than all the other species (P<0.05) and rhesus and cynomolgus were significantly different from the human values (p<0.05). The marmoset showed a large range of concentration. Significant differences were also found between the species for 1,25(OH)2D3 (F = 84, df = 3,88, P = 0.0001). Marmoset values were significantly higher than all the other species (P<0.05) and showed more variability. Additionally, both rhesus and cynomolgus had significantly higher values than human (P<0.05). Bartlett’s test for variances indicated that for each comparison the marmoset showed significantly more variability than the other species for the vitamin D forms (P<0.0001). The frequency distribution of 25(OH)D3 levels is shown for all three species (Fig. 2).
Fig 1.
Comparison of vitamin D levels between the species including humans
A. 25 hydroxyvitamin D3, B) 1,25 dihydroxyvitamin D3,for four primate species. Lines indicate medium for the samples of each species. Significance is indicated by: a is significantly different to b, c is significantly different to d.
Fig 2.
A histogram of the frequency distribution of samples with 25 hydroxyvitamin D3 values for the three nonhuman primate species indicating the variability found in the vitamin D levels: A) common marmoset, B) rhesus macaque, C) cynomolgus macaque.
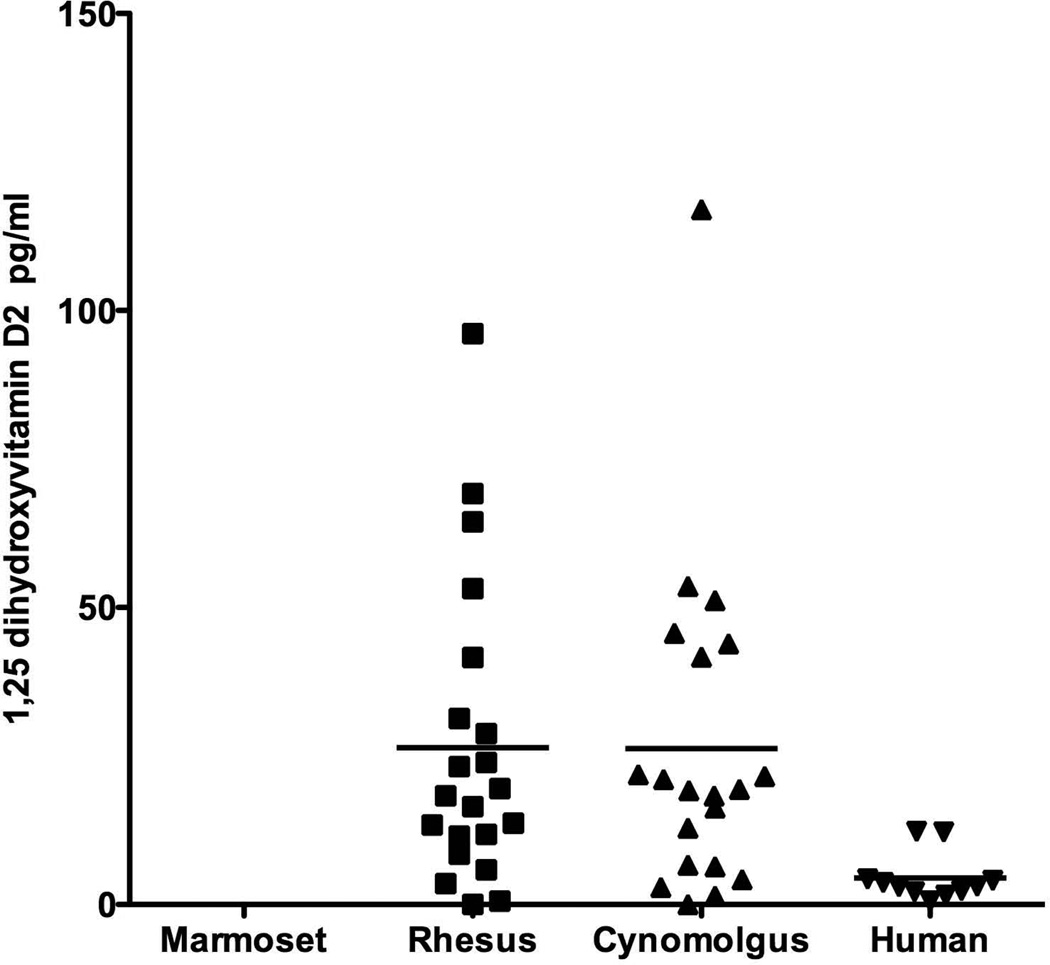
Vitamin D3 is the predominant form of vitamin D to supplement the diet in the nonhuman primates but Vitamin D2 is found in some food products. There was almost no detectable 25(OH)D2 measured by our selective measurements for any of the nonhuman primates. The marmosets did not show any detectable 25(OH)D2 measurement. For humans, however, all samples had detectable levels of 25(OH)D2 but only as 1/100 of the levels of D3. Conversely, with 1,25(OH)2D2 both the rhesus and cynomolgus monkeys had detectable levels and had higher levels than humans (Fig. 3).
Fig 3.
Comparison of the amount of 1,25 dihydroxyvitamin D2 found in the serum samples of the four species: marmoset, rhesus, cynomolgus and human. Horizontal lines indicate the mean for samples of each species. Marmosets did not show any D2 measurement while the macaques showed a higher range of variation than did humans.
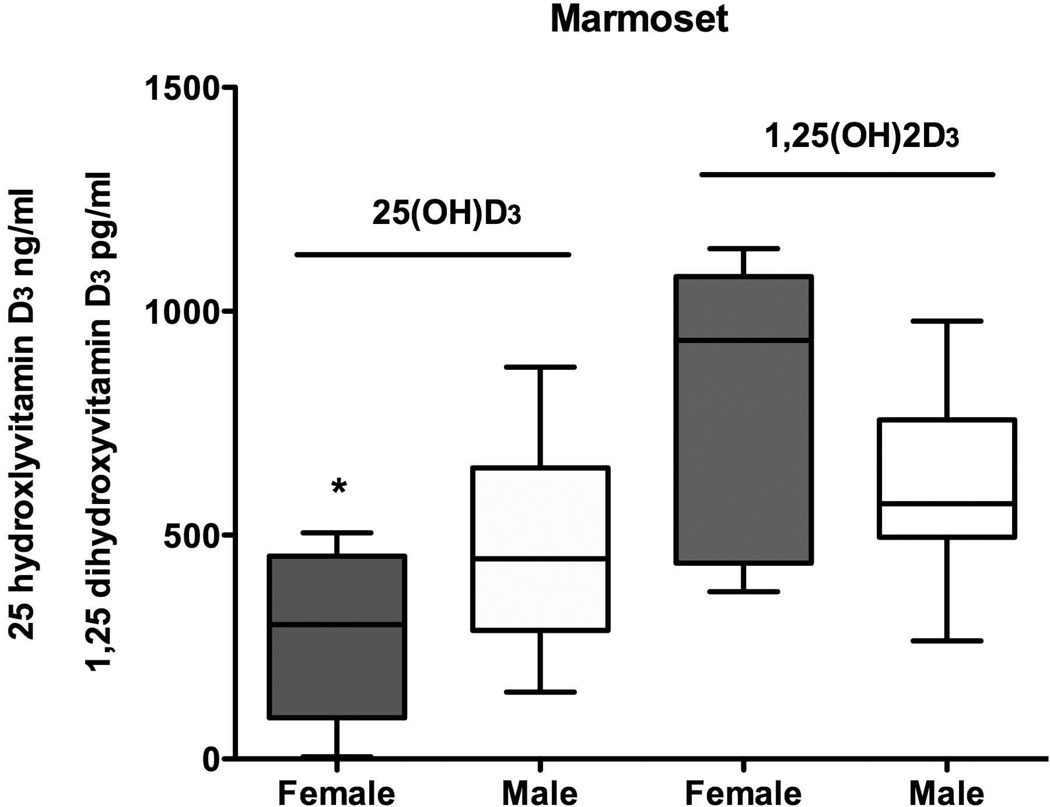
Marmosets and macaques were compared for sex differences in vitamin D metabolites (Fig. 4). Marmosets showed a significant sex difference in levels of 25(OH)D3 where females had significantly lower levels than males (t = 2.5, df = 23,P = 0.02). There was a trend towards a significant difference in 1,25(OH)2D3 for the marmosets but the trend was for higher 1,25(OH)2D3 levels in females (t = 2.0, df = 21, P = 0.06). The rhesus monkeys did not reveal sex differences for 25(OH)D3 (t = 0.54, df = 23, P = 0.59) or for 1,25(OH)2D3 (t =1.6, df = 22, P = 0.13).
Fig 4.
Comparison of values for male and female marmosets for 25 hydroxyvitamin D3 and 1,25 dihydroxyvitamin D3. Female levels were significantly lower than males for 25 hydroxyvitmain D3.
We then assessed whether age had an association with vitamin D metabolite levels. Age as a factor was compared with both 25(OH)D3 and 1,25(OH)2D3 concentrations to determine if there was a relationship that might explain some of the variance found in the vitamin D concentration. Age did not correlate with either 25(OH)D3 or 1,25(OH)2D3 neither in the marmoset, nor for rhesus age with 25(OH)D3. However, rhesus age significantly correlated with 1,25(OH)2D3 (r = −0.42, n = 24 P = 0.04) so that the older the rhesus monkeys the lower levels of 1,25(OH)2D3. For the cynomolgus monkeys there was no association of 25(OH)D3 with age but a weak correlation of age and 1,25(OH)2D3 (r = 0.39, n = 25, P = 0.05).
We examined the body weights of the monkeys at the time of the evaluation of the vitamin D levels and found no evidence of an association with any of the vitamin D metabolites for the weight of the marmoset, rhesus or cynomolgus monkeys.
Examination of the relationship between food allocation, an approximation of food intake, revealed a significant positive correlation with 25(OH)D3 (r = 0.44, P = 0.02) for rhesus macaques and marginally significant correlations with 1,25(OH)2D3 and total 1,25(OH)2D (r = 0.38 for both, P < 0.057) for cynomolgus macaques only.
DISCUSSION
Our data provides a comparison of vitamin D values within and between the three most commonly used laboratory nonhuman primate species and human subjects using the most sensitive and specific methods to date. With the use of LC/MS/MS we were able to make these comparisons for both of the most studied vitamin D metabolites and in both the D2 and D3 forms using the same methodology. Few nonhuman primate studies have values for D2 in any species. The values we have from this method allowed us to validate the significant differences in vitamin D concentration between species and to document the large variations that occur within species, as it has been reported for humans (Hilger et al., 2014).
In order to establish our methodology we have used human samples that are part of a reference laboratory (ARUP, USA, http://www.aruplab.com) and validated the method with a sensitive RIA and with the most recent sensitive LC/MS/MS technique [Hedman et al. 2014]. Furthermore, our 25(OH)D assay is compliant with the NIST program. Our reported results for these samples reflect the results from other nonhuman primate sample methodologies. While we had similar values to other human studies, our mean for 25(OH)D3 was higher than reported for other studies [Shinki et al. 1983; Orgaz-Molina et al. 2013]. The human samples we used are selected for the range they provide in values, and therefore, do not necessarily reflect the normal range found for humans. However, we can reliably report the values we have for the nonhuman primate species and compare them to reports in the literature that use different methodology. Our values for the marmoset, rhesus and cynomolgus monkeys were on the high end of reported values for both 25(OH)D3 and 1,25(OH)2D3 but not out of the range of values reported by other studies. This may be due to the mass spectrometry method having the ability to ionize the vitamin D compounds so effectively that it picks up more of the analyte than immunoassays or simply due to inaccuracies of the prior methods. As expected, we found that vitamin D metabolite values for the marmoset were significantly higher than other primate species and that rhesus and cynomolgus monkeys had values significantly higher than humans. We discuss our findings below by species.
Marmosets
Most New World primates have been reported to have extremely high levels of circulating vitamin D3 metabolites in both the 25(OH)D3 and 1,25(OH)2D3 forms with levels two to 10 times higher than those found in Old World primates and humans [Adams et al., 2003; Gacad et al. 1992; Crissey et al. 1999 with the exception of Callimico]. Several species of New World primates have a high expression of competitive binding proteins intracellular that bind to the vitamin D metabolites and require elevated levels of both 1,25(OH)2D3 for activation of cells and 25(OH)D3 as the substrate for 1,25(OH)2D3 production [Adams et al. 2003; Liberman et al. 1985; Teixeira et al. 2012]. Our study also indicated that levels were higher for New World primates for both vitamin D metabolites. Additionally, since our method measured both D2 and D3 forms for the metabolites, we also demonstrated that marmoset D2 was not measurable in our marmoset samples and therefore supports previous findings that D2 is not absorbed and reflected in 1,25(OH)2D [Marx et al. 1989].
Our data revealed high variability in vitamin D metabolite values for both 25(OH)D3 and 1,25(H)2D3 forms. The levels we have found for 25(OH)D3 were generally higher than the levels in Jarcho et al., [2013] by around 40% but showed a wide range in values. Their study demonstrated that marmosets with significantly reduced 25(OH)D3 values were associated with a significantly reduced digestive efficiency; indicating a lack of the ability to absorb food in the digestive tract. They suggest that marmosets have a wide range of digestive abilities that are reflected in the 25(OH)D3 levels and that low 25(OH)D3 levels may indicate marmosets that are more likely to exhibit metabolic bone disease in the future [Jarcho et al. 2013]. Our data only revealed very low 25(OH)D3 in two samples out of 25 and these samples did not show the concordant low levels in 1,25(OH)2D3. In fact no correlation between the levels of 25(OH)D3 and 1,25(OH)2D3 was found, indicating that 25(OH)D3 do not always predict 1,25(OH)2D3 levels from marmoset/=85 samples.
The marmosets have very high levels of vitamin D3 added to their diet. The WNPRC Marmoset Colony provides 30% more vitamin D supplement than the SWNPRC colony when compared to the Jarcho et al. [2013] study. This may explain why our marmosets did not show concordant low levels in 25(OH)D3. Marmosets that are free-living in the wild and exposed to natural light have a range of 25(OH)D3 between 20.1–103.3 ng/ml (Teixeira et al. 2012) which was much lower than the range we found: 4–875 ng/ml. It is also possible that the WNPRC marmosets were not only absorbing their vitamin D from the supplement but also generating it in their skin as a result of some exposure to UV exposure from the lighting in their rooms. Marmosets are well known for hanging from the top of their cage upside down, exposing their hairless abdomen to light, as they would hang from branches outdoors. Nonetheless, exposure to UV light under the conditions of our vivarium is not expected to be a major contributor to circulating vitamin D.
There could be a number of factors responsible for the wide variation found in the concentration in circulation, in particular, for 25(OH)D3. As mentioned previously, this variation could be due to the absorption problems that have been reported in marmosets or the lack of the 25(OH)D3 could be the cause of the lack of absorption [Jarcho et al. 2013]. Additionally, nonhuman primates, like other mammal species with hair/fur covering the majority of their bodies, do not have exposed skin visible for vitamin D absorption from UV radiation to initiate the cascade creating 25(OH)D3. However, as occurs with many mammals, it is likely that the vitamin D generated through the oily secretions deposited onto the fur can be obtained orally through grooming, thereby adding an additional source of 25(OH)D3 and the variability that occurs in both 25(OH)D3 and 1,25(OH)2D3 [Carpenter and Zhao 1999].
The higher amount of vitamin D3 in the diet of the WNPRC marmosets is reflected in the higher measurements of 25(OH)D3 and 1,25(OH)2D3 measured by our LC/MS/MS methods. New methods are currently being developed that will allow us to measure the breakdown products of vitamin D (24-OH-D, 24,25-OH D) as well as circulating levels of colecalciferol. These further measurements would provide insights into what are appropriate levels for the marmoset and what levels might compromise the liver’s ability to hydroxylate the vitamin.
Rhesus
Rhesus monkeys exhibited higher levels of both 25(OH)D3 and 1,25(OH)2D2 than humans as have previously been reported [Shinki et al. 1983; Marx et al. 1989]. Notably, our values for 25(OH)D3 were most similar to the values reported for rhesus macaques living outdoors and free-range at Cayo Santiago, Puerto Rico [Vieth et al. 1987]. The Cayo Santiago rhesus monkeys have access to UV from the sunlight consistently throughout the day and continuously throughout the year while also having 8.0 IU/g of vitamin D3 supplemented in their diet. If the methods can be reliably compared, then it would appear that the exposure to sunlight and vitamin D conversion in skin has not increased the levels of 25(OH)D3 in circulation over the dietary component. One possibility would be that the high levels of the dietary cholecalciferol inhibit skin conversion by UV. Alternatively, the dense hair covering the rhesus macaque body significantly lowers the skin’s ability to absorb UV rays needed for vitamin D conversion.
Levels of 1,25(OH)2D3 were also similar between the Cayo rhesus and our results. The WNPRC rhesus monkeys showed consistent results with little variation in 25(OH)D3 levels and more variation in the 1,25(OH)2D3 levels, which may indicate the importance of keeping a consistent level of 25(OH)D3 circulating and ready for hydroxylase conversion as demanded.
A significant decrease in 1,25(OH)2D3 levels with increased age was reported for the Cayo rhesus monkeys [Vieth et al. 1987]. While our study and the Cayo Santiago rhesus study did not show differences in 1,25(OH)2D3 levels by sex, they both showed it by age. However, the Cayo Santiago study found only male age influenced the decline of 1,25(OH)2D3 and our study did not see a sex difference in age effect. This could be due to the limited number of females that were in the study and lack of selection of rhesus monkeys to produce a continuum of ages. Aging in humans is associated with a significant decline in 25(OH)D3 in circulation(Resmini et al., 2013). There appears to be a reduction in the effective synthesis of vitamin D from the skin. A significant decrease in the amount of 7-dehydrocholesterol in the skin occurs with age in humans. Additionally, the conversion of UV light from the sun into pre-vitamin D3 is the main source of vitamin D as the amount of vitamin D2 in the diet is minimal.
Both the rhesus and the cynomolgus monkey showed measurable levels of 1,25(OH)2D2 without showing measurable levels of 25(OH)D2. This must indicate that the 25(OH)D2 was lower than the sensitivity of our 25(OH)D assay and therefore was not recorded. The source of the 1,25(OH)2D2 would have to be from the diet. Although no vitamin D2 was added to the diet, brewers dried yeast is a component of the diet. Ergosterol is produced in yeast and can be absorbed and converted into 25(OH)D2 [Fryberg et al. 1972]. Vitamin D2 is not as potent as vitamin D3, and not as readily available [Cranney et al. 2007], although at the tissue level differences appear to be minor since 1,25(OH)2D2 and 1,25(OH)2D3 are comparable in binding the vitamin D receptor [Bikle 2010]. In nonhuman primates there is variability in how vitamin D2 is absorbed and available for use.
Cynomolgus
As expected, the cynomolgus monkeys were very similar to the rhesus monkeys in vitamin D metabolite levels. At the WNPRC the macaques were fed identical diets and housed in the same type housing. Cynomolgus monkeys in this study fell into a relatively narrow range of age, were males, were Mauritian derived, and therefore expected to be very genetically similar. Yet, Fig. 2 indicates the wide variability of 25(OH)D3 found in the males. One possibility would be if differences in intestinal health influenced the absorption in vitamin D as occurs in the marmosets [Jarcho et al. 2013]. Investigators using cynomolgus as models for human related vitamin D research have documented that variability exists even when all the monkeys are fed a controlled diet [Schnatz et al. 2012]. They hypothesized that the variability is largely dependent on genetics. However, if in a genetically constrained species, such as Mauritian cynomolgus macaques, there is still much variability, then this may be an environmental or epigenetic influence affecting the levels of 25(OH)D3. We did, however, find a significant correlation between vitamin D levels and individual food allocations, a rough approximation of food intake. While our cynomolgus monkeys were all males for this study, the fetoplacental unit during pregnancy is a major site of 1,25(OH)2D production for both D2 and D3 [Markestad et al. 1984]. This exposure could influence the rate of synthesis in the offspring’s dihydroxylated vitamin D through maturity.
CONCLUSIONS
While there were different levels of the two forms of vitamin D metabolites observed between the species, the most notable differences were within each species. Humans show considerable variation in their 25(OH)D, some of which may come from measurement techniques, but recently these have been greatly reduced. All three of the nonhuman primate species we examined also showed significant variation between individuals within a species. Since much of the differences in exposure to UV radiation has been eliminated in laboratory primates the individuals within each species should be getting equal amounts of dietary vitamin D. This indicates there has to be other variables affecting the vitamin D concentration.
Genetics could explain differences in laboratory cynomolgus monkeys with controlled dietary vitamin D, sun exposure, and menopausal status [Schnatz et al. 2012]. Yet more factors than genetics could be in play. Absorption of dietary vitamin D can be variable per individual within a species. Binding to chylomicrons absorbs Vitamin D within the gastrointestinal tract. It is then transported by the lymphatic system to the blood circulatory system where it binds with vitamin D binding protein and is transported to the liver for metabolism or to adipose tissue for storage. The rate and extent of 25(OH)D production is dependent upon vitamin D hydroxylase and its inactivation by cyctochromes P-450 CYP24, CYP3A4 and the vitamin D binding protein. Markers in the corresponding genes may contribute to the variations found within a species [Mallah et al. 2011].
Environmental influences might also cause the variability within a species for vitamin D metabolite differences. In utero exposure to mother’s vitamin D levels may predispose an individual to levels that influence the absorption of dietary D or limit the rate of the hydroxylase needed to convert the 25(OH)D. Exposure to illness could influence the absorption ability of the intestinal tract as well. There are a number of factors that could explain the variability and these primate models offer the opportunity to examine these factors.
The rhesus macaque and the cynomolgus macaque would make ideal models for humans in studying the sources of the variability in vitamin D levels and how this influences the multitude of roles both 25(OH)D and 1,25(OH)2D3 have in the body, from bone homeostasis, neuroprotection and endocrine responses to immune function. The marmosets show the most variability and are possibly the most sensitive to disruptions in vitamin D levels. Determining the cause of the extremely low levels of 25(OH)D that can exist would greatly benefit the health of marmosets. Additionally, marmosets could be models for in utero and epigenetic effects on vitamin D variability due to their twinning and short generation time.
ACKNOWLEDGEMENTS
We gratefully acknowledge the members of the WNPRC Scientific Protocol Implementation Unit who obtained the blood samples and Megan Sosa, the WNPRC animal care staff and technicians for caring for the animals, Bonnie Friscino for providing information on the monkeys’ food intake. We thank AB SCIEX for providing the Amplifex Diene® derivatizer for 1,25-dihydroxyvitaman D analysis. Lauren Koenig provided helpful comments on the manuscript. This work was supported by NIH grants from the Office of Research Infrastructure Programs (P51 OD011106) to the Wisconsin National Primate Research Center. Additional support was made by a grant from the National Center for Advancing Translational Sciences (UL1TR000427).
REFERENCES
- Adams JS, Cacad MA, Baker AJ, Gonzales G, Rude RK. Serum concentrations of 1,25-dihydroxyvitamin D3 in Platyrrhini and Catarrhini: A phylogenetic appraisal. American Journal of Primatology. 1985;9:219–224. doi: 10.1002/ajp.1350090307. [DOI] [PubMed] [Google Scholar]
- Adams JS, Chen H, Chun RF, et al. Novel regulators of vitamin D action and metabolism: lessons learned at the Los Angeles zoo. Journal of Cell Biochemistry. 2003;88:308–314. doi: 10.1002/jcb.10333. [DOI] [PubMed] [Google Scholar]
- Aloia JF, Patel M, DiMaano R, et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. American Journal of Clinical Nutrition. 2008;87:1952–1958. doi: 10.1093/ajcn/87.6.1952. [DOI] [PubMed] [Google Scholar]
- Bikle DD. VITAMIN D: Newly discovered actions require reconsideration of physiologic requirements. Trends in Endocrinology & Metabolism. 2010;21:375–384. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkley N, Simpos CT. Standardizing vitamin D assays: the way forward. Journal of Bone Mineral Research. 2014;29:1709–1714. doi: 10.1002/jbmr.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkley N, Krueger D, Lensmeyer GL. 25-hydroxyvitamin D measurement 2009: A review for clinicians. Journal of Clinical Densitometry. 2009;12:417–427. doi: 10.1016/j.jocd.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Binkley N, Gemar D, Engelke J, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. Journal of Clinical Endocrinology and Metabolism. 2011;96:981–988. doi: 10.1210/jc.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A, Tilmont EM, Handy AM, et al. A nonhuman primate model of age-related bone loss: A longitudinal study in male and premenopausal female Rhesus monkeys. Bone. 2001;28:295–302. doi: 10.1016/s8756-3282(00)00452-x. [DOI] [PubMed] [Google Scholar]
- Burwitz BJ, Pendley CJ, Greene JM, et al. Maritian cynomolgus macaques share two exceptionally common major histocompatibility complex class I alleles that restrict simian immunodeficiency virus-specific CD8+ T Cells. Journal of Virology. 2009;83:6011–6019. doi: 10.1128/JVI.00199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KJ, Zhao L. Forgotten mysteries in the early history of vitamin D. Journal of Nutrition. 1999;129:923–927. doi: 10.1093/jn/129.5.923. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Lane MA, Binkley N, Wegner FH, Kemnitz Skeletal effects of aging in male rhesus monkeys. Bone. 1999;24:17–23. doi: 10.1016/s8756-3282(98)00147-1. [DOI] [PubMed] [Google Scholar]
- Cranny C, Horsely T, O’Donnell S, Weiler H, Ooi D, Atkinson S. Evidence Report/Technology Assessment No. 158. Agency for Healthcare Research and Quality; 2007. Effectiveness and safety of vitamin D. [PMC free article] [PubMed] [Google Scholar]
- Crissey SD, Meehan TP, Langman C, Pruett-Jones MA. Vitamin D metabolites 25(OH)D and 1,25(OH)D and kidney function indices and the relationship to diet in Goeldi’s monkeys (Callimico goeldii) Zoo Biology. 1999;18:565–574. [Google Scholar]
- Fryberg M, Oehlschlager AC, Unrau AM. Biosynthetic routes to ergosterol in yeast. Biochemical and Biophysical Research Communications. 1972;48:593–597. doi: 10.1016/0006-291x(72)90389-0. [DOI] [PubMed] [Google Scholar]
- Gacad MA, Deseran MW, Adams JS. Influence of ultraviolet B radiation on vitamin D3 metabolism in vitamin D3 resistant new world monkeys. American Journal of Primatology. 1992;28:263–270. doi: 10.1002/ajp.1350280404. [DOI] [PubMed] [Google Scholar]
- Hedman CJ, Wiebe DA, Dey S, Plath J, Kemnitz JW, Ziegler TE. Development of a sensitive LC/MS/MS method for vitamin D metabolites 1,25 dihydroxyvitamin D2&3 measurement using a novel derivatization agent. Journal of Chromatography B. 2014;953–954:62–67. doi: 10.1016/j.jchromb.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilger J, Friedel A, Herr R, et al. A systematic review of vitamin D status in populations worldwide. British Journal of Nutrition. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D Deficiency. New England Journal of Medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley N, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology and Metabolism. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- De la Hunty A, Wallace AM, Gibson S, et al. UK foods standards agency workshop consensus report: the choice of method for measuring 25-hydroxyvitamin D to estimate vitamin D status for the UK National Diet and Nutrition Survey. British Journal of Nutrition. 2010;104:612–619. doi: 10.1017/S000711451000214X. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Power ML, Layne-Colon DG, Tardif SD. Digestive efficiency mediated by serum calcium predicts bone mineral density in the common marmoset (Callithrix jacchus) American Journal of Primatology. 2013;75:153–160. doi: 10.1002/ajp.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivula MK, Matinlassi N, Laitinen P, Risteli J. Four automated 25-OH total vitamin D immunoassays and commercial liquid chromatography tandem-mass spectrometry in Finnish population. Clinical Lab. 2013;59:397–405. doi: 10.7754/clin.lab.2012.120527. [DOI] [PubMed] [Google Scholar]
- Lee DM, Vanderschueren D, Boonen S, et al. Association of 25-hydroxyvitmain D, 1,25-dihydroxyvitamin D and parathyroid hormone with mortality among middle-aged and older European men. Age and Aging. 2013;0:1–8. doi: 10.1093/ageing/aft206. [DOI] [PubMed] [Google Scholar]
- Liberman UA, de Grange D, Marx SJ. Low affinity of the receptor for 1alpha, 25-dihydroxyvitimain D3 in the marmoset, a New World monkey. Federation of European Biochemical Societies. 1985;182:385–388. doi: 10.1016/0014-5793(85)80338-0. [DOI] [PubMed] [Google Scholar]
- Mallah EM, Hamad MF, EiManaseer MA, et al. Plasma concentrations of 25-hydroxyvitamin D among Jordanians: Effect of biological and habitual factors on vitamin D status. BMC Clinical Pathology. 2011;11:1–6. doi: 10.1186/1472-6890-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markestad T, Aksnes L, Ulstein M, Asrskog D. 25-Hydroxyvitamin D and 1,25-dihydroxyvitamin D of D2 and D3 origin in maternal and umbilical cord serum after vitamin D2 supplementation in human pregnancy. American Journal of Clinical Nutrition. 1984;40:1057–1063. doi: 10.1093/ajcn/40.5.1057. [DOI] [PubMed] [Google Scholar]
- Marx SJ, Jones G, Weinstein RS, Chrousos GP, Renquist DM. Differences in mineral metabolism among nonhuman primates receiving diets with only vitamin D3 or only vitamin D2. Journal Clinical Endocrinology & Metabolism. 1989;69:1282–1290. doi: 10.1210/jcem-69-6-1282. [DOI] [PubMed] [Google Scholar]
- Netzel BC, Cradic KW, Bro ET, et al. Increasing liquid chromatography-tandem mass spectrometry throughput by mass tagging: A sample-multiplexed high-throughput assay for 25-hydroxyvitamin D2 and D3. Clinical Chemistry. 2011;57:431–440. doi: 10.1373/clinchem.2010.157115. [DOI] [PubMed] [Google Scholar]
- Novakovic B, Sibson M, Ng HK, Manuelpillai U, Rakyan V, Down T, Beck S, Fournier T, Evain-Brion D, Dimitridis E, Craig JM, Morley R, Saffery R. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. Journal of Biological Chemistry. 2009;284:14838–14848. doi: 10.1074/jbc.M809542200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgaz-Molina J, Magro-Checa C, Rosales-Alexander JL, et al. Association of 25-hydroxyvitamin D serum levels and metabolic parameters in psoriatic patients with and without arthritis. Journal of the American Academy of Dermatology. 2013;69:938–946. doi: 10.1016/j.jaad.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Resmini G, Tarantiono U, Iolascon G. Vitamin D: role and opportunity to prescribe. Aging Clinical and Experimental Research. 2013;25:125–127. doi: 10.1007/s40520-013-0108-8. [DOI] [PubMed] [Google Scholar]
- Ross AC, Taylor CL, Yakine AL, et al. Report on dietary reference intakes for vitamin D and calcium; Institute of Medicine. The National Academies Press; 2011. [PubMed] [Google Scholar]
- Schnatz PF, Marakovits KA, O’Sullivan DM, Ethun K, Clarkson TB, Appt SE. Response to an adequate dietary intake of vitamin D3 modulates the effect of estrogen therapy on bone density. Journal of Women’s Health. 2012;21:858–864. doi: 10.1089/jwh.2011.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempos CT, Vesper HW, Phinney KW, et al. Vitamin D status as an international issue: National surveys and the problem of standardization. Scandinavian Journal of Clinical and Laboratory Investigation. 2012;72:32–40. doi: 10.3109/00365513.2012.681935. [DOI] [PubMed] [Google Scholar]
- Shinki T, Shiina Y, Takahashi N, Tanioka Y, Koizumi H, Suda T. Extremely high circulating levels of 1alpha, 25-dihydroxyvitamin D3. Biochemical and Biophysical Research Communications. 1983;114:452–457. doi: 10.1016/0006-291x(83)90801-x. [DOI] [PubMed] [Google Scholar]
- Teixeira DS, Nobrega YKM, Valencia CEU, Gandolfi L, Pratesi R, Castro LCG. Evaluation of 25-hydroxy-vitamin D and parathyroid hormone in Callithrix penicillata primates living in their natural habitat in Brazil. Journal Medical Primatology. 2012;41:364–371. doi: 10.1111/jmp.12021. [DOI] [PubMed] [Google Scholar]
- Vieth R, Kessler MJ, Pritzker PH. Serum concentrations of vitamin D metabolites in Cayo Santiago rhesus macaques. Journal Medical Primatology. 1987;16:349–357. [PubMed] [Google Scholar]
- Yamaguchi A, Kohno Y, Uarnazaki T, et al. Bone in the marmoset: A resemblance to vitamin D-dependent rickets, Type II. Calcified Tissue International. 1986;39:22–27. doi: 10.1007/BF02555736. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Lucey AF, Horgan R, Kenny LC, Kiely M. Impact of pregnancy on vitamin D status: a longitudinal study. British Journal of Nutrition. 2014;112:1081–1087. doi: 10.1017/S0007114514001883. [DOI] [PubMed] [Google Scholar]