Abstract
A rapid growth in the overweight and obese population in the last few decades suggest that the current diet, exercise, awareness or drug strategies are still not effectively restraining the obesity epidemic. Obesity results from increased energy intake, and the body’s energy balance shifts towards energy abundance. Therefore, current research is focused on developing new strategies aimed at increasing energy expenditure. As a result, brown adipose tissue (BAT) is receiving tremendous attention since the major function of BAT is to dissipate energy as heat. For example, mouse models that have increased BAT activity or increased numbers of brown-like adipocytes within the white adipose tissue (WAT) are lean and protected from obesity. Alternatively, mouse models that lack BAT activity are more susceptible to age and diet-induced obesity. However, a significant loss of BAT mass during the natural growth process in humans has created enormous challenges in effectively utilizing this tissue to increase energy expenditure. New strategies are primarily focused on expanding the BAT mass and/or activating the existing BAT. In this regard, recent finding that expression of early B cell factor-2 (Ebf2) reprograms the white pre-adipocytes into brown adipocytes is a significant break-through in developing BAT-mediated strategies to treat obesity. Here we review the major biological functions of WAT and BAT, which play critical but opposing roles in the energy spectrum, energy storage versus energy expenditure, and we evaluate whether activation and/or expansion of BAT is practically achievable to treat obesity in humans.
Keywords: Leptin, Ucp1, Thermogenesis, PGC1α, Beige fat
Introduction
Obesity is rapidly emerging as the greatest challenge to human health worldwide. According to the World Health Organization (WHO), there are currently approximately 1.5 billion overweight adults in the world and of those ~500 million are obese. In addition, ~42 million children under the age of 5 are overweight or obese. The situation in the United States is rather alarming, with ~65% of US adults either overweight or obese. Obesity is a substantial risk factor for a number of diseases and numerous cancers (Hursting, 2014; Mirza, 2011; Poirier and Eckel, 2002; Vucenik and Stains, 2012). At the biological level, a shift in the energy balance of the body results in obesity. If the energy (food) intake consistently surpasses energy expenditure, the extra energy is transported and stored in the form of triglycerides in the white adipose (fat) tissue (WAT) (Hursting, 2014). A number of factors, such as genetic, environmental, behavioral and life style and biological factors, play a major role in increased food consumption and/or overeating disorders (Dhurandhar and Keith, 2014; Singh, 2014; Waalen, 2014). For example, according to recent studies, certain types of foods such as those found in fatty and sugary diets, are as addictive as sex and drugs and stimulate the pleasure centers of the brain (Avena et al., 2012; Volkow et al., 2013). In the course of time, this permanently alters feeding behavior, and individuals are addicted to consume high energy fatty diets. Therefore, understanding the physiological and molecular mechanisms of energy intake, storage, and expenditure has become an intense area of research. Although a number of metabolic, hormonal and neuronal signals arising from different organs contribute to the overall body energy homeostasis (Pang and Han, 2012; Sisley and Sandoval, 2011; Suzuki et al., 2010), in this review, we mainly focus on two tissues, WAT and BAT (brown adipose tissue), that play crucial roles on the opposite ends of the energy spectrum – storing energy versus wasting energy.
White adipose tissue (WAT)
The major function of WAT is to store extra energy in the form of triglycerides, which form large unilocular lipid droplets in white adipocytes. WAT represents ~10% of the body weight of healthy adult humans. WAT stores energy when there is a surplus and breaks down triglycerides and supplies fatty acids to other organs when needed. Thus, WAT functions as the energy storage and supply center of the body. Although WAT consists of a small fraction of immune and stromal cell populations (Gimble et al., 2011; Lolmede et al., 2011), it is predominantly composed of white adipocytes. In adults, most of these adipocytes are mature, fully differentiated cells and their size is directly proportional to the amount of stored lipids. The white adipocytes are capable of storing a large amount of energy by hypertrophy (MacKellar et al., 2010). WAT also consists of a preadipocyte population and/or adipose progenitors that reside along the adipose tissue vasculature. Under conditions of increased energy influx, when the existing adipocytes reach their maximum storage capacity, the preadipocytes are induced to differentiate into mature adipocytes in order to accommodate the incoming energy. Therefore, in addition to adipocyte hypertrophy, the preadipocyte/adipose progenitor population plays a predominant role in meeting the demands of increased energy influx (MacKellar et al., 2010; Wang et al., 2013b). Together, WAT has an enormous ability to expand by both hypertrophy and hyperplasia to house extra energy with little or no wastage (Hausman et al., 2001). This efficient capture and storage of energy appears to be an evolutionarily conserved phenomenon to cope with periods of food scarcity. However, in the current times of relatively easy availability of cheap energy-rich food, this energy storage efficiency is, not surprisingly, contributing to the process of moderate weight gain to overweight and ultimately to obesity.
It appears that the WAT has an unrestricted ability to expand; morbidly obese individuals can gain as much as several tens of kilograms of extra WAT tissue, which can exceed more than 50% of the body weight, suggesting that WAT continues to expand as long as excessive energy intake persists. What happens if we target and inhibit WAT expansion? Do we lose extra energy due to lack of storage space in the WAT? These questions are quite elegantly answered by two studies in mouse models. In the first study, a fatless mouse was generated by selectively expressing a dominant-negative protein, A-ZIP/F-1, in adipocytes (Moitra et al., 1998). A-ZIP/F-1 prevents DNA binding and thereby suppresses the function of several B-ZIP transcription factors in the C/EBP and AP1 families. These transcription factors are required for normal adipogenesis. As a result, the A-ZIP/F-1 mice have absolutely no white fat tissue and thus no energy storage capacity (Moitra et al., 1998). In the absence of WAT, these mice accumulate lipids in a number of tissues such as liver, muscle, heart and kidney and suffer increased levels of inflammation, develop diabetes, and are also susceptible to spontaneous and induced carcinogenesis (Nunez et al., 2006). In another study, PPARγ, a crucial regulator of adipocyte differentiation, was specifically deleted from the adipocytes. This causes severe loss of WAT (lipoatrophy) in these mice. Nevertheless, surprisingly, the body weights of adult adipocyte-specific PPARγ-null mice are similar to wild-type mice. The adipose-specific PPARγ-null mice suffer from severe insulin resistance, diabetes and a fatty liver due to ectopic accumulation of lipids. These mice also display abnormalities of bone, skin, and mammary glands, all of which contain adipose tissue (Wang et al., 2013a). These studies clearly indicate that in the absence of the energy store house, WAT, the extra energy may not be just eliminated from the body; thus interfering with adipogenesis and/or WAT expansion can have severe deleterious consequences. In the absence of WAT, cells of other tissues could be forced to capture and store energy, which in turn severely impairs their basic biological functions. It appears that the evolutionarily conserved mechanisms favor energy storage over energy loss even though abundant energy has detrimental effects on the overall function of the body.
Leptin vs Ghrelin
Is there a mechanism whereby WAT communicates stored energy levels to the brain, thereby suppressing energy intake? WAT is not just a simple storehouse of energy. It also functions as an active endocrine organ and secretes a number of cytokines, such as leptin, adiponectin, resistin, IL-6, IL-10 and TNFα (Guerre-Millo, 2004). Of these, leptin and adiponectin are predominantly secreted by adipocytes, and their circulating levels oscillate in response to stored energy levels. These adipokines communicate with other organs, such as brain, muscle, liver and other endocrine organs, to regulate energy homeostasis (Trayhurn and Wood, 2004). The adipokine that is primarily responsible for communicating WAT stored energy levels to the brain is leptin. Secreted from the adipocytes of the WAT, leptin signals the hypothalamus in the brain of energy abundance and thereby controls appetite (Attele et al., 2002). On the other side, ghrelin, a peptide hormone secreted from the endocrine cells of the stomach, signals hunger or energy insufficiency to the hypothalamus, thereby regulating short-term appetite and energy distribution. After a meal, when the stomach is full, ghrelin levels are reduced. Although ghrelin and leptin transmit opposite signals to the brain, they activate a number of overlapping signaling pathways (Klok et al., 2007). Does leptin play a role in suppressing ghrelin levels and ghrelin-mediated appetite signaling? It was shown that leptin, indeed, could suppress ghrelin-mediated signaling (Barazzoni et al., 2003; Kalra et al., 2005; Kohno et al., 2007). However, leptin appears to function mainly in long-term appetite control and might not regulate short-term hunger. For example, administration of leptin to animals starved overnight does not prevent them from consuming food, suggesting that leptin may not override the hunger signals initiated by ghrelin.
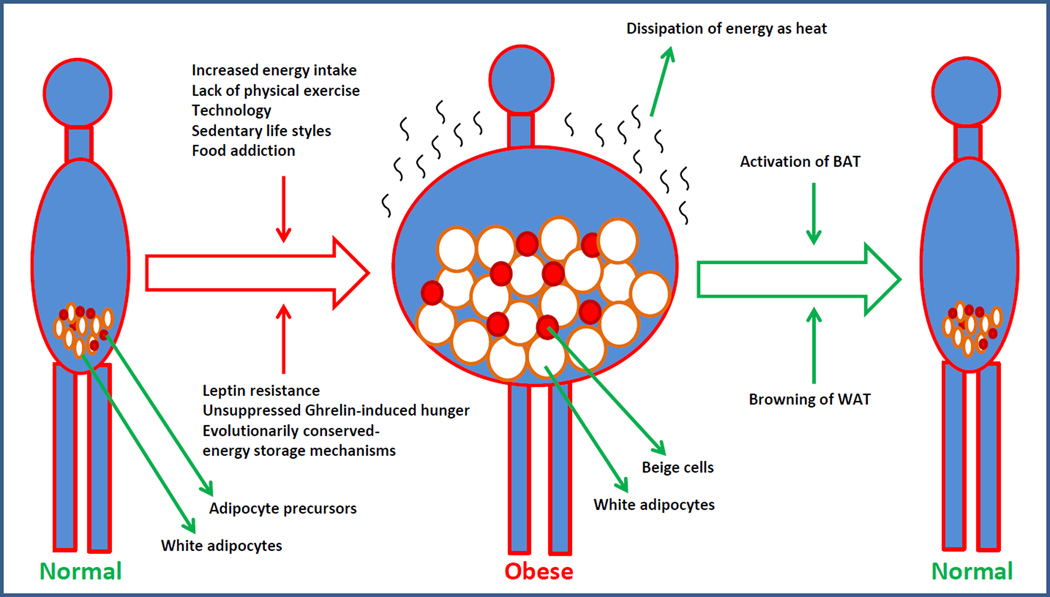
Leptin levels are directly proportional to the amount of stored energy, and its levels continue to rise as the storage increases. However, although in the obese condition the circulating levels of leptin are very high, leptin fails to suppress appetite. This is due to the development of leptin resistance (DePaoli, 2014; Myers et al., 2008). For example, daily administration of leptin to obese animals reduces their food intake for few weeks before they develop leptin resistance and resume their normal food consumption rates. If energy levels are very high, do ghrelin-mediated hunger signals continue to be elicited? Ghrelin levels are lower in the obese condition where the energy levels are very high (Tschop et al., 2001). In certain studies, it was described that ghrelin-mediated appetite is reduced in the obese condition; however, it was also shown that ghrelin stimulates appetite and food intake even more in obese than lean humans (Druce et al., 2005). It appears that ghrelin-mediated signaling fails to recognize the presence of enormous stored energy in the WAT and continues to signal hunger when the stomach is empty. This could be partly due to the breakdown of leptin signaling, and under conditions of leptin resistance, ghrelin might be more potent, even at low levels, in inducing appetite. The inability of leptin to sustain its action on ghrelin-mediated hunger and the relatively rapid development of leptin resistance suggest that the evolutionarily conserved mechanisms favor continuous energy intake and storage, resist tapping into the stored energy, and instead stimulate hunger. It appears that the intake and storage of energy are evolutionarily favored mechanisms that require a breakdown of leptin signaling. These rigid mechanisms that favor energy intake and storage contribute to weight gain and ultimately obesity (Figure 1). Therefore, the only path to balance body energy and prevent obesity is finding a way to waste energy.
Figure 1.
Cartoon showing an array of factors responsible for causing obesity and the brown adipocyte-mediated energy expenditure strategies to possibly reverse obesity in humans. The detailed mechanisms are discussed in the text.
Brown adipose tissue (BAT)
The most efficient way to waste body energy is by increasing physical activity. Rigorous physical activities, such as running, swimming and biking, can burn off hundreds of calories and thus facilitate balancing excessive energy intake with energy expenditure (Strasser, 2013). Although it is a very well-known fact that regular physical exercise prevents weight gain, the majority of the humans are unable to follow it due to a number biological, behavioral and lifestyle reasons. If we did, we would not have the problem of obesity. Therefore, we need to find a way to waste energy without extensively participating in physically demanding activities. Is BAT the solution for this problem? The primary function of BAT is to dissipate energy in the form of heat, a process called adaptive thermogenesis. For example, by effectively utilizing BAT, small mammals living in cold environments produce heat for their survival. Thus, the idea of wasting body energy by employing BAT is very appealing. Despite the fact that the existence of BAT in rodents and newborn humans has been very well known for a long time (Cannon and Nedergaard, 2004), its exploitation to increase energy expenditure and treat obesity has not been taken very seriously. This is mainly because of the belief that BAT mass is steadily lost during the natural human growth process, which leaves very little or no BAT in adults, and, therefore, strategies aimed at activating or expanding BAT in adults may not be feasible. By debunking this belief, recent PET-CT (positron emission tomography) studies demonstrate the existence of metabolically active BAT in adult healthy humans, and the mass and activity of this tissue is declined in obese and aged subjects (Virtanen et al., 2009). These findings reenergized the practical feasibility of increasing the amount and/or activity of BAT in the body in order to waste energy and thus treat obesity (Figure 1). The idea of activating BAT to treat obesity seems to be realistic, since in humans as little as 50 g of BAT (~0.1% of body weight) is estimated to burn ~20% of the basal caloric needs if fully stimulated (Rothwell and Stock, 1983). This is further supported in a number of mouse models where enhanced activity of BAT or increasing the number of brown-like cells or transplantation of BAT protected mice from diet and age-associated obesity (Seale et al., 2009).
What makes BAT so unique and how does it dissipate energy? BAT is specialized in performing a physiological mechanism called adaptive thermogenesis, during which energy is dissipated to generate heat in response to cold and/or diet (Cannon and Nedergaard, 2004; Seale et al., 2009). BAT is densely packed with mitochondria and executes heat production through a unique protein called uncoupling protein-1 (UCP1), which is located in the inner mitochondrial membrane. UCP1 uncouples mitochondrial oxidative phosphorylation from ATP production and dissipates chemical energy as heat, which significantly increases energy expenditure (Klingenberg, 1999; Kozak and Anunciado-Koza, 2008). Consequently, genetic deletion of Ucp1 leads to impaired ability to produce heat in response to cold exposure (Enerback et al., 1997), and the Ucp1 knockout mice gain more body weight when they were housed at thermo-neutral temperature (Feldmann et al., 2009). These observations suggest that UCP1-triggered BAT-mediated thermogenesis can be activated by both cold and diet. These findings in animal models further support the idea of expansion of BAT and activation of BAT-mediated thermogenesis to achieve energy balance and to treat obesity in humans (Costford et al., 2007; Kozak and Anunciado-Koza, 2008). But the most important question is how do we specifically expand and/or activate BAT?
Cold-induced thermogenesis
The easiest way to activate and expand BAT is exposure to cold temperatures. For example, exposing rodents to cold temperatures not only activates BAT in the short-term but also increases the total BAT mass in the long-term (Klingenspor, 2003; Morrison et al., 2012; Nakamura and Morrison, 2011). Cold exposure studies in human subjects also showed similar activation and an increase in BAT mass with a concomitant loss of body weight (Cypess et al., 2009; Nedergaard et al., 2010; van der Lans et al., 2013). Conversely, exposure to warmer temperatures results in suppression of thermogenesis due to a reduction in the sympathetic drive to BAT (van der Lans et al., 2013). How does cold temperature induce BAT activation? Exposure to cold causes release of catecholamines such as norepinephrine from the sympathetic nerve terminals that act on the β-adrenergic receptors of the brown adipocytes (Cannon and Nedergaard, 2004). Activated β-adrenergic receptors stimulate the cAMP/PKA/CREB signaling pathway, which ultimately induces peroxisome proliferator activated receptor γ coactivator 1α (PGC1α), the master regulator of UCP1-mediated thermogenesis (Herzig et al., 2001). The β-adrenergic receptor/cAMP pathway also induces PGC1α through p38 MAPK, which activates PGC1α by removing p160-mediated repression, thereby increasing PGC1α protein stability (Cao et al., 2004). Overall, PGC1α expression as well as its transcriptional activity is greatly induced in response to cold exposure. PGC1α in turn activates a number of nuclear and non-nuclear factors and functions as the central regulator of numerous pathways involved in mitochondrial biogenesis and thermogenesis (Austin and St-Pierre, 2012; Delerive et al., 2002; Finck and Kelly, 2006; Handschin and Spiegelman, 2006; Huss et al., 2002; Knutti et al., 2000; Lin et al., 2005a; Lin et al., 2005b; Puigserver et al., 2003; Puigserver et al., 1998; Vega et al., 2000; Wang et al., 2003). For example, PGC1α directly induces the expression of Ucp1, and it also coactivates nuclear respiratory factors 1 and 2 (NRF1 and NRF2), which regulate the expression of genes encoding respiratory chain subunits and other factors essential for mitochondrial biogenesis and oxidative phosphorylation (Austin and St-Pierre, 2012; Wu et al., 1999). By regulating all these factors, PGC1α has a strong impact on several aspects of mitochondrial energy metabolism. Consequently, deletion of Pgc1α in mice results in impaired thermogenesis in response to cold exposure (Lin et al., 2004; Uldry et al., 2006). In contrast, induced expression of PGC1α is sufficient to induce an array of genes involved in mitochondrial biogenesis and thermogenesis, including Ucp1 in white adipocytes (Puigserver et al., 1998; Tiraby et al., 2003), suggesting a central role for PGC1α in thermogenesis.
Is it practically feasible to conduct prescribed cold therapy procedures on humans to increase thermogenesis and to treat obesity? Although it has been known for some time that cold can effectively induce thermogenesis, so far there are not any reported cases of prescribed cold therapy procedures in clinics, and it might be very difficult to implement such procedures to treat obesity. For example, in most of the animal studies, mice were exposed to cold temperatures for prolonged periods of time (4–24h) in order to achieve significant BAT activation. Alternatively, animals were exposed to cold temperatures for shorter periods of time but it was done repeatedly to achieve significant BAT expansion (Fisher et al., 2012; Ravussin et al., 2014; Whittle et al., 2012). Therefore, similar prescribed cold therapy procedures might be difficult to implement in humans due to our inability to tolerate such cold conditions for such prolonged periods of time or multiple cold exposures. On the other hand, short tolerable levels of cold exposure might not be sufficient to achieve significant BAT activation. In addition, although cold exposure might burn some fat, it can also cause certain unintended medical complications such as stress, hypothermia, changes in blood pressure and respiratory issues. Can we achieve BAT activation without exposure to cold?
Activation of BAT
Is it possible to avoid cold exposure and directly activate the β-adrenergic receptor/PGC1α pathway and induce BAT-mediated thermogenesis? Subsequently, β3-adrenergic receptor (β3-AR) agonists have been identified that were shown to induce thermogenesis and increase metabolic rate in rodents (Arch et al., 1984; Connacher et al., 1992). These β3-AR agonists were able to increase energy expenditure and appeared to be beneficial in counteracting obesity and diabetes in rodents. However, utilizing β3-AR agonists to increase BAT-mediated thermogenesis in humans was unsuccessful due to the very low level of expression of β3-AR receptors on human brown adipocytes (Arch, 2002). Therefore, these agonists were not able to induce the desired thermogenic response in human BAT and caused several off-target effects (Arch, 2002). Alternatively, attempts to utilize non-specific sympathomimetics, such as ephedrine and sibutramine, to induce thermogenesis resulted in severe side effects, such as stroke and cardiovascular complications, due to broad and nonspecific action of adrenergic stimulation (Di Dalmazi et al., 2013). Therefore, strategies that bypass β-adrenergic stimulation and directly activate PGC1α in BAT might be necessary to develop approaches to pharmacologically activate BAT in humans. However, developing such an approach may or may not be possible because, although PGC1α is the central regulator of UCP1-mediated thermogenesis in the BAT, it is also deeply involved in a number of other cellular mechanisms such as glucose and fatty acid metabolism, mitochondrial biogenesis and oxidative metabolism in other organs such as muscle, liver, heart and brain (Austin and St-Pierre, 2012; Puigserver et al., 2003). Therefore, it will be extremely challenging to identify a pharmacological agent that specifically targets and activates PGC1α only in the BAT without causing inadvertent side effects since it also plays an active role in other organs such as liver and muscle. An alternative approach could be identifying the specific molecular regulators of PGC1α and UCP1 in BAT and target these factors to modulate PGC1α/UCP1-mediated thermogenesis. Due to their critical role in thermogenesis, the expression and activities of PGC1α and UCP1 are precisely controlled by a number of proteins that either positively or negatively regulate PGC1α and UCP1, thus serving as accelerators or brakes and controling thermogenesis. Subsequently, a number of factors such as FOXC2, SRC1, CREB, SIRT3, ERRα and p38 MAPK were identified as positive regulators of PGC1α (Seale et al., 2009). Specific deletion of these factors in mice resulted in impaired PGC1α/UCP1-mediated thermogenesis and reduced energy expenditure, and the mice are prone to diet-induced obesity, indicating the importance of these factors in the activation of PGC1α/UCP1-mediated thermogenesis in BAT (Cao et al., 2004; Picard et al., 2002). Therefore, instead of directly targeting PGC1α, pharmacological activation of these factors could serve as an alternative approach to enhance thermogenesis since they ultimately mediate their effects through PGC1α. Conversely, factors such as pRB, p107, RIP140, Cidea, LXRα, Id1, Twist1 and TRPV4 were identified to function as negative regulators of PGC1α and/or UCP1 and thus suppress BAT-associated thermogenesis. Specific deletion of these factors in mice resulted in increased BAT activation and/or expansion and elevated energy expenditure, and the mice are protected from diet and age-associated obesity (Kiskinis et al., 2007; Pan et al., 2009; Satyanarayana et al., 2012; Scime et al., 2005; Sharma et al., 2014; Villena et al., 2007; Ye et al., 2012; Zhou et al., 2003). Therefore, pharmacological intervention of these factors might function as an alternative approach to enhance BAT thermogenesis. However, unfortunately, most of these positive and negative regulators are not only expressed in BAT and function in PGC1α/UCP1-mediated thermogenesis but they are also expressed in various other tissues and participate in a wide range of cellular processes. Therefore, strategies to specifically target one or more of these factors to modulate thermogenesis in BAT might be extremely challenging, and other organs will face unintended consequences from drugs that are directed towards these factors to activate BAT.
Browning of WAT
Another strategy that is under active investigation is to specifically target preadipocyte and/or adipose progenitors in the WAT and induce them to differentiate into brown-like cells, a process called browning of WAT. This will potentially increase the amount of brown adipocytes/brown-like cells, enhance energy expenditure and reduce body weight (Figure 1). In response to various stimuli such as cold and β-AR or PPARγ agonists, pools of UCP1-expressing brown-like adipocytes were detected in mouse WAT, especially in inguinal WAT (Barbatelli et al., 2010; Himms-Hagen et al., 2000; Vitali et al., 2012). These adipocytes are called ‘beige’ or ‘brite’ and, similar to brown adipocytes, these cells have high mitochondrial content and express thermogenic genes such as Pgc1α and Ucp1. Although beige adipocytes participate in thermogenesis similarly to brown adipocytes, their precise origin is not completely understood. Conversely, the developmental origins of brown adipocytes are relatively well known and are derived from a Pax7+Myf5+-expressing progenitor population of the mesoderm that also gives rise to skeletal muscle cells. In these progenitors, by co-operating with PPARγ, EBF2 induces the expression of PRDM16, which determines the brown adipocyte cell fate (Seale et al., 2008; Seale et al., 2007; Timmons et al., 2007). PRDM16 can bind to and modulate the transcriptional activity of numerous factors such as PPARα, PPARγ and PGC1α, thus functioning as a critical regulator of brown adipocyte cell fate (Kajimura et al., 2009; Seale et al., 2007). With respect to beige adipocytes, recent evidence suggests that these adipocytes do not appear to be derived from Pax7+Myf5+ precursor cells (Rosen and Spiegelman, 2014; Seale et al., 2008). Initial studies suggested that the mature differentiated white adipocytes in WAT can transdifferentiate into beige adipocytes when stimulated by cold or β3-AR agonists (Barbatelli et al., 2010; Vitali et al., 2012). However, subsequent lineage tracing studies revealed that the beige adipocytes do not arise from the existing differentiated white adipocytes and are indeed derived from the adipose progenitor/precursor cells (Wang et al., 2013b). The adipose progenitors in the WAT are believed to be bi-potential and, under conditions of excessive energy influx, they differentiate into white adipocytes, whereas, in response to cold or β-AR stimulus, they differentiate into beige adipocytes (Wu et al., 2012). As a result, thermogenic genes such as Ucp1 and the PGC1α-network of genes are expressed in beige adipocytes only when there is an external stimulus such as cold or β-AR agonists. This is in contrast to brown adipocytes where the thermogenic genes are expressed all the time, at least at basal levels (Wu et al., 2013). Consequently, the thermogenic capacity of beige adipocytes is reversible, and these cells can lose this ability when the stimulus is withdrawn (Rosenwald et al., 2013). It appears that the beige adipocytes are susceptible to transdifferentiation into white adipocytes when there is high energy influx. Similar to mouse WAT, it is also known that human WAT contains adipose progenitor cells that express UCP1 in response to PPAR-γ activation and display beige adipocyte characteristics (Elabd et al., 2009). Can we force adipose progenitors in WAT to differentiate into brown-like cells in humans? Obviously, cold exposures and β-AR/ PPAR-γ agonists can induce browning of WAT in humans. However, both of these approaches are difficult to implement in humans due to the non-specific nature of β-AR agonists and the inability of humans to tolerate cold conditions for prolonged periods of time. Can we avoid cold exposure and β-AR agonists and still be able to induce browning in WAT? Recent studies successfully identified some of the important genes, such as Prdm16 and Ebf2, that participate in brown adipocyte programing (Rajakumari et al., 2013; Seale et al., 2008; Seale et al., 2009). These findings will potentially allow us to think beyond cold and agonists and formulate more specific targeted approaches to induce browning of WAT. Not surprisingly, due to their dominant role in brown adipocyte programing, it was demonstrated that induced expression of Prdm16 or Ebf2 is sufficient to convert white adipose precursors into brown-like UCP1-expressing cells. Similarly, overexpression of Prdm16 in mouse adipose causes browning of WAT and protects mice against diet-induced obesity (Seale et al., 2011). Based on these studies, it is clear that strategies aimed at inducing the expression of PRDM16 or EBF2 are sufficient to cause browning in WAT, which will have profound implications in the treatment of obesity. Additionally, a number of other factors have been identified to have a positive or negative regulatory effect not only on BAT thermogenesis but also on the browning of WAT. Some of these factors include FOXC2, RIP140, LXR, p107 and pRB, and activation of positive regulators or inhibition of suppressors function as alternative strategies to stimulate WAT browning, enhance energy expenditure and treat obesity. However, so far, pharmacological agents that specifically target any of these known factors to increase browning of WAT in humans are not available and are still an active area of investigation.
Conclusions
A steady growth of the overweight and obese population in the world implies that current diet, exercise and drug strategies are clearly not working in curtailing the obesity epidemic. Obesity results from a shift towards energy abundance in the body; therefore, new strategies must be aimed at enhancing body energy expenditure and/or identifying a way to waste energy. Since the predominant function of BAT is to dissipate energy as heat, new strategies should be designed to exploit this tissue to raise body energy expenditure. Under natural conditions, BAT mass steadily declines during the growth process, and adult humans have a significantly less percentage of BAT compared to newborns. BAT mass is also mostly lost in obese individuals, suggesting that its existence is susceptible to high energy influx. For example, the presence of significant levels of childhood obesity and 65% of adults in the US are overweight or obese clearly indicates that under normal conditions BAT is definitely unable to handle the excessive energy influx. If it did, we would not have the problem of obesity. Moreover, in energy excessive conditions, BAT appears to be prone to transdifferentiation into white-like cells. For example, feeding mice with a high-fat diet for just a few weeks can lead to significant lipid accumulation in BAT, and it appears like WAT, although mice have a much higher percentage of BAT compared to humans. Therefore, strategies to either increasing the amount of BAT or activating the existing BAT are currently under active investigation with the expectation that it will counter-balance higher energy influx. Activation and/or expansion of BAT could potentially work to treat obesity since it has been demonstrated that an increase in the amount and/or activation of BAT causes a lean and healthy phenotype in numerous animal models. Moreover, recent advances in identifying genes that play critical roles not only in the developmental origins of BAT and its activation but also the differentiation and browning of WAT is providing new opportunities to develop specific strategies at the molecular level to activate the existing BAT or to convert WAT into BAT. For example, approaches such as specific activation of PGC1α in the brown adipocytes or induction of Ebf2 or Prdm16 in preadipocytes of WAT to generate more brown-like adipocytes in an obese individual could increase energy expenditure and reduce body fat. However, whether an obese patient still has any brown adipose tissue left to activate is questionable. In addition, converting adipocyte progenitors in the hypertrophic WAT of an obese individual into brown-like cells may not be easy because when there is a constant influx of energy into the body the adipose progenitors might prefer to differentiate into white adipocytes to store incoming energy rather than differentiate into beige cells to burn off the extra energy. At this moment, although BAT-mediated energy expenditure strategies seem to be theoretically possible, it will be extremely challenging to implement any of these strategies in humans to reduce WAT mass and treat obesity. This is mainly due to the fact that not only lifestyle but also biological factors such as development of leptin resistance, unsuppressed continuous ghrelin-induced appetite and unlimited ability of WAT to expand and store energy all favor energy storage over energy expenditure. It will be a monumental challenge for BAT to fight against all these factors and reduce WAT mass and restore body energy balance.
Acknowledgments
Due to space limitations, we are unable to cite a number of important contributions to the field, and we sincerely apologize to the authors of those publications. We thank the members of the Ande lab for their participation in the discussions and their useful critics. The authors also thank Dr. Rhea-Beth Markowitz for critically reviewing and editing the manuscript. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health under the award number DP2DK105565.
Abbreviations
- β3-AR
Beta 3-adrenergic receptor
- BAT
Brown adipose tissue
- B-ZIP
Basic leucine zipper
- C/EBP
CCAAT/enhancer binding protein
- cAMP
Cyclic adenosine monophosphate
- Cidea
Cell death-inducing DNA fragmentation factor, alpha subunit-like effector A
- CREB
cAMP response element binding protein
- Ebf2
Early B cell factor 2
- ERRα
Estrogen-related receptor alpha
- FOXC2
Fork-head box C2
- Id1
Inhibitor of differentiation 1
- IL-6
Interleukin 6
- IL-10
Interleukin 10
- LXRα
Liver-X-receptor alpha
- Myf-5
Myogenic factor 5
- MAPK
Mitogen activated protein kinase
- NRF1
Nuclear respiratory factor 1
- NRF2
Nuclear respiratory factor 2
- p107
Pocket protein 107
- PAX7
Paired box 7
- PET-CT
Positron emission tomography-computed tomography
- PGC1α
Peroxisome proliferator activated receptor gamma coactivator 1 alpha
- PKA
Protein kinase alpha
- PPARγ
Peroxisome proliferator-activated receptor gamma
- pRB
Retinoblastoma protein
- RIP140
Receptor-interacting protein 140
- PRDM16
PR domain containing 16
- SIRT3
Sirtuin-3
- SRC1
Src tyrosine kinase 1
- TNFα
Tumor necrosis factor alpha
- TRPV4
Transient receptor potential cation channel, subfamily 5, member 4
- Twist1
Twist family basic helix-loop-helix transcription factor 1
- UCP1
Uncoupling protein 1
- WAT
White adipose tissue
Footnotes
Conflict of interest
The authors declare no competing financial conflicts of interest.
References
- Arch JR. beta(3)-Adrenoceptor agonists: potential, pitfalls and progress. European journal of pharmacology. 2002;440:99–107. doi: 10.1016/s0014-2999(02)01421-8. [DOI] [PubMed] [Google Scholar]
- Arch JR, Ainsworth AT, Ellis RD, Piercy V, Thody VE, Thurlby PL, Wilson C, Wilson S, Young P. Treatment of obesity with thermogenic beta-adrenoceptor agonists: studies on BRL 26830A in rodents. Int J Obes 8 Suppl. 1984;1:1–11. [PubMed] [Google Scholar]
- Attele AS, Shi ZQ, Yuan CS. Leptin, gut, and food intake. Biochemical pharmacology. 2002;63:1579–1583. doi: 10.1016/s0006-2952(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Austin S, St-Pierre J. PGC1alpha and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. Journal of cell science. 2012;125:4963–4971. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- Avena NM, Gold JA, Kroll C, Gold MS. Further developments in the neurobiology of food and addiction: update on the state of the science. Nutrition. 2012;28:341–343. doi: 10.1016/j.nut.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzoni R, Zanetti M, Stebel M, Biolo G, Cattin L, Guarnieri G. Hyperleptinemia prevents increased plasma ghrelin concentration during short-term moderate caloric restriction in rats. Gastroenterology. 2003;124:1188–1192. doi: 10.1016/s0016-5085(03)00281-6. [DOI] [PubMed] [Google Scholar]
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. American journal of physiology Endocrinology and metabolism. 2010;298:E1244–E1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Molecular and cellular biology. 2004;24:3057–3067. doi: 10.1128/MCB.24.7.3057-3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connacher AA, Bennet WM, Jung RT. Clinical studies with the beta-adrenoceptor agonist BRL 26830A. The American journal of clinical nutrition. 1992;55:258S–261S. doi: 10.1093/ajcn/55.1.258s. [DOI] [PubMed] [Google Scholar]
- Costford S, Gowing A, Harper ME. Mitochondrial uncoupling as a target in the treatment of obesity. Current opinion in clinical nutrition and metabolic care. 2007;10:671–678. doi: 10.1097/MCO.0b013e3282f0dbe4. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delerive P, Wu Y, Burris TP, Chin WW, Suen CS. PGC-1 functions as a transcriptional coactivator for the retinoid X receptors. The Journal of biological chemistry. 2002;277:3913–3917. doi: 10.1074/jbc.M109409200. [DOI] [PubMed] [Google Scholar]
- DePaoli AM. 20 years of leptin: leptin in common obesity and associated disorders of metabolism. The Journal of endocrinology. 2014;223:T71–T81. doi: 10.1530/JOE-14-0258. [DOI] [PubMed] [Google Scholar]
- Dhurandhar EJ, Keith SW. The aetiology of obesity beyond eating more and exercising less. Best practice & research Clinical gastroenterology. 2014;28:533–544. doi: 10.1016/j.bpg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Di Dalmazi G, Vicennati V, Pasquali R, Pagotto U. The unrelenting fall of the pharmacological treatment of obesity. Endocrine. 2013;44:598–609. doi: 10.1007/s12020-013-9983-1. [DOI] [PubMed] [Google Scholar]
- Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, Ghatei MA, Small C, Bloom SR. Ghrelin increases food intake in obese as well as lean subjects. International journal of obesity. 2005;29:1130–1136. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- Elabd C, Chiellini C, Carmona M, Galitzky J, Cochet O, Petersen R, Penicaud L, Kristiansen K, Bouloumie A, Casteilla L, et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem cells. 2009;27:2753–2760. doi: 10.1002/stem.200. [DOI] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell metabolism. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. The Journal of clinical investigation. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Bunnell BA, Chiu ES, Guilak F. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: let's not get lost in translation. Stem cells. 2011;29:749–754. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes & metabolism. 2004;30:13–19. doi: 10.1016/s1262-3636(07)70084-8. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocrine reviews. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. American journal of physiology Cell physiology. 2000;279:C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- Hursting SD. Obesity, energy balance, and cancer: a mechanistic perspective. Cancer treatment and research. 2014;159:21–33. doi: 10.1007/978-3-642-38007-5_2. [DOI] [PubMed] [Google Scholar]
- Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. The Journal of biological chemistry. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra SP, Ueno N, Kalra PS. Stimulation of appetite by ghrelin is regulated by leptin restraint: peripheral and central sites of action. The Journal of nutrition. 2005;135:1331–1335. doi: 10.1093/jn/135.5.1331. [DOI] [PubMed] [Google Scholar]
- Kiskinis E, Hallberg M, Christian M, Olofsson M, Dilworth SM, White R, Parker MG. RIP140 directs histone and DNA methylation to silence Ucp1 expression in white adipocytes. The EMBO journal. 2007;26:4831–4840. doi: 10.1038/sj.emboj.7601908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Uncoupling protein--a useful energy dissipator. Journal of bioenergetics and biomembranes. 1999;31:419–430. doi: 10.1023/a:1005440221914. [DOI] [PubMed] [Google Scholar]
- Klingenspor M. Cold-induced recruitment of brown adipose tissue thermogenesis. Experimental physiology. 2003;88:141–148. doi: 10.1113/eph8802508. [DOI] [PubMed] [Google Scholar]
- Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Knutti D, Kaul A, Kralli A. A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Molecular and cellular biology. 2000;20:2411–2422. doi: 10.1128/mcb.20.7.2411-2422.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno D, Nakata M, Maekawa F, Fujiwara K, Maejima Y, Kuramochi M, Shimazaki T, Okano H, Onaka T, Yada T. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology. 2007;148:2251–2263. doi: 10.1210/en.2006-1240. [DOI] [PubMed] [Google Scholar]
- Kozak LP, Anunciado-Koza R. UCP1: its involvement and utility in obesity. International journal of obesity. 2008;32(Suppl 7):S32–S38. doi: 10.1038/ijo.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell metabolism. 2005a;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005b;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Lolmede K, Duffaut C, Zakaroff-Girard A, Bouloumie A. Immune cells in adipose tissue: key players in metabolic disorders. Diabetes & metabolism. 2011;37:283–290. doi: 10.1016/j.diabet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- MacKellar J, Cushman SW, Periwal V. Waves of adipose tissue growth in the genetically obese Zucker fatty rat. PloS one. 2010;5:e8197. doi: 10.1371/journal.pone.0008197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza MS. Obesity, Visceral Fat, and NAFLD: Querying the Role of Adipokines in the Progression of Nonalcoholic Fatty Liver Disease. ISRN gastroenterology. 2011;2011:592404. doi: 10.5402/2011/592404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, et al. Life without white fat: a transgenic mouse. Genes & development. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Frontiers in endocrinology. 2012;3 doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annual review of physiology. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. The Journal of physiology. 2011;589:3641–3658. doi: 10.1113/jphysiol.2011.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Annals of the New York Academy of Sciences. 2010;1212:E20–E36. doi: 10.1111/j.1749-6632.2010.05905.x. [DOI] [PubMed] [Google Scholar]
- Nunez NP, Oh WJ, Rozenberg J, Perella C, Anver M, Barrett JC, Perkins SN, Berrigan D, Moitra J, Varticovski L, et al. Accelerated tumor formation in a fatless mouse with type 2 diabetes and inflammation. Cancer research. 2006;66:5469–5476. doi: 10.1158/0008-5472.CAN-05-4102. [DOI] [PubMed] [Google Scholar]
- Pan D, Fujimoto M, Lopes A, Wang YX. Twist-1 is a PPARdelta-inducible, negative-feedback regulator of PGC-1alpha in brown fat metabolism. Cell. 2009;137:73–86. doi: 10.1016/j.cell.2009.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Han W. Regulation of synaptic functions in central nervous system by endocrine hormones and the maintenance of energy homoeostasis. Bioscience reports. 2012;32:423–432. doi: 10.1042/BSR20120026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Gehin M, Annicotte J, Rocchi S, Champy MF, O'Malley BW, Chambon P, Auwerx J. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111:931–941. doi: 10.1016/s0092-8674(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Poirier P, Eckel RH. Obesity and cardiovascular disease. Current atherosclerosis reports. 2002;4:448–453. doi: 10.1007/s11883-002-0049-8. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, Reed RR, Seale P. EBF2 determines and maintains brown adipocyte identity. Cell metabolism. 2013;17:562–574. doi: 10.1016/j.cmet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin Y, Xiao C, Gavrilova O, Reitman ML. Effect of intermittent cold exposure on brown fat activation, obesity, and energy homeostasis in mice. PloS one. 2014;9:e85876. doi: 10.1371/journal.pone.0085876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nature cell biology. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. Effects of age on diet-induced thermogenesis and brown adipose tissue metabolism in the rat. Int J Obes. 1983;7:583–589. [PubMed] [Google Scholar]
- Satyanarayana A, Klarmann KD, Gavrilova O, Keller JR. Ablation of the transcriptional regulator Id1 enhances energy expenditure, increases insulin sensitivity, and protects against age and diet induced insulin resistance, and hepatosteatosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:309–323. doi: 10.1096/fj.11-190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scime A, Grenier G, Huh MS, Gillespie MA, Bevilacqua L, Harper ME, Rudnicki MA. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell metabolism. 2005;2:283–295. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. The Journal of clinical investigation. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes & development. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell metabolism. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma BK, Patil M, Satyanarayana A. Negative regulators of brown adipose tissue (BAT)-mediated thermogenesis. Journal of cellular physiology. 2014;229:1901–1907. doi: 10.1002/jcp.24664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. Mood, food, and obesity. Frontiers in psychology. 2014;5:925. doi: 10.3389/fpsyg.2014.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisley S, Sandoval D. Hypothalamic control of energy and glucose metabolism. Reviews in endocrine & metabolic disorders. 2011;12:219–233. doi: 10.1007/s11154-011-9189-x. [DOI] [PubMed] [Google Scholar]
- Strasser B. Physical activity in obesity and metabolic syndrome. Annals of the New York Academy of Sciences. 2013;1281:141–159. doi: 10.1111/j.1749-6632.2012.06785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocrine journal. 2010;57:359–372. doi: 10.1507/endocrj.k10e-077. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. The Journal of biological chemistry. 2003;278:33370–33376. doi: 10.1074/jbc.M305235200. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. The British journal of nutrition. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell metabolism. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jorgensen JA, Wu J, Mottaghy FM, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. The Journal of clinical investigation. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Molecular and cellular biology. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena JA, Hock MB, Chang WY, Barcas JE, Giguere V, Kralli A. Orphan nuclear receptor estrogen-related receptor alpha is essential for adaptive thermogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1418–1423. doi: 10.1073/pnas.0607696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. Journal of lipid research. 2012;53:619–629. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Annals of the New York Academy of Sciences. 2012;1271:37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalen J. The genetics of human obesity. Translational research : the journal of laboratory and clinical medicine. 2014;164:293–301. doi: 10.1016/j.trsl.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Wang F, Mullican SE, DiSpirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARgamma. Proceedings of the National Academy of Sciences of the United States of America. 2013a;110:18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature medicine. 2013b;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes & development. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, Cohen P, Khandekar MJ, Bostrom P, Mepani RJ, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151:96–110. doi: 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, Ponniah S, Lin SC, Hong W, Li P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nature genetics. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]