Abstract
Traumatic brain injury (TBI), which can lead to disability, dysfunction, and even death, is a prominent health problem worldwide. Effective therapy for this serious and debilitating condition is needed. Human umbilical cord matrix, known as Wharton's jelly (WJ), provides a natural, interface scaffold that is enriched in mesenchymal stem cells. In this study, we tested the efficacy of WJ tissue transplantation in a weight drop model of TBI in rats. WJ tissue was cultured and transplanted into the injury site 24h after TBI. The modified neurologic severity score, body weight, brain edema, and lesion volume were evaluated at various time points after TBI. Cognitive behavior was assessed by the novel object recognition test and the Morris water maze test. Expression of brain-derived neurotrophic factor (BDNF) in the perilesional brain area was measured at day 14 after TBI. We found that WJ tissue transplantation lessened TBI-induced brain edema (day 3), reduced lesion volume (day 28), improved neurologic function (days 21 to 28), and promoted memory and cognitive recovery. Additionally, expression of BDNF mRNA and protein was higher in WJ tissue-treated rats than in sham-operated or vehicle-treated rats. These data suggest that WJ tissue transplantation can reduce TBI-induced brain injury and may have therapeutic potential for the treatment of TBI.
Keywords: Cognition, Traumatic brain injury, Wharton's jelly, Transplantation, BDNF
Introduction
Traumatic brain injury (TBI) remains a major health problem worldwide. It causes considerable disability, mortality, and functional impairment that severely affects quality of life (Bennett et al. 2012). Despite extensive research, no available treatment can effectively repair the biostructural damage of TBI. However, stem cell therapies have offered some promise. Studies have shown that transplantation of neural stem cells provides neuroprotection after TBI (Riess et al. 2002; Bakhtiary et al. 2011; Arien-Zakay et al. 2012; Wang et al. 2013). Primarily through paracrine and endocrine mechanisms (Camussi et al. 2010), stem cells regulate inflammatory response, decrease free radical production, reduce apoptosis, and promote endogenous neuronal growth, synaptic connections, and neural repair (Joyce et al. 2010). Of the various stem cell types, human umbilical cord mesenchymal stem cells (hUC-MSCs) have many advantages (Arien-Zakay et al. 2012). They display strong self-renewal and differentiation abilities (Karahuseyinoglu et al. 2007); can be obtained from a wide variety of sources; are easy to collect, culture, and transport; have high reproductive activity; cause no risk of allograft rejection; and pose no ethical controversy (Wang et al. 2013). Therefore, hUC-MSCs are one of the most promising types of seed cells for treating TBI. However, researchers have not yet tested the efficacy of umbilical cord matrix in TBI.
Umbilical cord matrix, also known as Wharton's jelly (WJ) (Malkowski et al. 2007), originates from extra-embryonic mesoderm at day 13 of embryonic development and is able to produce hUC-MSCs. Umbilical cord is composed of two arteries and one vein surrounded by a unique connective tissue stroma that is rich in proteoglycans and mucopolysaccharides. The mucoprotein around human umbilical cord blood vessels composes WJ, which contains abundant hyaluronic acid and collagen. Because of its anti-extrusion and anti-stretching properties, WJ not only supports cell growth, but also acts as a tissue scaffold to produce hUC-MSCs (Sobolewski et al. 2005).
Although WJ tissue provides a good source of hUC-MSCs, its therapeutic potential has not been tested in animal models. Several groups reported that neural stem cell or MSC transplantation upregulates synaptic protein expression, stimulates secretion of brain-derived neurotrophic factor (BDNF) and other neurotrophic factors, and promotes functional recovery after TBI (Ma et al. 2012; Kim et al. 2010). In this study, we tested the hypothesis that transplantation of WJ tissue would reduce lesion volume and improve neurologic and cognitive function after TBI in rats. To elucidate the potential mechanism of protection, we measured the expression of BDNF at 2 weeks after WJ tissue transplantation.
Materials and Methods
Animals
This study was conducted in accordance with the National Institutes of Health guidelines for the use of experimental animals. Animal protocols were approved by the Zhengzhou University Animal Care and Use Committee. Ninety-eight male Sprague-Dawley rats (240 ± 20 g) were used. All efforts were made to minimize the numbers of animals used and ensure minimal suffering. All studies described below and the data analyses were conducted in a blinded manner.
Preparation of WJ Tissue from Human Umbilical Cord
We obtained human umbilical cords postpartum from full-term, healthy infants delivered via normal vaginal delivery. The mothers provided informed consent according to institutional guidelines under an approved protocol. The umbilical cord membrane was stripped, and the umbilical cord blood vessels (two arteries and one vein) were removed to retain the WJ, which was cut into pieces (around 1mm3) and then cultured in DMEM/F12 (GIBCO, Carlsbad, CA) containing 10% fetal bovine serum and 0.1% penicillin-streptomycin. The tissues were cultured at 37°C in an incubator with 5% CO2 atmosphere. WJ tissue became increasingly swollen and enlarged in the medium and attached to the surface of culture flasks.
After WJ tissue pieces had been in culture for 5 days, visible single-spindled or triangular cells initiated adhesion around the tissue. Cells gradually multiplied and grew into a radial-like array around the adherent tissue. After 14 days, adherent cells reached 80-90% confluence. These cells were passaged while the WJ tissue remained in the medium. The WJ tissue grew against the wall of the flask and generated new hUC-MSCs in less than 24 h. We used WJ tissue that had produced three passages of hUC-MSCs for transplantation.
We used flow cytometry to detect the cell surface markers of the hUC-MSCs at passage 3. The cells were labeled with the following human antibodies: CD29-PE, CD44-FITC, CD133-FITC, HLA-ABC-FITC, HLA-DR-FITC, CD34-PE, CD45-PerC, FITC-IgG1, PerCP-IgG1, and PE-IgG2a (BD Bioscience, USA). Approximately 10,000 cells were measured on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA), and the results were analyzed with ModFit software, as described previously (Zhou et al. 2011). We performed three independent experiments to confirm the results.
TBI Models
To produce a contusion injury of the left parietal cortex of rats, we used a weight-drop model of TBI in which a weight falls from a fixed distance onto the exposed cranial dura. Each rat was anesthetized with an intraperitoneal injection of 10% chloral hydrate (3 ml/kg), and its head was fixed in a stereotactic device. Under aseptic conditions, a dental drill was used to create a left parietal bone window (5 mm in diameter) 3mm behind the anterior fontanel and 2 mm to the left. A 50-g steel rod with a flat end (4 mm in diameter) was released from a height of 30 cm onto a piston resting on the dura (Liu et al. 2014). The piston was allowed to compress the tissue a maximum of 5 mm. The cut was then sutured. After this procedure, the rats were returned to their cages and maintained at room temperature (23±1°C). Heart rate, arterial blood pressure, and rectal temperature were monitored throughout the procedure, and rectal temperature was kept at 37±0.5°C.
Experimental Groups
Rats were randomly divided into three groups: sham group (32 rats), vehicle-treated TBI group (33 rats), and WJ tissue-treated TBI group (33 rats). All rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate before treatment. For the sham group, we only opened the skull. For the TBI + vehicle group, we re-opened the skull at 24 h after TBI and injected fresh, uncultured WJ tissue (approximately 1 mm3) into the middle of the bone window (stereotactic coordinates: 3 mm behind the anterior fontanelle and 2.5 mm to the left of centerline) through a 1 ml syringe with a 25 G needle. We then replaced the skull and sutured the opening. For the WJ tissue-treated TBI group, we placed the activated WJ tissue that had produced three passages of hUC-MSCs into the middle of the bone window at 24 h after TBI. We sacrificed 6, 18 and 8 rats of each group at 3, 14, and 28 days after TBI, respectively.
Brain Water Content
Brain water content was measured on day 3 after TBI, as described previously (Wu et al. 2010). Rats were sacrificed by decapitation. Ipsilateral hemispheres were weighed immediately on an analytical balance to obtain the wet weight and then dried for 48 h at 100°C to obtain the dry weight. Water content was calculated as: Water content (%) = (wet weight – dry weight)/wet weight×100%.
Lesion Volume
On day 28 after TBI, rats were deeply anesthetized and perfusion-fixed with 4% paraformaldehyde. Brains were frozen-sectioned at 40μm. Coronal cryostat sections 400μm apart between bregma -0.5 mm and bregma -4.5mm were stained with hematoxylin and eosin. Image J software (NIH, Bethesda, MD, USA) was used to quantify lesion volumes (Zhu et al. 2014). The volume of the lesion in cubic millimeters was calculated as the sum of the damaged areas of each section multiplied by the interslice distance (Wu et al. 2012).
Neurologic Function: Neurologic Deficit Scoring
On days 1, 3, 7, 14, 21, and 28 after TBI, neurologic deficit was evaluated with the modified neurologic severity score (mNSS), which measures motor, sensory, reflex, and balance deficits (Zhang et al. 2009; Jiang et al. 2013). The mNSS test is graded on a scale of 0 (normal) to 18 (maximal deficit).
Cognitive Function: Novel Object Recognition Test
The novel object recognition test was used to evaluate recognition memory. The experiments were carried out in a black, open-field box measuring 50 ×25 ×50 cm. On day 27 post-TBI, a rat was placed in the cage and habituated for 10 min. The next day, two identical novel objects (red cubes, 4×4×4 cm) were placed in the arena, and the rat was allowed to explore them for 10 min. After 1 h, one novel object (green ball, 4 cm in diameter) and one old object (red cube) were placed in the box and the rat was allowed to explore for 5 min. A camera recorded each test. We compared the total time spent exploring the old and new objects. The exploration time included time in direct contact with the object and time within the object area; a discrimination index (total time spent with new object/total time devoted to exploration of objects) was also calculated for each rat (Zhu et al. 2014).
Cognitive Function: Morris Water Maze Test
On days 24–28, we measured acquisition of memory retention and cognitive functions with the Morris water maze. A pool 120 cm in diameter and 60 cm deep was filled with water (22±2°C) to a depth of 27 cm and divided into quadrants (Zhang et al. 2009). A round Plexiglas platform (10-cm diameter) was placed 1 cm below the water surface in the first quadrant. Testing involved four trials per day. Rats were placed in randomly chosen positions of the pool facing a wall and were allowed up to 90 s to find the invisible platform. Upon locating the platform (or being guided to the platform after 90s), the animal was permitted to remain on the platform for 10 s before the next trial. The average latency time of four trials (Fang et al. 2014) and the percentage of time spent in the correct quadrant were recorded.
Western Blot Analysis
On day 14 after TBI, rats (6/group) were killed by deep anesthesia and their brains were removed. A continuous segment of brain tissue surrounding the lesion within 1 mm was dissected out by a needle and used to extract total proteins. Approximately 100 mg of brain tissue from the ipsilateral perilesional cortex and subcortical regions was collected. Tissue homogenate was centrifuged at 12,000 rpm for 10 min at 4°C, and the supernatant was stored at -80°C for later use. Protein concentration was determined by a BCA kit (Pierce, Rockford, IL). Fifty-microgram protein samples were separated by 10% SDS-PAGE and transferred to PVDF membranes using a semidry electrotransferring unit (Bio-Rad, Hercules, CA). The membranes were blocked and then probed with primary antibody: rabbit anti-rat BDNF (1:1000; Abcam, Cambridge, MA) and β-actin (1:2500; Santa Cruz Biotechnology, Dallas, TX) overnight at 4°C. The membranes were washed and incubated with horseradish peroxidase-linked anti-rabbit secondary antibodies (1:1000; Santa Cruz Biotechnology) for 1 h. Chemiluminescence was detected by exposure to x-ray film. Experiments were performed in triplicate. Resulting protein bands were analyzed with Image J software (version 1.4.1).
Real-Time Quantitative RT-PCR
At 14 days after TBI, total RNA was extracted from the ipsilateral perilesional cortex and subcortical regions with Trizol reagent (Life Technologies, Rockville, MD). The Prime Script 1stStrand cDNA Synthesis Kit (Takara, Japan) was used for the reverse transcription reactions. cDNA was amplified on a Fast 7500 real-time PCR instrument (Bio-Rad) using SYBR Premix Ex Taq (Prefect Real Time, Takara, Japan). The reactions contained primers for BDNF forward (GGTCACAGTCCTGGAGAAAG) and reverse (GTCTATCCTTATGAACCGCC). The primers and probes were obtained from Sangon Biotech (Shanghai, China). Reactions were incubated at 95°C for 30s, followed by 40 cycles of 95°C for 5 s, 60°C for 30 s, and 72°C for 60 s. BDNF mRNA level was normalized to the endogenous control glyceraldehyde-3-phosphate dehydrogenase in the same sample. The 2-ΔΔ Ct method was used for relative quantification of gene expression as described (Chang et al. 2014).
Immunohistochemistry
Immunohistochemistry was carried out as described previously (Zan et al. 2014). After being blocked, coronal sections (n=6 rats/group) were incubated with rabbit anti-MAP2 (a mature neuronal marker, 1:1000, Abcam) at 4°C overnight and then with biotinylated goat anti-rabbit IgG (1:1000, Abcam) for 1 h at room temperature. The immunoreactions were visualized with diaminobenzidine-H2O2 solution. Control sections were processed without the primary antibody and showed no positive signals. MAP2-positive cells were counted around the lesion under a 40× objective from 15 randomly selected locations per rat (5 fields per section × 3 sections per rat). Quantifications were averaged and expressed as positive cells per field. Sections were selected and analyzed by an investigator blinded to the experimental cohort.
Statistical Analyses
All data are presented as mean ± standard deviation (SD) and were analyzed by SPSS 18.0 software (SPSS, Chicago, IL). We evaluated mNSS score, body weight, and Morris water maze results by two-way repeated measure ANOVA to detect significant differences. One-way ANOVA followed by Newman-Keuls test was used for brain water content, protein expression, and mRNA expression. Differences between two groups at one time point were analyzed with independent sample t-tests. The criterion for statistical significance was p<0.05.
Results
During this study, the mortality was 3.33% (1/33) in the vehicle group and 3.33% (1/33) in the WJ tissue-treated group. The two dead rats were excluded from the final blind data analysis.
Characterization of WJ Tissue
At the third passage, hUC-MSCs had a typical fibroblastic morphology. They had strong proliferative ability, were primarily spindle-shaped or triangular, adhered rapidly, and expanded without visible changes in the growth pattern or morphology (Fig. 1A) (Zhou et al. 2011). Flow cytometry analysis showed that hUC-MSCs expressed high levels of matrix marker CD44 and integrin marker CD29 (Fig. S1.A) but did not express hematopoietic lineage markers (CD133, CD34, and CD45) or HLA-DR (MHCII) (Fig. S1.B, C).
Fig. 1.
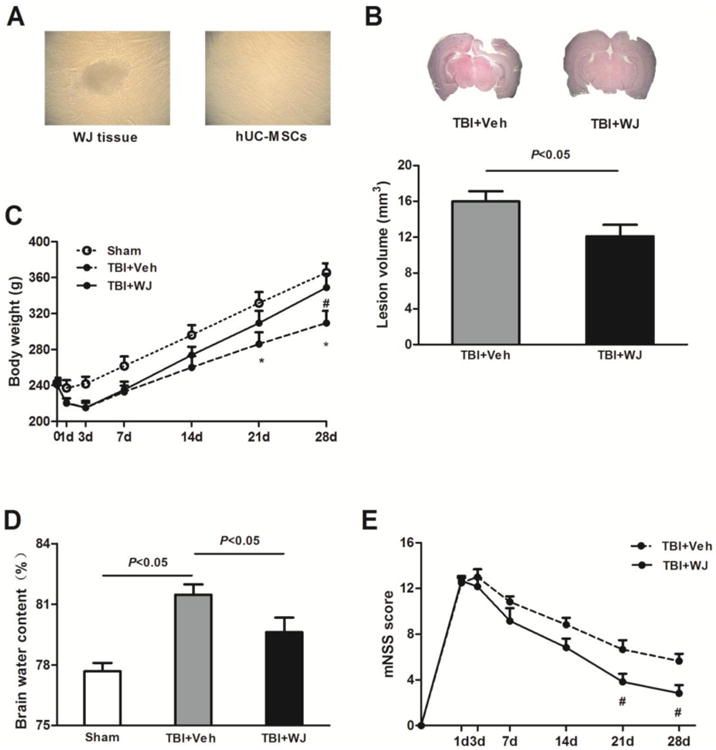
Wharton's jelly (WJ) tissue transplantation decreases lesion volume and brain edema and improves neurologic deficits. (A) Single-spindled or triangular hUC-MSCs adhered rapidly around the WJ tissue and proliferated rapidly without visible changes in growth pattern or morphology. (B) Top: Representative brain sections from WJ tissue-treated and vehicle-treated rats on day 28 after traumatic brain injury (TBI). Bottom: Lesion volume was smaller in WJ tissue-treated rats than in vehicle-treated rats (n=6 rats per group, t-test). (C) The body weight of WJ tissue-treated TBI rats recovered faster and increased more than that of vehicle-treated rats during the 28 days after TBI (n=6 rats/group, *p<0.05 vs. sham group, #p<0.05 vs. vehicle-treated TBI group, two-way ANOVA followed by Bonferroni post-hoc tests). (D) Brain water content was greater in the vehicle-treated TBI group than in the sham group but was reduced by WJ tissue treatment.n=6 rats/group, one-way ANOVA followed by Newman-Keulstest. (E) WJ tissue-treated rats had significantly less neurologic deficit than did vehicle-treated rats on days 21 and 28 after TBI. n=6 rats/groups, #p<0.05, two-way ANOVA followed by Bonferroni post-hoc test. Values are mean ± SD, mNSS, modified neurologic severity score.
WJ Tissue Treatment Improves Physiologic Recovery
On day 28 after TBI, brain lesions were smaller in rats treated with WJ tissue (12.10±1.28 mm3) than in those treated with vehicle (16.00±1.14 mm3; n=6 rats/group, p<0.05; Fig. 1B). No obvious lesion was observed in the sham-operated rats.
Both vehicle- and WJ tissue-treated rats lost body weight from base line on days 1 and 3 after TBI but began to gain weight on day 7. Rats in the WJ tissue-treated group had gained more body weight than vehicle-treated rats by day28 (n=6 rats/group, p<0.05; Fig. 1C).
Brain water content on day 3after TBI was significantly greater in the vehicle-treated TBI group (81.47±0.52%) than in the sham group (77.68±0.42%, n=6 rats/group, p<0.05). WJ tissue treatment significantly reduced brain water content (79.62±0.72%) compared to that in the vehicle-treated group (n=6 rats/group, p<0.05; Fig. 1D).
We found no significant difference in mNSS between vehicle- and WJ tissue-treated groups on days 1, 3, 7, and 14 after TBI; however on days 21 and 28, mNSS scores were significantly lower in WJ tissue-treated rats than in vehicle-treated rats (n=6 rats/group, F=11.07, p<0.05; Fig. 1E).
WJ Tissue Treatment Improves Cognitive Function
Rats that underwent TBI exhibited cognitive impairment on day 28 after TBI. In the novel object recognition test, sham rats spent more time exploring the novel object than the old object (n=8 rats/group, p<0.05; Fig. 2A), but vehicle-treated TBI rats explored novel and old objects for similar lengths of time (n=8 rats/group, p>0.05; Fig. 2A). However, after WJ tissue treatment, TBI rats spent significantly more time exploring the novel object than the old object (n=8 rats/group, p<0.05; Fig. 2A). Additionally, the discrimination index was significantly higher in the sham group (75.32±1.66) and in the WJ tissue-treated TBI group (64.24±2.13) than in the vehicle-treated TBI group (53.85±2.38; n=8 rats/group, both p<0.05; Fig. 2B).
Fig. 2.
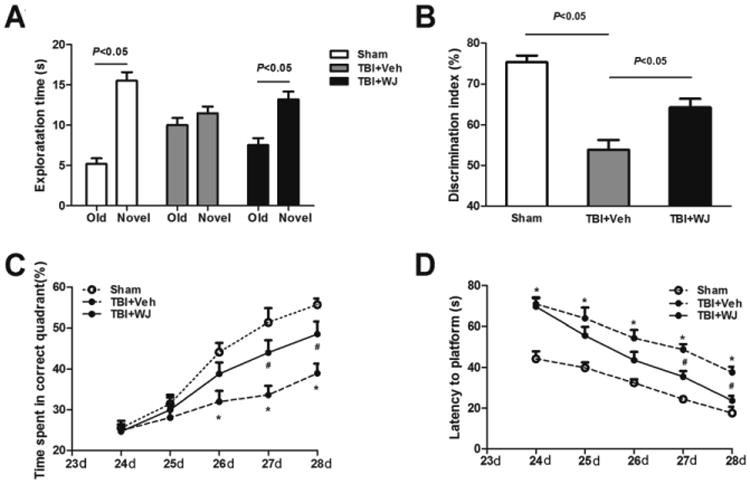
Wharton's jelly (WJ) tissue transplantation improves cognitive function after traumatic brain injury (TBI). In the novel object recognition test (A, B), sham rats spent more time exploring the novel object than the old object, but the TBI rats spent similar amounts of time exploring the novel and old objects. WJ tissue-treated rats spent more time exploring the novel object than the old object (n=8 rats/group, t-test; A). WJ tissue-treated rats also had a higher discrimination index than did vehicle-treated rats (n=8 rats/group, one-way ANOVA followed by Newman-Keulstest; B). TBI rats spent less time in the correct quadrant of the Morris water maze than did sham-operated rats (C, D). However, WJ tissue-treated rats spent more time in the correct quadrant on days 27 and 28 after TBI than did vehicle-treated rats (C). Similarly, TBI rats that received WJ tissue had shorter latency to find the platform (improved learning) than did vehicle-treated rats on days 27 and 28 (D). n=6 rats/group.*p<0.05 vs. sham group, #p<0.05 vs. vehicle-treated TBI group, two-way ANOVA followed by Bonferroni post-hoc test; values are mean ± SD.
Rats in the vehicle-treated group spent less time in the correct quadrant of the Morris water maze than did rats in the sham group on days 26–28 after TBI (n=6 rats/group, F=17.96, all p<0.05, Fig. 2C). The mean percentage of time spent in the correct quadrant was higher in the WJ tissue-treated TBI group than in the vehicle-treated TBI group on days 27 and 28 (n=6 rats/group, p<0.05; Fig. 2C). In addition, the latency to find the platform was significantly longer in the vehicle-treated TBI group than in the sham group from day 24 to 28 (n=6 rats/group, F=40.66, p<0.05); however, WJ tissue treatment reduced the time required for rats to find the platform on days 27 and 28 (n=6 rat/group, p<0.05; Fig. 2D).
WJ Tissue Treatment Increases BDNF Protein and mRNA Expression
To elucidate the underlying mechanism by which WJ tissue provides neuroprotection after TBI, we examined BDNF protein and mRNA expression in the perilesional cortex and subcortical regions on day 14 after TBI. Vehicle-treated TBI rats tended to have higher expression levels of BDNF protein and mRNA than sham-operated rats did, but the difference was not significant (n=6 rats/group, both p>0.05; Fig. 3A, B); however, BDNF protein and mRNA were significantly higher in the WJ tissue-treated TBI rats than in the vehicle-treated TBI rats on day 14 after treatment (n=6 rats/group, F=11.66, 5.101, both p<0.05; Fig. 3A, B).
Fig. 3.
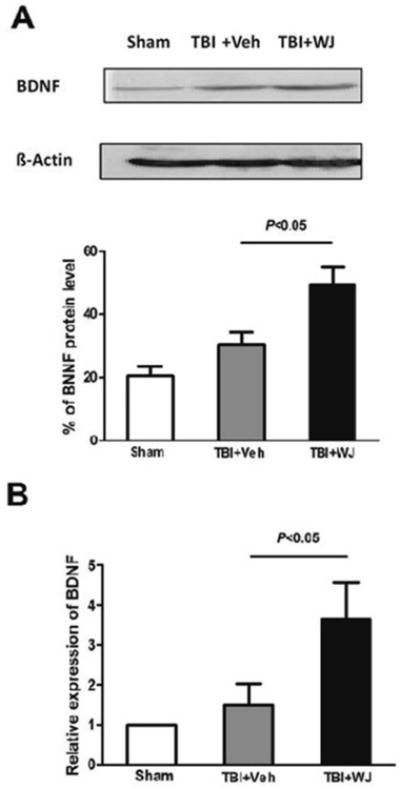
Wharton's jelly (WJ) tissue transplantation increases brain-derived neurotrophic factor (BDNF) protein and mRNA expression after traumatic brain injury (TBI). Expression of BDNF protein (A) and mRNA (B) was greater in the WJ tissue-treated TBI group than in the vehicle-treated TBI group. Values are mean ± SD; n=6 rats/group. One-way ANOVA followed by Newman-Keuls test.
WJ Tissue Treatment Increases the Number of MAP2-Positive Neurons in the Perilesional Area
To determine whether WJ tissue has direct neuroprotective effects, we measured the number of neurons by MAP2 immunohistochemistry. We found that the number of MAP2-positive neurons was increased in the perilesional cortex and subcortex of the WJ tissue-treated group compared with that of the vehicle-treated group. Additionally, the WJ tissue-treated group had more neurons with normal morphology than did the vehicle-treated group on day 14 after TBI (n=6 rats/group, p<0.05, Fig.4A, B).
Fig. 4.
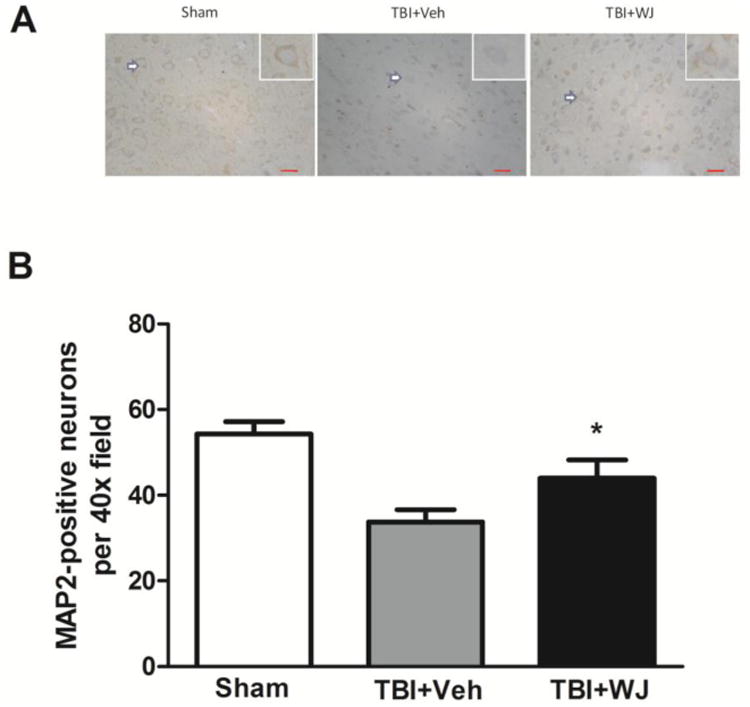
Wharton's jelly (WJ) tissue transplantation increases the number of MAP2-positive neurons in the perilesional cortex and subcortex after traumatic brain injury (TBI). The shape of the neurons in the WJ tissue-treated rats was better than that in the vehicle-treated rats (A). Scale bars: 100 μm. The insets show representative MAP2-positive neurons at higher magnification. More MAP2-positive neurons were present in the perilesional area of the WJ tissue-treated TBI rats than in that of the vehicle-treated TBI rats on day 14 (B). Values are mean ± SD; n=6 rats/group. One-way ANOVA followed by Newman-Keuls test.
Discussion
Our findings indicate that WJ tissue transplantation after TBI can reduce brain edema and lesion volume while increasing MAP2-positive neurons and promoting sensorimotor and cognitive recovery. Studies have shown that intravenous or in situ transplantation of WJ cells promotes the functional recovery of rats after spinal cord injury (Yang et al. 2008) or TBI (Wang et al. 2013). Additionally, we found previously that WJ tissue can produce hUC-MSCs less than 24 h after the first passage and that the hUC-MSCs at the first, third, and fifth passages exhibited no differences in proliferation capability (Zhou et al. 2011). Therefore, we speculated that the WJ tissue, which contains both stromal microenvironment and hUC-MSCs, could be more appropriate for treating TBI than hUC-MSCs alone. In this study, we placed the WJ tissue on the surface of the injured brain through a bone window to facilitate recovery of the microenvironment. This procedure produced significant protection and improved both histologic and functional outcomes after TBI. One study has shown that collagen scaffolds improve early engraftment and support the survival of the grafted cells post-transplantation (Guan et al. 2013). Future studies will be required to determine how long the transplanted tissue can survive and continue to produce hUC-MSCs.
BDNF helps to support the survival of existing neurons and encourages the growth and differentiation of new neurons and synapses (Lu et al. 2014); such growth correlates with cognitive function (Shen et al. 2013; Vatansever et al. 2013). In this study, rats that underwent TBI and received WJ tissue had higher levels of BDNF and more healthy neurons in the perilesional region than did rats treated with vehicle, suggesting that an increase in BDNF might be involved in post-TBI neuroprotection and improvement of sensorimotor and cognitive functions.
Our data indicate that WJ tissue transplantation has potential as a therapeutic approach for TBI, possibly via up regulation of BDNF, which might play a role in histologic and functional recovery. If WJ tissue transplantation after TBI can be shown to improve functional recovery in large clinical trials, it may offer an economic and practical treatment for patients with TBI. However, we do need to identify the phenotype of the hUC-MSCs after transplantation into the injured brain and determine whether they differentiate into neurons and/or glial cells. We also need to identify the active components of the WJ that provide brain protection and elucidate the underlying molecular and cellular mechanisms.
Supplementary Material
Acknowledgments
This study was supported by Natural Science Foundation of China (81071008), Excellent Youth Foundation of Henan Scientific Committee (114100510005), Technology Foundation for Selected Overseas Chinese Scholar, Ministry of Personnel of China, Key Programs for Science and Technology Development of the Department of Science and Technology of Henan Province (122102310400), and NIH K01AG031926, R01NS078026, and R01AT007317. T.C. is the recipient of the China Scholarship Council Joint PhD Training award. We thank Jiarui Wang and Claire Levine for assistance with this manuscript.
Footnotes
Conflicts of Interest: The authors declare no competing financial interests.
References
- Arien-Zakay H, Shohami E, Nagler A, Lazarovici P. Human umbilical cord blood stem cells for treatment of traumatic brain injury. J Mol Neurosci. 2012;48:S66–S66. [Google Scholar]
- Bakhtiary M, Marzban M, Mehdizadeh M, Joghataei MT, Khoei S, Tondar M, Mahabadi VP, Laribi B, Ebrahimi A, Hashemian SJ, Modiry N, Mehrabi S. Combination of stem cell mobilized by granulocyte-colony stimulating factor and human umbilical cord matrix stem cell: therapy of traumatic brain injury in rats. Iran J Basic Med Sci. 2011;14(4):327–339. [PMC free article] [PubMed] [Google Scholar]
- Bennett MH, Trytko B, Jonker B. Hyperbaric oxygen therapy for the adjunctive treatment of traumatic brain injury. Cochrane Database Syst Rev. 2012;12:CD004609. doi: 10.1002/14651858.CD004609.pub3. [DOI] [PubMed] [Google Scholar]
- Camussi G, Deregibus MC, Tetta C. Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Curr Opin Nephrol Hypertens. 2010;19(1):7–12. doi: 10.1097/MNH.0b013e328332fb6f. [DOI] [PubMed] [Google Scholar]
- Chang CF, Cho S, Wang J. (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann Clin Transl Neurol. 2014;1(4):258–271. doi: 10.1002/acn3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang B, Liang M, Yang G, Ye Y, Xu H, He X, Huang JH. Expression of S100A6 in rat hippocampus after traumatic brain injury due to lateral head acceleration. Int J Mol Sci. 2014;15(4):6378–6390. doi: 10.3390/ijms15046378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J, Zhu Z, Zhao RC, Xiao Z, Wu C, Han Q, Chen L, Tong W, Zhang J, Gao J, Feng M, Bao X, Dai J, Wang R. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials. 2013;34(24):5937–5946. doi: 10.1016/j.biomaterials.2013.04.047. [DOI] [PubMed] [Google Scholar]
- Jiang C, Wang J, Yu L, Ou C, Liu X, Zhao X, Wang J. Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia. Behav Brain Res. 2013;250:222–229. doi: 10.1016/j.bbr.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5(6):933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25(2):319–331. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Kim SH. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. Journal of neurotrauma. 2010;27(1):131–138. doi: 10.1089/neu.2008-0818. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zou Y, Belegu V, Lv LY, Lin N, Wang TY, McDonald JW, Zhou X, Xia QJ, Wang TH. Co-grafting of neural stem cells with olfactory en sheathing cells promotes neuronal restoration in traumatic brain injury with an anti-inflammatory mechanism. J Neuroinflammation. 2014;11:66. doi: 10.1186/1742-2094-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- Ma H, Yu B, Kong L, Zhang Y, Shi Y. Neural stem cells over-expressing brain-derived neurotrophic factor (BDNF) stimulate synaptic protein expression and promote functional recovery following transplantation in rat model of traumatic brain injury. Neurochemical research. 2012;37(1):69–83. doi: 10.1007/s11064-011-0584-1. [DOI] [PubMed] [Google Scholar]
- Malkowski A, Sobolewski K, Jaworski S, Bankowski E. FGF binding by extracellular matrix components of Wharton's jelly. Acta Biochim Pol. 2007;54(2):357–363. [PubMed] [Google Scholar]
- Riess P, Zhang C, Saatman KE, Laurer HL, Longhi LG, Raghupathi R, Lenzlinger PM, Lifshitz J, Boockvar J, Neugebauer E, Snyder EY, McIntosh TK. Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery. 2002;51(4):1043–1052. doi: 10.1097/00006123-200210000-00035. discussion 1052-1044. [DOI] [PubMed] [Google Scholar]
- Shen X, Li A, Zhang Y, Dong X, Shan T, Wu Y, Jia J, Hu Y. The effect of different intensities of treadmill exercise on cognitive function deficit following a severe controlled cortical impact in rats. Int J Mol Sci. 2013;14(11):21598–21612. doi: 10.3390/ijms141121598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolewski K, Malkowski A, Bankowski E, Jaworski S. Wharton's jelly as a reservoir of peptide growth factors. Placenta. 2005;26(10):747–752. doi: 10.1016/j.placenta.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Vatansever F, Xuan W, Huang YY, Hamblin MR. Transcranial low-level light therapy produces neuroprotection, neurogenesis and BDNF after TBI in mice. Proc SPIE. 2013;8569:85690E (85611)–85690E (85611). doi: 10.1117/12.2001900. [DOI] [Google Scholar]
- Wang S, Cheng H, Dai G, Wang X, Hua R, Liu X, Wang P, Chen G, Yue W, An Y. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013;1532:76–84. doi: 10.1016/j.brainres.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Wu H, Wu T, Li M, Wang J. Efficacy of the lipid-soluble iron chelator 2,2′-dipyridyl against hemorrhagic brain injury. Neurobiol Dis. 2012;45(1):388–394. doi: 10.1016/j.nbd.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhang Z, Li Y, Zhao R, Li H, Song Y, Qi J, Wang J. Time course of upregulation of inflammatory mediators in the hemorrhagic brain in rats: correlation with brain edema. Neurochem Int. 2010;57(3):248–253. doi: 10.1016/j.neuint.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS One. 2008;3(10):e3336. doi: 10.1371/journal.pone.0003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zan L, Zhang X, Xi Y, Wu H, Song Y, Teng G, Li H, Qi J, Wang J. Src regulates angiogenic factors and vascular permeability after focal cerebral ischemia-reperfusion. Neuroscience. 2014;262:118–128. doi: 10.1016/j.neuroscience.2013.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiong Y, Mahmood A, Meng Y, Qu C, Schallert T, Chopp M. Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit. Brain Res. 2009;1294:153–164. doi: 10.1016/j.brainres.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Yang B, Tian Y, Jiao H, Zheng W, Wang J, Guan F. Immunomodulatory effect of human umbilical cord Wharton's jelly-derived mesenchymal stem cells on lymphocytes. Cell Immunol. 2011;272(1):33–38. doi: 10.1016/j.cellimm.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Gao Y, Chang CF, Wan JR, Zhu SS, Wang J. Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. Plos One. 2014;9(5):e97423. doi: 10.1371/journal.pone.0097423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


