Background: The yeast tRNA splicing endonuclease (Sen) complex is located on the mitochondrial outer membrane and splices precursor tRNAs.
Results: The Sen complex cleaves the mitochondria-localized CBP1 mRNA in vivo and in vitro.
Conclusion: Mitochondrial localization of the Sen complex is required for the cleavage of the CBP1 mRNA.
Significance: This study shows a novel role of the tRNA splicing endonuclease complex in the cleavage of mitochondria-localized mRNA.
Keywords: endonuclease; mitochondria; mRNA decay; transfer RNA (tRNA); translation; mRNA decay, endonuclease; mRNA localization; quality control; tRNA splicing
Abstract
The tRNA splicing endonuclease (Sen) complex is located on the mitochondrial outer membrane and splices precursor tRNAs in Saccharomyces cerevisiae. Here, we demonstrate that the Sen complex cleaves the mitochondria-localized mRNA encoding Cbp1 (cytochrome b mRNA processing 1). Endonucleolytic cleavage of this mRNA required two cis-elements: the mitochondrial targeting signal and the stem-loop 652–726-nt region. Mitochondrial localization of the Sen complex was required for cleavage of the CBP1 mRNA, and the Sen complex cleaved this mRNA directly in vitro. We propose that the Sen complex cleaves the CBP1 mRNA, which is co-translationally localized to mitochondria via its mitochondrial targeting signal.
Introduction
Cells have translation-dependent surveillance systems that eliminate aberrant mRNAs to prevent the production of potentially harmful protein products. The nonstop decay quality control system rapidly degrades aberrant mRNAs lacking a termination codon (nonstop mRNAs), which are produced mainly by premature polyadenylation within an ORF (1, 2). Perturbation of translation elongation by stable RNA secondary structures, rare codons, or positively charged nascent peptides induces both no-go decay (NGD),4 in which mRNAs in the vicinity of stalled ribosomes undergo endonucleolytic cleavage (3, 4), and ribosome quality control, in which the arrested protein product is co-translationally degraded by the proteasome (5–12).
In Saccharomyces cerevisiae, Cbp1 (cytochrome b mRNA processing 1) is a nuclear-encoded protein that is imported into the mitochondria. It was proposed that Cbp1 plays a role in post-transcriptional regulation and translation of the mRNA encoding cytochrome b by interacting with its 5′-UTR (13, 14). The CBP1 nonstop mRNA, which is produced by polyadenylation in the ORF, is rapidly degraded by nonstop decay (2). The ratio of nonstop to normal CBP1 mRNA is regulated by the carbon source and is increased during the switch to respiratory growth (15), suggesting that the production of nonstop mRNA plays a role in down-regulation of the CBP1 mRNA and protein, as well as the mRNA encoding cytochrome b, during the induction of respiration (14).
The tRNA splicing endonuclease (Sen) complex is localized to the nucleus in vertebrates (16) and the mitochondria in S. cerevisiae (17, 18). The yeast Sen complex splices tRNAs on the mitochondrial surface; although nuclear-localized Sen complex mutants are capable of tRNA splicing in yeast (18, 19), they cannot complement the lethality caused by depletion of the native Sen complex (19). Hence, the biological significance of mitochondrial localization of this complex is still largely unknown. Here, we report that the Sen complex cleaves the CBP1 mRNA on the mitochondrial outer membrane. To our knowledge, this study is the first description of the potential correlation between mitochondrial localization of mRNAs and their endonucleolytic cleavage.
Experimental Procedures
Strains and Other Methods
The yeast gene disruption mutants were constructed using kanMX4 (20) or natMX4 (21), as described previously (22). The strains and plasmids used in this study are listed in Table 1, and the oligonucleotides used for plasmid construction are listed in Table 2. Northern and Western blot analyses were performed as described previously (22). The DIG-labeled probes used in this study are listed in Table 3.
TABLE 1.
Yeast strains and plasmids used in this study
| Strain/plasmids | Genotype/plasmid | Source |
|---|---|---|
| Strains | ||
| W303-1a | MATa ade2 his3 leu2 trp1 ura3 can1 | Lab stock |
| YIT2013 | W303-1a ski2Δ::kanMX | Ref. 6 |
| YIT2019 | W303-1a xrn1Δ::kanMX | Ref. 29 |
| YIT2020 | W303-1a xrn1Δ::kanMX dom34Δ::hygtMX | Ref. 4 |
| TYSC360 | W303-1a sen54Δ::HIS3, p314–54 CEN6-ARSH4 TRP1 SEN54 | Ref. 17 |
| TYSC361 | W303-1a sen54Δ::HIS3, p314–200 CEN6-ARSH4 TRP1 sen54-Δ200–232 | Ref. 17 |
| COSC05 | W303-1a sen2Δ::LEU2, pCOSC05 CEN6-ARSH4 TRP1 SEN2 | Ref. 17 |
| COSC06 | W303-1a sen2Δ::LEU2, pCOSC05 CEN6-ARSH4 TRP1 sen2–41 | Ref. 17 |
| YTS1612 | W303-1a sen54Δ::HIS3, p314–54 CEN6-ARSH4 TRP1 SEN54, ski2Δ::kanMX | This study |
| YTS1613 | W303-1a sen54Δ::HIS3, p314–200 CEN6-ARSH4 TRP1 sen54-Δ200–232, ski2Δ::kanMX | This study |
| YTS1614 | W303-1a sen54Δ::HIS3, p314–54 CEN6-ARSH4 TRP1 SEN54, xrn1Δ::kanMX | This study |
| YTS1615 | W303-1a sen54Δ::HIS3, p314–200 CEN6-ARSH4 TRP1 sen54-Δ200–232, xrn1Δ::kanMX | This study |
| YTS1616 | W303-1a sen2Δ::LEU2, | This study |
| pCOSC05 CEN6-ARSH4 TRP1 SEN2, ski2Δ::kanMX | ||
| YTS1617 | W303-1a sen2Δ::LEU2, pCOSC05 CEN6-ARSH4 TRP1 sen2–41, ski2Δ::kanMX | This study |
| YTS1638 | W303-1a sen2Δ::LEU2, pCOSC05 CEN6-ARSH4 TRP1 SEN2, xrn1Δ::kanMX | This study |
| YTS1639 | W303-1a sen2Δ::LEU2, pCOSC05 CEN6-ARSH4 TRP1 sen2–41, xrn1Δ::kanMX | This study |
| YTS1798 | W303-1a tom20Δ::natMX | This study |
| YTS1801 | W303-1a tom20Δ::natMX ski2Δ::kanMX | This study |
| YTS1803 | W303-1a puf3Δ::hygMX ski2Δ::kanMX | This study |
| YTS1806 | W303-1a tom20Δ::hygMX xrn1Δ::kanMX | This study |
| Plasmids | ||
| p416GPDp | URA3 CEN | Ref. 30 |
| p416GAL1p | URA3 CEN | Ref. 30 |
| p415GAL1p | LEU2 CEN | Ref. 30 |
| p414GAL1p | TRP1 CEN | Ref. 30 |
| p413GAL1p | HIS3 CEN | Ref. 30 |
| yCplac33 | URA3 CEN | Ref. 31 |
| pSA144 | p416GPDp-GFP-FLAG-HIS3 | Ref. 32 |
| pTYSC155 | CEN6-ARSH4 URA3 SEN54 | Ref. 17 |
| pTS152 | p416GAL1p-CBP1 | This study |
| pTS220 | p416GAL1p-CBP1-Δ652–678 | This study |
| pTS221 | p416GAL1p-CBP1-Δ679–702 | This study |
| pTS223 | p416GAL1p-CBP1-Δ703–726 | This study |
| pTS256 | p416GAL1p-CBP1-Δ727–738 | This study |
| pTS257 | p416GAL1p-CBP1-Δ739–762 | This study |
| pTS258 | p416GAL1p-CBP1-Δ763–789 | This study |
| pTS343 | p416GAL1p-CBP1-FS (+652–726) | This study |
| pTS344 | p416GAL1p-CBP1-FS (+727–789) | This study |
| pTS244 | p416GAL1p-CBP1-M1 | This study |
| pTS247 | p416GAL1p-CBP1-M2 | This study |
| pTS184 | p416GAL1p-stemloop-CBP1 | This study |
| pTS260 | p416GAL1p-stemloop-CBP1-ΔMTS | This study |
| pTS179 | p416GAL1p-CBP1-ΔMTS | This study |
| pTS225 | p416GAL1p-CBP1-ΔuORF | This study |
| pTS173 | p416GAL1p-CBP1-MMH2 | This study |
| pTS229 | p416GPDp-GFP-CBP1 (+643–738)-FLAG-HIS3 | This study |
| pTS231 | p416GPDp-CBP1 (+1–99)-GFP-FLAG-HIS3 | This study |
| pTS236 | p416GPDp-CBP1 (+1–99)-GFP-CBP1 (+643–738)-FLAG-HIS3 | This study |
| pTS324 | p416GPDp-CBP1 (+1–99)-GFP-CBP1 (FS 643–738)- FLAG-HIS3 | This study |
| pTS332 | p416GPDp-CBP1 (+1–99)-GFP-CBP1 (M1 643–738)- FLAG-HIS3 | This study |
| pTS318 | p415GAL1p-SEN2 | This study |
| pTS299 | p415GAL1p-His6-flag-SEN2 | This study |
| pTS300 | p415GAL1p-His6-flag-sen2 H297A | This study |
| pTS305 | p413GAL1p-SEN15 | This study |
| pTS314 | p414GAL1p-SEN54 | This study |
| pTS320 | p416GAL1p-SEN34 | This study |
| pTS351 | yCplac33TOM20p-TOM20 | This study |
TABLE 2.
List of oligonucleotides used for plasmid construction
| Description | Sequence |
|---|---|
| CBP1 | 5′-GCACTAGTATTTTGCACGTTTCCCTTTCCATGCAATGT-3′ |
| 5′-GCGTCGACATATCGTAAATGTGCGTTTGGCCGTTCATC-3′ | |
| CBP1-FS(+652–726) | 5′-ATTAGAAAGGAGTTTCTAATTGAAACCTCGTTAACTTTATTGACCGCCTCCAAGGTTATACTTTCGAACAGAGAGTTCAG-3′ |
| 5′-CGAGGTTTCAATTAGAAACTCCTTTCTAATAAGGCCCTCGGCTTACGTAATGAAGCCACCGCAATTATGCAAGGCTATAC-3′ | |
| CBP1-FS(+727–789) | 5′-AAAGAATAGTTTATATTCATCAGTTTGTTCAGTATAGCCTTGCATAATTGCGGTGGCTTCATTACTTAAGCCGAGGGCCT-3′ |
| 5′-GGCTATACTGAACAAACTGATGAATATAAACTATTCTTTGACTATACGATATTGAAGCTTCGGGATGATCAAGTGCTGCT-3′ | |
| CBP1-M1 | 5′-CAACTGAACTCTCTGTTCGAAAGTATAACCGGAGGCGGAATAAAGTTAACCAGATTTCAATTAGAAACTCTTAGTAATAAAGCCTTAGGCTTAAGTAATGAAGCCCCGCAATTATGC-3′ |
| 5′-GGTTATACTTTCGAACAGAGAGTTCAGTTG-3′ | |
| CBP1-M2 | 5′-CAACTGAACTCTCTGTTCGAAAGTATAACCGGAGGTGGAATAAAGTTAACTAGATTCCAGCTAGAGACATTGAGTAACAAAGCCTTAGGATTAAGTAATGAAGCCCCGCAATTATGCAAG-3′ |
| 5′-GGTTATACTTTCGAACAGAGAGTTCAGTTG-3′ | |
| CBP1-ΔMTS | 5′-GACTAGTATGGATCCAATTCAAAAACAGGTCTTGGC-3′ |
| 5′-GCGTCGACATATCGTAAATGTGCGTTTGGCCGTTCATC-3′ | |
| CBP1-Δupstream ORF | 5′-GACTAGTATGTTTTTACCTCGTCTCGTTCGG-3′ |
| 5′-GCGTCGACATATCGTAAATGTGCGTTTGGCCGTTCATC-3′ | |
| CBP1-MMH2 | 5′-GATCTGCAGCGAGATGTTCTGGCAGCTGACCCCCGAGTACTACTGCAACAACCCACTGATCCTGCCGGCCATCATCGACTTCATCACCAAGCAGGACAGCCTCACCATGGCCAAGGAACTCATGCAGAACATTAACAGATAC-3′ |
| 5′-GTCAGCTGCCAGAACATCTCGCTGCAGATCGATCGGTAGGCCCGGTTGTGGGTGCAGATCTTGAAGACCTTGTAGATGATCCTGCTGTCGTGCGCGCTAATGGAAACATAGTGCTCTTTGATTTTGTTCC-3′ | |
| CBP1(+643–738) | 5′-CTAGAAGTATAACCGGAGGCGGAATAAAGTTAACGAGGTTTCAATTAGAAACTCTTTCTAATAAGGCCCTCGGCTTAAGTAATGAAGCCCCGCAATTATGCA-3′ |
| 5′-CTAGTGCATAATTGCGGGGCTTCATTACTTAAGCCGAGGGCCTTATTAGAAAGAGTTTCTAATTGAAACCTCGTTAACTTTATTCCGCCTCCGGTTATACTT-3′ | |
| CBP1(+1–99) | 5′-CTAGAATGTTTTTACCTCGTCTCGTTCGGTACAGGACCGAGAGGTTTATAAAAATGGTACCTACCAGGACCTTGCGACGAATCAACCACAGCAGCAGGGATCCAA-3′ |
| 5′-CTAGTTGGATCCCTGCTGCTGTGGTTGATTCGTCGCAAGGTCCTGGTAGGTACCATTTTTATAAACCTCTCGGTCCTGTACCGAACGAGACGAGGTAAAAACATT-3′ | |
| FS 643–738 | 5′-CTAGTAAGTATAACTCGGAGGCGGTCAATAAAGTTAACGAGGTTTCAATTAGAAACTCCTTTCTAATAAGGCCCTCGGCTTACGTAATGAAGCCCCGCAATTATGCAT-3′ |
| 5′-CTAGATGCATAATTGCGGGGCTTCATTACGTAAGCCGAGGGCCTTATTAGAAAGGAGTTTCTAATTGAAACCTCGTTAACTTTATTGACCGCCTCCGAGTTATACTTA-3′ | |
| M1 643–738 | 5′-CTAGAAGTATAACCGGAGGCGGAATAAAGTTAACCAGATTTCAATTAGAAACTCTTAGTAATAAAGCCTTAGGCTTAAGTAATGAAGCCCCGCAATTATGCA-3′ |
| 5′-CTAGTGCATAATTGCGGGGCTTCATTACTTAAGCCTAAGGCTTTATTACTAAGAGTTTCTAATTGAAATCTGGTTAACTTTATTCCGCCTCCGGTTATACTT-3′ | |
| His6-flag-SEN2 | 5′-GACTAGTATGCATCACCATCACCATCACCATCACGACTACAAGGACGACGATGACAAGATGTCTAAAGGGAGGvGTCAATCAGAAGCGTTAC-3′ |
| 5′-CCCAAGCTTCTAGTCTCTATTTCTTCCGGGAACCCATCTCTTATAC-3′ | |
| His6-flag-sen2 H297A | 5′-GATTATTTATTATATAAGAGAGGGCCACCATTTCAAGCCGCTGAATTTTGTGTTATGGGTCTTGAC-3′ |
| 5′-GTCAAGACCCATAACACAAAATTCAGCGGCTTGAAATGGTGGCCCTCTCTTATATAATAAATAATC-3′ | |
| Tom20 −500F and +500R | 5′-CGAGCTCGGCAGCTATGCCATCGTTAAGTGTGGATTTCCC-3′ |
| 5′-CCGCTCGAGATAAAATTGGCGGTAAGAAAGGATCTGAAGG-3′ |
TABLE 3.
List of oligonucleotides used for DIG-labeled probes
| Probe name | DIG labeling method | Oligonucleotide sequences |
|---|---|---|
| GFP | Internal label (PCR) | 5′-GCTCTAGAATGAGTAAAGGAGAAGAACTTTTCAC-3′ |
| 5′-GGACTAGTTTTGTATAGTTCATCCATGCCA-3′ | ||
| HIS3 | Internal label (PCR) | 5′-GCTCTAGATGACAGAGCAGAAAGCCCTAG-3′ |
| 5′-CGGGATCCCATAAGAACACCTTTGGTGG-3′ | ||
| CBP1 168–210 | 5′ end label | 5′-CCGAAGACTTTTGTAGTCCGCCATGTCAGACCAATATTTCCG-3′ |
| CBP1 457–504 | 5′ end label | 5′-CAGCAGGAAAAGGTCGGCAGCCATAACTATGTCTCCTGTGCCGATGGC-3′ |
| CBP1 786–835 | 5′ end label | 5′-CAATGGACTTGTACGCAAGCAGCACTTGATCATCCCGAAGCTTCAAATCG-3′ |
| CBP1 1189–1258 | 5′ end label | 5′-GTTAAGCCAGACAATATGATGGTTTTCGGGTAAGTGTATCTGTTAATGTTCTGCATGAGTTCCTTGGCC-3′ |
| CBP1 −26–889 | Internal label (PCR) | 5′-GCACTAGTATTTTGCACGTTTCCCTTTCCATGCAATGT-3′ |
| 5′-TGCTCTTTGATTTTGTTCCAA-3′ | ||
| CBP1 1101–1965 | Internal label (PCR) | 5′-CATGCAGAACATTAACAGATACAC-3′ |
| 5′-GCGTCGACATATCGTAAATGTGCGTTTGGCCGTTCATC-3′ | ||
| CBP1 1–300 | Internal label (PCR) | 5′-GACTAGTATGTTTTTACCTCGTCTCGTTCGG-3′ |
| 5′-TGAGGTTTTTGTGCGATTGATGAAACTG-3′ |
Primer Extension Experiments
Primer extension experiments were performed using a 5′-fluorescently labeled primer (LI-COR Biosciences) complementary to the +786–815-nt region of the CBP1 mRNA. PrimeScript reverse transcriptase (TaKaRa) was used to synthesize a single-stranded DNA toward the 5′ end of the RNA. The reaction contained 5 μg of RNA and 0.1 pmol of 5′-fluorescently labeled primer. The size of the labeled single-stranded DNA was determined relative to a sequencing ladder on a sequencing gel.
Western Blotting
Yeast cells were grown in minimal medium containing 2% glucose or galactose. The cells were harvested when the culture reached an A600 of 0.6. The protein products of various GFP-HIS3 reporter genes were detected by Western blotting using an anti-GFP antibody (sc-9996; Santa Cruz Biotechnology) and an anti-mouse IgG, horseradish peroxidase-conjugated secondary antibody (GE Healthcare). The intensities of the bands on the blots were quantified using the LAS4000 imaging system and Multi-Gauge version 3.0 software (Fujifilm). The relative levels of the protein products were determined by comparison with a standard curve prepared using a series of dilutions.
In Vitro tRNA Splicing Assay
FLAG-tagged wild-type Sen2 and FLAG-tagged Sen2-H297A were purified from cell extracts and examined for endonucleolytic activity. Nontagged wild-type Sen2 was used as a negative control. The RNA products were separated on a polyacrylamide gel containing 7 m urea, dried, and then exposed to an imaging plate (Fujifilm). Identification of all RNA species was confirmed by UTP labeling of precursor RNA substrates. All procedures were performed as described previously (23, 24).
Plasmid Construction
Recombinant DNA procedures were performed as described previously (25). Plasmids expressing various CBP1 mRNAs were constructed as follows: To construct pTS152 (p416GAL1p-CBP1), a SpeI-SalI fragment was amplified by PCR using the two CBP1 primers listed in Table 2 and then inserted into the SpeI-SalI sites of p416GAL1p. To construct the CBP1 deletion constructs pIT220, 221, 223, and 256–258, deletions were introduced by PCR-based site-directed mutagenesis. An oligonucleotide (5′-GATCCGATATCCCGTGGAGGGGCGCGTGGTGGCGGCTGCAGCCGCCACCACGCGCCCCTCCACGGGATATCG-3′) was self-annealed and inserted into the SpeI site of pTS152 or pTS179 to create the pTS184 or pTS260 reporter, respectively.
The plasmids expressing various GFP-HIS3 reporters were constructed as follows: The two CBP1(+643–738) oligonucleotides listed in Table 2 were annealed and inserted into the SpeI site of pSA144 (pGPDp-GFP-FLAG-HIS3-CYC1ter) to create the pTS229 (p416GPDp-GFP-CBP1(+643–738)-FLAG-HIS3) reporter. The two CBP1(+1–99) oligonucleotides were annealed and inserted into the XbaI site of pSA144 and pTS229 to create pTS231 (p416GPDp-CBP1(+1–99)-GFP-FLAG-HIS3) and pTS236, respectively. Two FS 643–738 or M1 643–738 oligonucleotides were annealed and inserted into the SpeI site of pTS231 to create pTS324 and pTS332, respectively.
The plasmids expressing various Sen complex proteins were constructed as follows: To construct pTS318, 305, 314, and 320, the ORF fragments of each protein were amplified by PCR and inserted into p415GAL1p, p413GAL1p, p414GAL1p, or p416GAL1p, respectively. To construct pTS299, the SpeI-HindIII fragment of SEN2 was amplified by PCR using the two His6-flag-SEN2 primers listed in Table 2 and then inserted into the SpeI-HindIII sites of p415GAL1p. To construct pTS300, a point mutation was introduced by PCR-based site-directed mutagenesis using the His6-flag-sen2 H297A primers.
The plasmids expressing the Tom20 protein were constructed as follows. To construct pTS351, a SacI-XhoI ORF fragment including TOM20 was amplified by PCR using the Tom20 −500F and +500R primers listed in Table 2 and then inserted into the SacI-XhoI sites of yCplac33.
Results
The CBP1 mRNA Undergoes Endonucleolytic Cleavage
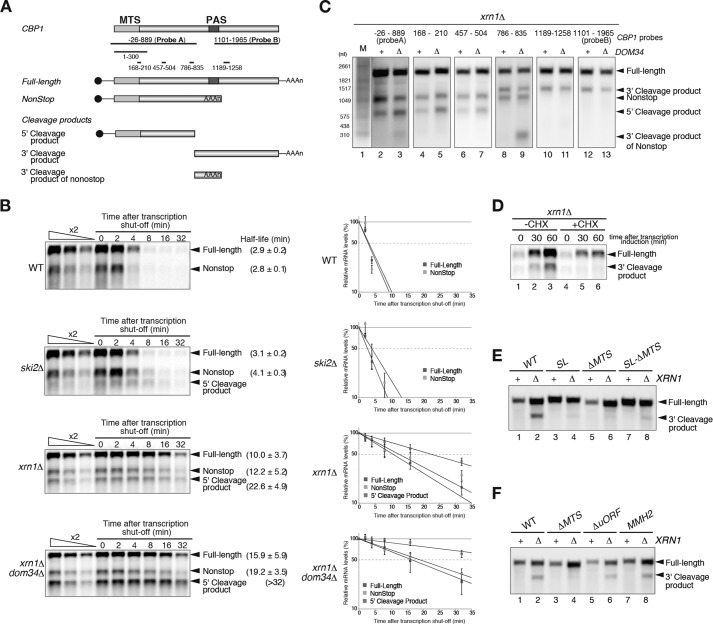
The CBP1 nonstop mRNA is an endogenous target of nonstop decay that is rapidly degraded by the exosome, a 3′ to 5′ exoribonuclease complex (1, 2). The Dom34-Hbs1 complex stimulates the degradation of nonstop mRNAs by dissociating ribosomes that are stalled at the 3′ ends of mRNAs (Fig. 1A) (4). The CBP1 nonstop mRNA was more stable in xrn1Δdom34Δ (t½ = 19.2 min) cells than xrn1Δ cells (t½ = 12.2 min) (Fig. 1B), indicating that the Dom34-Hbs1 complex facilitates the degradation of the CBP1 nonstop mRNA. Unexpected transcripts were detected in ski2Δ or xrn1Δ mutant cells, but not wild-type cells, indicating that endonucleolytic cleavage may occur within the ORF of the CBP1 nonstop mRNA (Fig. 1B). Northern blotting with probes corresponding to various regions of the CBP1 mRNA revealed that the 700-nt 5′ fragment (5′ cleavage product) was detected even in ski2Δ mutant cells that were deficient in 3′ to 5′ degradation pathways (Fig. 1, A–C). Two 3′ fragments of 1100 nt (3′ cleavage product) and 300 nt (3′ cleavage product of nonstop) were detected only in xrn1Δ mutant cells that were deficient in 5′ to 3′ degradation pathways (Fig. 1, A–C). These results indicate that endonucleolytic cleavage occurs in the vicinity of the 600–780-nt region of the CBP1 ORF. The 5′ cleavage product lacked a termination codon, and the Dom34-Hbs1 complex facilitated its degradation as expected (t½ > 32.0 min and t½ = 22.6 min in xrn1Δdom34Δ and xrn1Δ cells, respectively; Fig. 1B).
FIGURE 1.
Translation of the MTS is required for endonucleolytic cleavage of the CBP1 mRNA. A, a schematic illustration of the CBP1 mRNA. The filled box indicates the ORF, the line represents nontranslated regions, and the tract of As denotes the poly(A) tail. The MTS region encodes the mitochondrial targeting signal. PAS indicates the polyadenylation site. B, the CBP1 mRNA undergoes endonucleolytic cleavage. Left panels, the half-lives of the mRNAs are shown as the means ± standard deviation of three independent experiments. The detection of the 5′ cleavage product by Northern blotting with a DIG-labeled CBP1 1–300 probe. Right panels, graphs of the half-life analysis. The relative amounts of the mRNAs in the indicated cells are shown as the means ± standard deviation of three independent experiments. C, Northern blotting with probes corresponding to various regions of the CBP1 mRNA identified a number of cleavage products. W303xrn1Δ and W303xrn1Δdom34Δ mutants were grown in minimal medium containing 2% galactose. RNA samples were analyzed by Northern blotting with the DIG-labeled CBP1 probes depicted in A. The results shown are representative of three independent experiments. D, translation is required for endonucleolytic cleavage of the CBP1 mRNA. After translation inhibition by the addition of cycloheximide for 10 min, transcription from the GAL1p-CBP1 plasmids was induced by the addition of galactose. The cells were harvested at the indicated time points after induction. The 3′ cleavage product was detected by Northern blotting with a DIG-labeled CBP1 1101–1965 probe, which is depicted as probe B in A. The results shown are representative of three independent experiments. E, the mitochondrial targeting signal of the CBP1 mRNA is required for its cleavage. W303 and W303xrn1Δ mutant cells were transformed with the indicated CBP1 plasmids. SL, the stem-loop structure was inserted into the 5′-UTR. ΔMTS, deletion of the mitochondrial targeting signal. The 3′ cleavage product was detected as described for D. The results shown are representative of three independent experiments. F, neither the upstream ORF nor polyadenylation within the ORF are required for cleavage of the CBP1 mRNA. MMH2, CBP1 mutant defective in polyadenylation within the ORF. ΔuORF, deletion of the upstream ORF. The 3′ cleavage product was detected as described for D. The results shown are representative of three independent experiments.
To determine whether translation is required for endonucleolytic cleavage of the CBP1 mRNA, translation was inhibited by adding cycloheximide to the cells or inserting a stem-loop structure into the 5′-UTR of the mRNA. After the induction of CBP1 transcription from the GAL1 promoter in the presence of cycloheximide, the 3′ and 5′ cleavage products were only barely observed in xrn1Δ cells (Fig. 1D) and ski2Δ cells (data not shown), respectively. Endonucleolytic cleavage of the CBP1 mRNA was also abrogated by the insertion of a stem-loop structure into the 5′-UTR (Fig. 1E, lanes 3 and 4). To identify the region of the CBP1 mRNA that must be translated to allow cleavage, CBP1 mutants that lacked the mitochondrial targeting signal (MTS) or upstream ORF were generated. The 3′ cleavage product was detected in mutants that lacked an upstream ORF, but not in those that lacked the MTS (Fig. 1F, lanes 1–6). These results indicate that translation of the region of the CBP1 mRNA encoding the MTS is required for its endonucleolytic cleavage. The 3′ cleavage product was also detected in an MMH2 mutant that was defective in premature polyadenylation in the ORF (Fig. 1F, lanes 7 and 8) (14), indicating that polyadenylation within the CBP1 ORF to produce nonstop mRNA is dispensable for its endonucleolytic cleavage.
The Secondary Structure of the cis-Element in the CBP1 mRNA Is Crucial for Its Cleavage
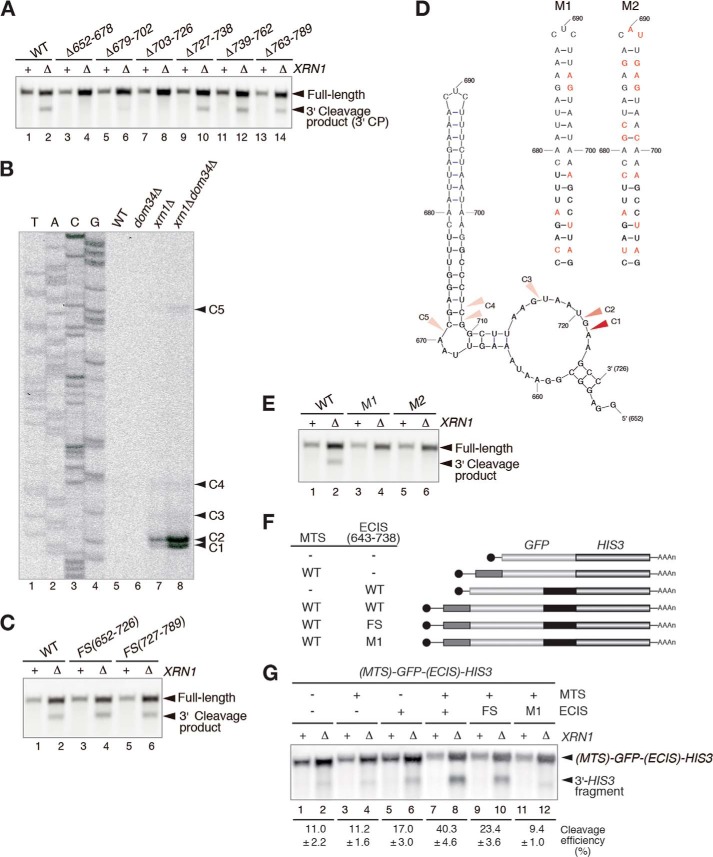
A deletion analysis revealed that the +652–726 nt region of the CBP1 ORF is indispensable for cleavage of the mRNA (Fig. 2A). Hence, this region was named the endonucleolytic cleavage-inducible sequence (ECIS). Next, a primer extension analysis was performed to analyze the 5′ ends of the 3′ cleavage products. The major cleavage sites, +720 and +721 nt, were located close to the 3′ end of the ECIS (C2 and C1 in Fig. 2B), and additional minor cleavage sites were located upstream of the major sites (C3, C4, and C5 in Fig. 2B). To address the significance of nascent peptides to the cleavage of CBP1, a frameshift mutation was introduced at the ECIS or the +727–789-nt region. The 3′ and 5′ endonucleolytic cleavage products derived from the frameshift CBP1 mutants were detected (Fig. 2C and data not shown), indicating that the translational product of the ECIS is not essential for the mRNA cleavage. Analysis of the secondary structure of the ECIS using the MFOLD program (26) identified a stem-loop structure (Fig. 2D); therefore, the CBP1 mRNA sequence was mutated (M1 and M2) to disrupt the secondary structure but retain the amino acid sequence of the ECIS (Fig. 2D). The 3′ endonucleolytic cleavage products derived from the M1 and M2 mutants were not detected in xrn1Δ cells (Fig. 2E), indicating that the secondary structure of the CBP1 mRNA is indispensable for its cleavage.
FIGURE 2.
Endonucleolytic cleavage of the CBP1 mRNA requires a stem-loop structure in the ORF. A, the +652–726-nt sequence of the CBP1 mRNA is indispensable for its cleavage. RNA samples extracted from W303 and W303xrn1Δ cells harboring the indicated deletion plasmids were analyzed by Northern blotting with DIG-labeled probe B, as described for Fig. 1D. B, analysis of the 5′ ends of the CBP1 mRNA 3′ cleavage products. The arrowheads indicate the 5′ ends of the 3′ endonucleolytic cleavage products derived from the reporter genes. C, the amino acid sequence encoded by the ECIS is not essential for cleavage of the CBP1 mRNA. The 3′ cleavage product was detected by Northern blotting with DIG-labeled probe B, as described for Fig. 1D. D, the predicted secondary structure of the CBP1 ECIS (left). The arrowheads indicate the positions of the 5′ ends of the 3′ endonucleolytic cleavage products determined in the primer extension experiments. The secondary structure of the RNA was predicted using MFOLD (26). The CBP1 nucleotides that were mutated in the M1 (middle) and M2 (right) plasmids are shown in red. E, analysis of the 3′ endonucleolytic cleavage products derived from the M1 and M2 mutants. RNA samples extracted from W303 and W303xrn1Δ cells harboring the mutant plasmids were analyzed by Northern blotting with DIG-labeled probe B, as described for Fig. 1D. F, schematic illustration of the GFP-HIS3 reporter mRNA. The boxes indicate the open-reading frames, the lines represent nontranslated regions, and the tract of As denotes the poly(A) tail. The gray and black boxes indicate the MTS region encoding the mitochondrial targeting signal and the ECIS region that is essential for the endonucleolytic cleavage, respectively. G, the ECIS of CBP1 is sufficient for its endonucleolytic cleavage. Northern blotting with a DIG-labeled HIS3 probe to detect the 3′ cleavage product. The levels of full-length mRNAs and 3′ cleavage products are shown as the mean values of three independent experiments. All results shown in the figure are representative of three independent experiments.
To determine whether they are able to induce endonucleolytic cleavage of the CBP1 mRNA, reporter genes containing the MTS and/or the ECIS were generated. The +643–738-nt sequence containing the ECIS (+652–726 nt) was inserted between the GFP and HIS3 genes, and the MTS was inserted at the 5′ end of GFP to construct the MTS-GFP-ECIS-HIS3 fusion (Fig. 2F). Northern blotting with a DIG-labeled GFP probe to detect the 5′ cleavage product (data not shown) or a DIG-labeled HIS3 probe to detect the 3′ cleavage product (Fig. 2G) revealed that insertion of both the ECIS and the MTS of CBP1, but not either alone, induced the endonucleolytic cleavage of the GFP-HIS3 reporter gene.
The Sen Complex Is Responsible for Cleavage of the CBP1 mRNA in Vivo and in Vitro
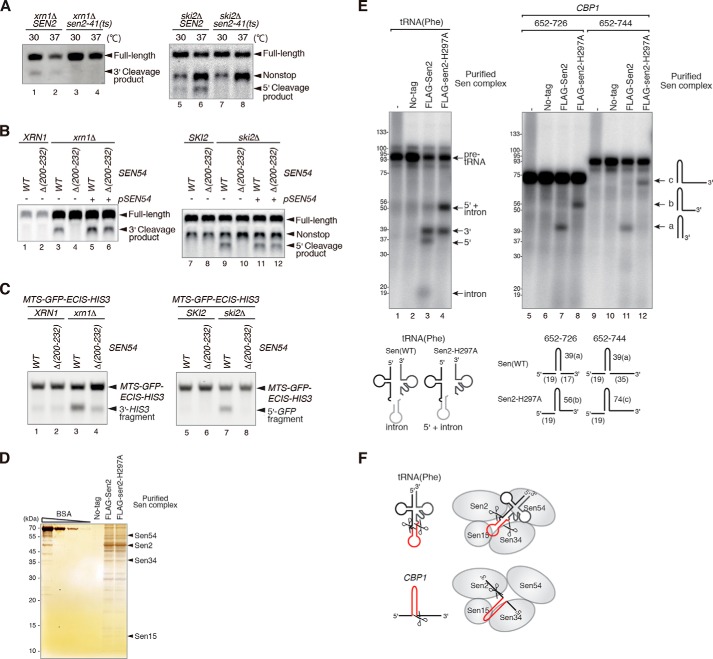
The MTS of the CBP1 mRNA was required for its endonucleolytic cleavage, suggesting that this mRNA is cleaved on the mitochondrial surface. Because the Sen complex localizes to the mitochondria in S. cerevisiae, we examined its role in cleavage of the CBP1 mRNA. Endonuclease activity is impaired severely in sen2–41 temperature-sensitive mutant cells at the restrictive temperature (18); therefore, sen2Δski2Δ and sen2Δxrn1Δ mutants harboring either wild-type SEN2 or sen2–41 mutant plasmids were generated. Total RNAs were prepared from cells grown at the permissive (30 °C) or restrictive (37 °C) temperature and then analyzed by Northern blotting with CBP1 probes. Endonucleolytic cleavage was abrogated in sen2–41 cells under both conditions, indicating that the Sen complex cleaves the CBP1 mRNA in vivo (Fig. 3A, lanes 2 and 6).
FIGURE 3.
The Sen complex cleaves the CBP1 mRNA. A, the Sen complex cleaves the CBP1 mRNA in vivo. Left panel, W303sen2Δxrn1Δ cells harboring wild-type SEN2 or the temperature-sensitive sen2–41 mutant were grown under normal (30 °C) or restrictive (37 °C) conditions. The 3′ cleavage product was detected by Northern blotting with DIG-labeled probe B. Right panel, Northern blot analyses of RNA samples from W303sen2Δski2Δ mutants harboring the wild-type SEN2 or sen2–41 plasmids. The cells were grown under normal conditions (30 °C) or were grown under normal conditions and then shifted to the restrictive temperature (37 °C) for 2 h prior to analysis. Northern blotting was performed using probe A (depicted in Fig. 1A) to detect the 5′ cleavage product. B, the mitochondrial location of Sen54 is required for cleavage of the CBP1 mRNA. Northern blot analyses of the indicated mutant cells were performed as described for A. A complementation assay was also performed. C, cleavage of the ECIS-containing reporter mRNA requires mitochondrial localization of the Sen complex. Left panel, Northern blotting using a HIS3 probe to detect the 3′ cleavage product. Right panel, Northern blotting using a GFP probe to detect the 5′ cleavage product. D, purification of the Sen complex. Samples were affinity-purified from cell extracts expressing Flag-Sen2-WT or Flag-Sen2-H297A and then subjected to 15% SDS-PAGE followed by silver staining. E, the Sen complex cleaves the ECIS in vitro. Analyses of the tRNA splicing activities of FLAG-tagged wild-type Sen2 and a FLAG-tagged Sen2-H297A mutant. F, a model for cleavage of the precursor tRNA or ECIS of the CBP1 mRNA by the Sen complex. All results shown in the figure are representative of three independent experiments.
The sen54-Δ(200–232) mutant is defective in mitochondrial localization of the Sen complex (16). Neither the 3′ cleavage products (Fig. 3B, lane 4) nor the 5′ cleavage products (Fig. 3B, lane 10) of the CBP1 mRNA were detected in sen54-Δ(200–232) mutant cells. These defects were complemented by transforming the cells with a plasmid harboring wild-type SEN54 (Fig. 3B, lanes 6 and 12). These results indicate that endonucleolytic cleavage of the CBP1 mRNA requires mitochondrial localization of the Sen complex. Complementary results were also obtained using the MTS-GFP-ECIS-HIS3 reporter gene (Fig. 3C), indicating that the reporter mRNA localized to mitochondria by the CBP1 MTS underwent cleavage at the ECIS by the Sen complex.
Next, we examined whether the Sen complex cleaves NGD-targeted mRNAs. NGD caused by translation arrest-inducing sequences was intact in sen2–41 and sen54-Δ(200–232) mutant cells (data not shown). Moreover, cleavage of NGD target mRNAs was not stimulated by the presence of a MTS at the amino terminus (data not shown), suggesting that the CBP1 mRNA and NGD-targeted mRNAs might be cleaved by different mechanisms. To determine whether it cleaves the CBP1 mRNA directly in vitro, the Sen complex was affinity-purified from cells expressing FLAG-tagged wild-type Sen2 or a FLAG-tagged Sen2-H297A mutant, which is deficient in 5′ endonuclease activity toward tRNA (23, 24) (Fig. 3D). The partially purified Sen complexes were tested in endonucleolytic cleavage assays using [32P]UTP-labeled RNAs. In the assays using the wild-type Sen complex, fragments of 41 nt were cleaved from every RNA species containing the ECIS sequence (Fig. 3E, lanes 7 and 11). We speculated that these fragments might correspond to the stem-loop region of the ECIS and that the cleavage site may correspond to the C4 site shown in Fig. 1D. Cleavage of these fragments did not occur in assays using the Sen2-H297A complex (Fig. 3E, lanes 8 and 12), indicating that this mutant is deficient in endonucleolytic cleavage of the 3′ region of the ECIS (Fig. 3F). Overall, these results are consistent with the in vivo data and indicate that the Sen complex cleaves the CBP1 mRNA directly in vitro. We noticed that the in vitro experiment using the mutant Sen complex produced a longer cleavage product, which may have resulted from the catalytic activity of Sen34, the Sen subunit harboring the other endonucleolytic center. In addition, the 3′ cleavage site in vitro is different from the major site in vivo (Figs. 2 and 3). These results suggest that processing of the ECIS in vivo requires the collaboration of several endonucleolytic activities, including the Sen activities.
Recognition of the Cbp1-translating Ribosome-mRNA Complex by Components of the Mitochondrial Translocation Machinery Is Crucial for Cleavage of the CBP1 mRNA
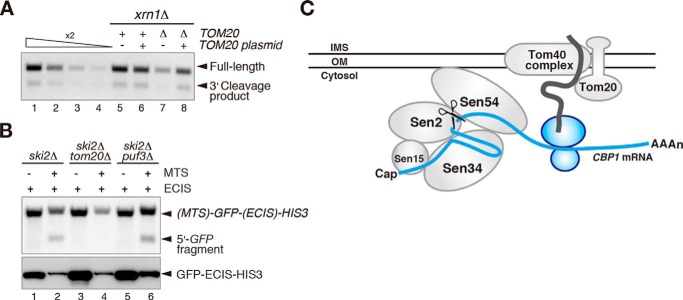
The MTS of a nascent peptide emerging from the ribosome is recognized by the mitochondrial translocator component Tom20, which is a MTS receptor that targets the translating mRNA to the mitochondrial outer membrane (7, 27). In addition, the Pumilio family protein Puf3 binds to the ORF and the 3′-UTR of specific mRNAs to localize them to the mitochondrial outer membrane (7). To determine whether Tom20 or Puf3 affects endonucleolytic cleavage of the CBP1 mRNA, tom20Δ or puf3Δ mutant cells were constructed in a ski2Δ or xrn1Δ mutant background. Cleavage of the CBP1 mRNA was reduced in both the tom20Δxrn1Δ and tom20Δski2Δ mutant cells, and this defect was complemented by transformation of the cells with a plasmid expressing Tom20 (Fig. 4A and data not shown). In addition, cleavage of the MTS-GFP-ECIS-HIS3 reporter mRNA was reduced in the ski2Δtom20Δ mutant cells, but not in the ski2Δpuf3Δ mutant cells (Fig. 4B, lane 4). These results suggest that Tom20 is required for mitochondrial localization of the CBP1 mRNA, thereby facilitating its endonucleolytic cleavage by the Sen complex.
FIGURE 4.
Tom20 is involved in endonucleolytic cleavage of the CBP1 mRNA. A, cleavage of the CBP1 mRNA is reduced in tom20Δ cells. W303xrn1Δ and W303tom20Δxrn1Δ cells harboring a control or wild-type TOM20 plasmid were analyzed by Northern blotting with probe B to detect the 3′ cleavage product, as described for Fig. 1D. B, cleavage of the MTS-GFP-ECIS-HIS3 reporter mRNA requires Tom20. RNA samples were analyzed by Northern blotting with a GFP probe (upper panel), and protein samples were analyzed by Western blotting with an anti-GFP antibody (lower panel). C, proposed model showing the mechanism by which the Sen complex controls the endonucleolytic cleavage of the CBP1 mRNA on the mitochondrial outer membrane. All of the results shown in the figure are representative of three independent experiments.
Discussion
The Cytoplasmic CBP1 mRNA Undergoes Endonucleolytic Cleavage by the tRNA Splicing Complex on the Outer Membrane of the Mitochondria
The results presented here demonstrate that the tRNA splicing endonuclease Sen complex cleaves the CBP1 mRNA on the mitochondrial outer membrane. The CBP1 mRNA was endonucleolytically cleaved in vivo (Fig. 1). Deletion analyses revealed that a MTS and an ECIS, the latter of which is located at the +652–726-nt region and forms a secondary structure, were indispensable for the cleavage (Figs. 1 and 2). In support of this finding, translation inhibition of the MTS and ECIS inhibited the endonucleolytic cleavage of the CBP1 mRNA (Fig. 1). Furthermore, insertion of the +643–738-nt region of the CBP1 mRNA, which included the ECIS, into a reporter mRNA induced endonucleolytic cleavage when a MTS was also present at the amino terminus (Fig. 2). Cleavage of the CBP1 mRNA was eliminated in a temperature-sensitive sen2 mutant that lacked endonuclease activity, and in a sen54 mutant displaying a defect in localization of Sen54 to the mitochondria (Fig. 3). In vitro tRNA splicing assays revealed that the Sen complex cleaves the CBP1 mRNA directly (Fig. 3). In addition, the endonucleolytic cleavage of this mRNA was regulated by Tom20 (Fig. 4). These results suggest that the CBP1 mRNA is localized to the mitochondria to enable cleavage by the tRNA splicing endonuclease Sen complex and that the mechanism of localization to the mitochondria is mediated by the MTS nascent peptide and Tom20 (Fig. 4C).
A Model for Endonucleolytic Cleavage of the CBP1 mRNA
Based on the results presented here, we propose a model of the mechanism of endonucleolytic cleavage of the CBP1 mRNA (Fig. 4C). In this model, Tom20 recognizes the MTS, an amino-terminal region of the nascent peptide derived from the CBP1 mRNA, and localizes the translation elongation complex composed of the ribosome, mRNA, and nascent polypeptide to the mitochondrial outer membrane. The Sen complex then recognizes and cleaves the secondary structure of the ECIS in the CBP1 mRNA. Cleavage by the Sen complex requires formation of the stem-loop structure and translation of the ECIS sequence (Fig. 2). Alternatively, translation of the ECIS may be required to dissociate a putative factor that binds to this region and protect it against cleavage by the Sen complex. The Sen complex splices tRNA on the mitochondrial surface in S. cerevisiae, and nuclear-localized Sen complex mutants are also able to splice tRNA (19). However, depletion of endogenous mitochondrial Sen proteins cannot be complemented by nuclear-localized Sen protein mutants (19). Therefore, the biological significance of localization of the Sen complex to the mitochondria is still unknown.
Overall, the results presented here demonstrate that the Sen complex localized on mitochondria cleaves the CBP1 mRNA. To our knowledge, this study is the first description of a correlation between mitochondrial localization and endonucleolytic cleavage of mRNAs. A previous study showed that the CBP1 mRNA has a mitochondria localization ratio of ∼20% (7). Because the efficiency of endonucleolytic cleavage of CBP1 mRNA is less than 20% (Figs. 1–4), we suspect that only a proportion of the mitochondria-targeted CBP1 mRNA undergoes endonucleolytic cleavage by the Sen complex localized on mitochondria. Genome-wide analyses identified a number of mitochondria-targeted mRNAs (7, 28), and the identification of mitochondria-localized mRNAs cleaved by the Sen complex will help to reveal the biological roles of this complex.
Acknowledgments
We thank members of our laboratories for discussion and critical comments on the manuscript.
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science 20112006 and 26291002 and by Research Grants in the Natural Sciences from the Sumitomo Foundation (to TI). The authors declare that they have no conflicts of interest with the contents of this article.
- NGD
- no-go decay
- ECIS
- endonucleolytic cleavage-inducible sequence
- MTS
- mitochondrial targeting signal
- Sen
- tRNA splicing endonuclease
- DIG
- digoxigenin.
References
- 1. van Hoof A., Frischmeyer P. A., Dietz H. C., Parker R. (2002) Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 295, 2262–2264 [DOI] [PubMed] [Google Scholar]
- 2. Frischmeyer P. A., van Hoof A., O'Donnell K., Guerrerio A. L., Parker R., Dietz H. C. (2002) An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science 295, 2258–2261 [DOI] [PubMed] [Google Scholar]
- 3. Doma M. K., Parker R. (2006) Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440, 561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsuboi T., Kuroha K., Kudo K., Makino S., Inoue E., Kashima I., Inada T. (2012) Dom34:hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3′ end of aberrant mRNA. Mol. Cell 46, 518–529 [DOI] [PubMed] [Google Scholar]
- 5. van den Elzen A. M., Henri J., Lazar N., Gas M. E., Durand D., Lacroute F., Nicaise M., van Tilbeurgh H., Séraphin B., Graille M. (2010) Dissection of Dom34-Hbs1 reveals independent functions in two RNA quality control pathways. Nat. Struct. Mol. Biol. 17, 1446–1452 [DOI] [PubMed] [Google Scholar]
- 6. Kuroha K., Akamatsu M., Dimitrova L., Ito T., Kato Y., Shirahige K., Inada T. (2010) Receptor for activated C kinase 1 stimulates nascent polypeptide-dependent translation arrest. EMBO Rep. 11, 956–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saint-Georges Y., Garcia M., Delaveau T., Jourdren L., Le Crom S., Lemoine S., Tanty V., Devaux F., Jacq C. (2008) Yeast mitochondrial biogenesis: a role for the PUF RNA-binding protein Puf3p in mRNA localization. PLoS One 3, e2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shoemaker C. J., Green R. (2011) Kinetic analysis reveals the ordered coupling of translation termination and ribosome recycling in yeast. Proc. Natl. Acad. Sci. U.S.A. 108, E1392–E1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shao S., von der Malsburg K., Hegde R. S. (2013) Listerin-dependent nascent protein ubiquitination relies on ribosome subunit dissociation. Mol. Cell 50, 637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lyumkis D., Doamekpor S. K., Bengtson M. H., Lee J. W., Toro T. B., Petroski M. D., Lima C. D., Potter C. S., Carragher B., Joazeiro C. A. (2013) Single-particle EM reveals extensive conformational variability of the Ltn1 E3 ligase. Proc. Natl. Acad. Sci. U.S.A. 110, 1702–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsuda R., Ikeuchi K., Nomura S., Inada T. (2014) Protein quality control systems associated with no-go and nonstop mRNA surveillance in yeast. Genes Cells 19, 1–12 [DOI] [PubMed] [Google Scholar]
- 12. Brandman O., Stewart-Ornstein J., Wong D., Larson A., Williams C. C., Li G. W., Zhou S., King D., Shen P. S., Weibezahn J., Dunn J. G., Rouskin S., Inada T., Frost A., Weissman J. S. (2012) A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell 151, 1042–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Islas-Osuna M. A., Ellis T. P., Marnell L. L., Mittelmeier T. M., Dieckmann C. L. (2002) Cbp1 is required for translation of the mitochondrial cytochrome b mRNA of Saccharomyces cerevisiae. J. Biol. Chem. 277, 37987–37990 [DOI] [PubMed] [Google Scholar]
- 14. Sparks K. A., Mayer S. A., Dieckmann C. L. (1997) Premature 3′-end formation of CBP1 mRNA results in the downregulation of cytochrome b mRNA during the induction of respiration in Saccharomyces cerevisiae. Mol. Cell. Biol. 17, 4199–4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sparks K. A., Dieckmann C. L. (1998) Regulation of poly(A) site choice of several yeast mRNAs. Nucleic Acids Res. 26, 4676–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paushkin S. V., Patel M., Furia B. S., Peltz S. W., Trotta C. R. (2004) Identification of a human endonuclease complex reveals a link between tRNA splicing and pre-mRNA 3′ end formation. Cell 117, 311–321 [DOI] [PubMed] [Google Scholar]
- 17. Yoshihisa T., Yunoki-Esaki K., Ohshima C., Tanaka N., Endo T. (2003) Possibility of cytoplasmic pre-tRNA splicing: the yeast tRNA splicing endonuclease mainly localizes on the mitochondria. Mol. Biol. Cell 14, 3266–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshihisa T., Ohshima C., Yunoki-Esaki K., Endo T. (2007) Cytoplasmic splicing of tRNA in Saccharomyces cerevisiae. Genes Cells 12, 285–297 [DOI] [PubMed] [Google Scholar]
- 19. Dhungel N., Hopper A. K. (2012) Beyond tRNA cleavage: novel essential function for yeast tRNA splicing endonuclease unrelated to tRNA processing. Genes Dev. 26, 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wach A., Brachat A., Pöhlmann R., Philippsen P. (1994) New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10, 1793–1808 [DOI] [PubMed] [Google Scholar]
- 21. Goldstein A. L., McCusker J. H. (1999) Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15, 1541–1553 [DOI] [PubMed] [Google Scholar]
- 22. Inada T., Aiba H. (2005) Translation of aberrant mRNAs lacking a termination codon or with a shortened 3′-UTR is repressed after initiation in yeast. EMBO J. 24, 1584–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trotta C. R., Miao F., Arn E. A., Stevens S. W., Ho C. K., Rauhut R., Abelson J. N. (1997) The yeast tRNA splicing endonuclease: a tetrameric enzyme with two active site subunits homologous to the archaeal tRNA endonucleases. Cell 89, 849–858 [DOI] [PubMed] [Google Scholar]
- 24. Trotta C. R., Paushkin S. V., Patel M., Li H., Peltz S. W. (2006) Cleavage of pre-tRNAs by the splicing endonuclease requires a composite active site. Nature 441, 375–377 [DOI] [PubMed] [Google Scholar]
- 25. Sambrook J., Russell D. W. (2006) The Condensed protocols. From Molecular Cloning: A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 26. Zuker M. (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abe Y., Shodai T., Muto T., Mihara K., Torii H., Nishikawa S., Endo T., Kohda D. (2000) Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell 100, 551–560 [DOI] [PubMed] [Google Scholar]
- 28. Marc P., Margeot A., Devaux F., Blugeon C., Corral-Debrinski M., Jacq C. (2002) Genome-wide analysis of mRNAs targeted to yeast mitochondria. EMBO Rep. 3, 159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsuboi T., Inada T. (2010) Tethering of poly(A)-binding protein interferes with non-translated mRNA decay from the 5′ end in yeast. J. Biol. Chem. 285, 33589–33601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mumberg D., Müller R., Funk M. (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156, 119–122 [DOI] [PubMed] [Google Scholar]
- 31. Gietz R. D., Sugino A. (1988) New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74, 527–534 [DOI] [PubMed] [Google Scholar]
- 32. Dimitrova L. N., Kuroha K., Tatematsu T., Inada T. (2009) Nascent peptide-dependent translation arrest leads to Not4p-mediated protein degradation by the proteasome. J. Biol. Chem. 284, 10343–10352 [DOI] [PMC free article] [PubMed] [Google Scholar]