Background: The involvement of miRNAs in the host mucosal immune response to gut microbes in colitis is still unclear.
Results: miR-193a-3p down-regulates PepT1, and intracolonic-delivery of miR-193a-3p ameliorated the severity of colitis.
Conclusion: miRNA-193a-3p can target colonic PepT1 and reduce intestinal inflammation.
Significance: Our study illustrates the new role of miRNAs in regulating the host immune response to microbes during colitis.
Keywords: gene regulation, inflammatory bowel disease (IBD), microRNA (miRNA), mucosal immunology, peptide transport
Abstract
Intestinal inflammation is characterized by epithelial disruption, leading to the loss of barrier function, recruitment of immune cells, and host immune responses to gut microbiota. PepT1, a di/tripeptide transporter that uptakes bacterial products, is up-regulated in inflamed colon tissue, which implies its role in bacterium-associated intestinal inflammation. Although microRNA (miRNA)-mediated gene regulation has been found to be involved in various processes of inflammatory bowel disease (IBD), the biological function of miRNAs in the pathogenesis of IBD remains to be explored. In this study we detected miRNA expression patterns in colon tissues during colitis and investigated the mechanism underlying the regulation of colonic PepT1 by miRNAs. We observed an inverse correlation between PepT1 and miR-193a-3p in inflamed colon tissues with active ulcerative colitis, and we further demonstrated that miR-193a-3p reduced PepT1 expression and activity as a target gene and subsequently suppressed the NF-κB pathway. Intracolonic delivery of miR-193a-3p significantly ameliorated dextran sodium sulfate-induced colitis, whereas the overexpression of colonic PepT1 via PepT1 3′-untranslated region mutant lentivirus vector abolished the anti-inflammatory effect of miR-193a-3p. Furthermore, antibiotic treatment eliminated the difference in the dextran sodium sulfate-induced inflammation between the presence and absence of miR-193a-3p. These findings suggest that miR-193a-3p regulation of PepT1 mediates the uptake of bacterial products and is a potent mechanism during the colonic inflammation process. Overall, we believe miR-193a-3p may be a potent regulator of colonic PepT1 for maintaining intestinal homeostasis.
Introduction
Inflammatory bowel disease (IBD),4 comprising Crohn's disease (CD) and ulcerative colitis (UC), are chronic inflammatory conditions that affect the gastrointestinal (GI) tract. In UC, the mucosal inflammation is limited to the colon beginning from the rectum, whereas in CD, inflammation primarily affects the ileum but may also occur at random locations in the GI tract, extending through the bowel wall (1). However, both diseases are thought to feature alterations in the immune response to environmental and GI microbiota in individuals genetically predisposed to IBD, which is characterized by a disruption in the intestinal epithelial barrier and an influx of immune cells (2).
PepT1 (encoded by the solute carrier family 15 member 1 gene, SLC15A1) is a di/tripeptide transporter that is highly expressed in normal small intestinal epithelial cells but not in normal colonic epithelia, resulting in low uptake of small bacterial peptides in a normal colon (3). IBD patients, however, express high levels of PepT1 in inflamed colon tissue, implying that PepT1 plays a critical role in the pathogenesis of IBD (4, 5). Colonic PepT1 can mediate excessive transportation of commensal bacterial products, such as N-formylated peptides, N-formylmethionylleucyl-phenylalanine (fMLF), and muramyl-dipeptide, into the cytosol of colonic epithelial cells (CECs), thereby switching from a symbiotic to a pathogenic relationship between intestinal microbes and the host (6, 7). Given the above, the regulation of PepT1 is a promising therapeutic target for intestinal anti-inflammatory studies (8).
MicroRNAs (miRNAs) are a class of endogenous small non-coding RNAs (∼22 nucleotides in length) that are involved in post-transcriptional gene regulation by either inducing translation repression or promoting the degradation of messenger RNAs (mRNAs) (9). miRNAs are important elements in the development of the innate and adaptive immune systems, and dysregulated miRNAs have been described in many immune diseases, including IBD, rheumatoid arthritis, asthma, and systemic lupus erythematosus (10, 11). Certain miRNAs have been recognized to make substantial contributions to the pathogenic mechanisms of UC or CD by directly repressing their target genes (12–14). Our previous study revealed differential expression of several miRNAs in dextran sodium sulfate (DSS)-induced colitis, in which miR-150 and its targeting of c-Myb may serve as a mechanism underlying the colonic epithelial disruption in DSS-induced murine experimental colitis and in active UC (15). However, the involvement of miRNAs in the interaction between the host mucosal immune response and microbes in IBD has not been completely elucidated, and the attempt to employ miRNAs as a potential anti-inflammatory agent remains to be further investigated.
In this study we identified the differential expression of miRNAs in inflamed colon tissues from active IBD patients. Among those miRNAs, miR-193a-3p is down-regulated, leading to the elevated expression and activity of colonic PepT1 and triggering the bacterial peptide-induced inflammatory response. Restoration of miR-193a-3p significantly decreased the intestinal inflammation in vivo using murine models of colitis.
Experimental Procedures
Patients
Colonic tissue samples were obtained by punch biopsies during endoscopic examination of patients with active UC and screening colonoscopies of normal control subjects in Ruijin Hospital associated with Shanghai Jiaotong University School of Medicine (Shanghai, China).
Plasmid Construction and Luciferase Reporter Assay
A luciferase reporter assay was performed as previously described (16). The full length of the 3′-untranslated region (3′-UTR) of human PepT1, containing a presumed miR-193a-3p complementary site (seed sequence, GGCCAGTA), was amplified by PCR using human genomic DNA as a template. The PCR products were inserted into the pMIR-REPORT plasmid (Applied Biosystems, Foster City, CA), and efficient insertion was confirmed by sequence analysis. To test for binding specificity, the seed sequence of miR-193a-3p was mutated from GGCCAGTA to CCGGTCAT. For the luciferase reporter assays, 4 μg of firefly luciferase reporter plasmid, 4 μg of β-galactosidase (β-gal) expression vector (Applied Biosystems), and 100 pmol of miR-193a-3p mimic (synthetic RNA oligonucleotides mimicking miR-193a-3p precursors; GenePharma, Shanghai, China), miR-193a-3p inhibitor (chemically modified antisense oligonucleotides designed to specifically target against mature miR-193a-3p; GenePharma), mimic-control (synthetic scrambled control oligonucleotides, GenePharma), or inhibitor control (synthetic scrambled control oligonucleotides; GenePharma) were transfected into 293T or Caco2 cells cultured in 6-well plates using Lipofectamine 2000 according to the manufacturer's instructions. Twenty-four hours after transfection, the cells were analyzed using a luciferase assay kit (Promega, Madison, WI).
Cell Culture
Human intestinal epithelial Caco2, HT29 cells, and 293T cells were purchased from the China Cell Culture Center (Shanghai, China). Caco2 cells were cultured in high glucose (4.5 g/liter) DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco), 1% penicillin and streptomycin, and 1% non-necessary amino acid (Gibco). HT29 cells were cultured in McCoy's 5A (Gibco), 1% penicillin and streptomycin, and 10% FBS. 293T cells were cultured in high glucose (4.5 g/liter) DMEM supplemented with 10% FBS and 1% penicillin and streptomycin. For the transport study, Caco2 cells were seeded on 6-well plates at a density of 5 × 104 cells/cm2. Caco2 cell monolayers cultured for 12 to 14 days were used in the experiments. The quality of the monolayers grown on the permeable membrane was assessed by measuring the transepithelial electrical resistances using the Millicell-ERS (Millipore, Bedford, MA).
Cell Transfection/Infection
Either miRNA mimic or siRNA was transfected into cells using Lipofectamine 2000 according to the manufacturer's instructions. siRNA sequences targeting the human PepT1 gene were designed and synthesized by RiboBio (RiboBio Biotechnology, Guangzhou, China). A scrambled siRNA, which could not target the human PepT1 gene, was used as a negative control.
The lentivirus vector encoding PepT1 and its 3′-UTR containing miR-193a-3p binding site (PepT1 vector) and the lentivirus vector encoding PepT1 and its 3′-UTR containing miR-193a-3p mutant binding site (PepT1 3′-UTR mutant lentivirus) were generated by the GenePharma Company (Shanghai, China). An empty backbone vector was used as a control. Caco2 cells were pretreated with a PepT1 lentivirus or PepT1 3′-UTR mutant lentivirus vector at a multiplicity of infection of 5:1 for 72 h before the transfection with miR-193a-3p mimic or mimic-control (100 pmol for 1 well in 6-well plates). The cells were harvested 24 h after the transfection.
RNA Isolation and Quantitative RT-PCR (qRT-PCR)
Total RNA from cells or tissues was extracted using the TRIzol reagent (Invitrogen). The expression of miRNAs was examined by qRT-PCR using TaqMan miRNA probes (Applied Biosystems) according to the manufacturer's instructions. The relative expression of mRNAs was examined by qRT-PCR using LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostics) according to the manufacturer's protocol. Primers for β-actin were used as internal controls. All of the reactions were run in triplicate. A comparative threshold cycle (ΔCT) method was used to compare each condition with the controls, and values are expressed as 2−ΔΔCT or -fold change. In these experiments, the relative levels of miRNAs in cells or tissues were normalized to U6 snRNA, a ubiquitously expressed small nuclear RNA.
Western Blotting
Proteins from whole cell or tissue lysates were separated by 12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Bio-Rad); membranes were probed at 4 °C overnight with antibodies of PepT1 (Santa Cruz, Dallas, TX), NF-κB/p65 (Santa Cruz), phospho-IκB-α (Ser32, Santa Cruz), or IκB-α (Santa Cruz). Normalization was performed by blotting the same membranes with an antibody against GAPDH (Santa Cruz).
Uptake Experiments
PepT1-mediated uptake and transport of cephalexin and fMLF in Caco2 cells was measured as previously described (17, 18). Caco2 cells were cultured in 6-well plates for 12–14 days after the formation of a complete monolayer of cells, with medium replacement every 2 days. The cells were transfected with 100 pmol of miR-193a-3p mimic/mimic-control/human PepT1 siRNA/scramble siRNA/PepT1 expression lentivirus vector with or without miR-193a-3p mimic/control vector with or without miR-193a-3p mimic for 24 h before uptake experiments.
On the day of the experiment, cell monolayers were washed 3 times with modified Krebs buffer (5.4 mm KCl, 2.8 mm CaCl2, 1 mm MgSO4, 0.3 mm NaHPO4, 137 mm NaCl, 0.3 mm KH2PO4, and 10 mm MES, pH 6) at 37 °C. Only the monolayers with transepithelial electrical resistance values >150 ohms/cm2 were used. The cells were then incubated in the modified Krebs buffer, pH 6.0, for 15 min with soft circular shaking at 37 °C. Then the buffer was replaced with modified Krebs buffer, pH 6.0, containing 1 mm cephalexin (Sigma) or 1 μm fMLF (Sigma). The cells were incubated for 30 min with soft circular shaking at 37 °C. At the end of the incubation period, cell monolayers were washed three times with ice-cold PBS to remove extracellular cephalexin or fMLF. The PepT1-mediated transport of cephalexin or fMLF into cells was calculated by determining the concentration of intracellular cephalexin or fMLF with high performance liquid chromatography (HPLC) after the cells were lysed with 500 μl of 1% NaOH, 0.1% SDS.
HPLC Determination of Cephalexin and fMLF Concentration
The concentration of cephalexin was determined using a C18 Supelcosil 250 × 4 mm, 4-μm particle column (Sigma) with concentration monitored at λ = 260 nm. The mobile phase was composed of 0.01 m sodium acetate buffer, pH 5.2, and acetonitrile (91.5:8.5, v/v). The flow rate was 1 ml/min, and the volume injected was 20 μl. Pure cephalexin (Sigma) diluted into a concentration gradient was used to establish a standard curve.
The concentration of fMLF was determined using a C8 Supelcosil 250 × 4.6 mm, 5-μm column (Sigma) with concentration monitored at λ = 215 nm. Acetonitrile was used as the mobile phase at a flow rate of 1 ml/min. Pure fMLF (Sigma) diluted into a concentration gradient was used to establish a standard curve.
Induction of Colitis
Eight-week-old female C57BL/6 mice (18–22 g) were purchased from the Model Animal Research Center (Nanjing, China) and maintained in a specific pathogen-free facility for use according to the guidelines for experimental animals at Nanjing University. DSS-induced colitis was carried out by the addition of 3% (w/v) DSS (36–50 kDa; MP Biomedicals) to filter-purified and sterilized drinking water for 8 days. Control mice were given normal drinking water.
The dnTGFβRII mice were generous gifts from Dr. Xiong Ma (Shanghai Institute of Digestive Disease, Shanghai, China) and maintained in a specific pathogen-free facility. C57BL/6 mice were used as controls. At 20 weeks of age, the dnTGFβRII mice and control mice were sacrificed, and the colons were excised for macroscopic observation, histopathological analysis, qRT-PCR, and Western blot analysis. Mice in both of the models were monitored daily for weight loss, rectal bleeding, and diarrhea.
Treatment and Assessment of Colonic Inflammatory Changes
Polyethylenimine (PEI; 25 kDa, Sigma)/miRNA mimic or mimic-control complexes were prepared by mixing an aqueous solution of PEI with a solution of miRNA at a molar ratio of PEI nitrogen to miRNA mimic phosphate (N/P) of 2:1. The solution was given to mice via a rectal enema in a total volume of 100 μl. DSS-treated mice were divided into three groups, and the mice in each group received treatment with either PEI/miR-193a-3p mimic, PEI/mimic-control, or saline at a dose of 3 mg of miRNA mimic or mimic-control/kg body weight every 2 days in DSS-induced colitis or from the age of 12 weeks to 20 weeks in dnTGFβRII mice. At the end of the treatment period, the lengths of the colons were measured, and the colonic histology was evaluated in a blinded fashion.
To analyze the cellular localization of miRNA precursors, frozen sections of the colons harvested 24 h after the intracolonic administration of the Cy3-labeled miRNA mimic (Life Technologies)-PEI complex were subjected to immunofluorescence staining. To examine the mature-miR-193a-3p in the colon, in situ hybridization and immunofluorescence were used.
To examine the influence of colonic PepT1 overexpression on the therapeutic effect of miR-193a-3p mimic, mice were treated with 109 PFU of PepT1 3′-UTR mutant lentivirus, control lentivirus, or saline as a 100-μl rectal infusion under sedation 24 h before the intracolonically administration of miR-193a-3p mimic/PEI (3 mg/kg body weight).
To investigate the role of miR-193a-3p in PepT1-mediated bacterial products in inducing intestinal inflammation, mice were given broad-spectrum antibiotics (1 g/liter) in drinking water for 4 weeks (ampicillin, vancomycin, neomycin sulfate, and metronidazole; Sigma). After 4 weeks of antibiotic treatment, 3% DSS was given in drinking water, and miR-193a-3p mimic/PEI was intracolonically administered.
Immunofluorescence Staining and miRNA in Situ Hybridization
Frozen sections of the mouse colonic tissues were sectioned (5–10 μm thick) using a Leica CM3050S cryostat cryosectioning system (Leica Microsystems, Wetzlar, Germany) and mounted on cover slides followed by air-drying before immunostaining was performed. For cell localization of miRNA precursors, frozen sections of the colons, harvested 24 h after the Cy3-labeled miRNA precursor-PEI complex administration, were stained with a mouse-anti mouse/human PCK antibody (Abcam, Cambridge, MA) at 4 °C overnight. The secondary antibody FITC-labeled goat anti-mouse (Invitrogen) was applied at room temperature for 45 min followed by staining of the nuclei with 4,6-diamidino-2-phenylindole (DAPI; Sigma).
For miRNA in situ hybridization and immunofluorescent staining, a 5′,3′-digoxin-conjugated miR-193a-3p probe (Exiqon, Vedbaek, Denmark) was used for the in situ hybridization of miR-193a-3p. Sections were blocked in prehybridization buffer for 20 min at room temperature, and then the miR-193a-3p probe (10 ng/ml) in the hybridization buffer was hybridized with tissue sections for 1 h. After washing the slides with washing buffer, the sections were stained with rabbit anti-digoxin antibody (Biorbyt, Cambridge, UK) at 4 °C overnight. Then the sections were stained with a mouse-anti mouse PCK antibody (BioLegend, San Diego, CA) at 4 °C for 12 h. The TRITC-labeled goat anti-rabbit antibody and FITC-labeled goat anti-mouse/rat antibody (Invitrogen) was applied at room temperature for 45 min followed by the nuclear DAPI staining.
Disease Activity Index Evaluation
The disease activity index (DAI) score ranged from 0 to 4 based on the following parameters: change in weight (0, ≤ 1%; 1, 1–5%; 2, 5–10%; 3, 10–15%; 4, > 15%), rectal bleeding (0, no blood using hemoccult; 2, positive hemoccult; 4, gross bleeding), and stool consistency (0, normal; 2, loose stools; 4, diarrhea). The combined scores were then averaged to obtain the final disease activity index score.
Histopathological Analysis
For histopathological examination, a sample of colonic tissue was harvested, fixed in Bouin solution composed of picric acid, 40% formaldehyde, and glacial acetic acid (15:5:1, v/v), embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Inflammation was blindly scored from 0 to 4 as follows: 0, no signs of inflammation; 1, low leukocyte infiltration; 2, moderate leukocyte infiltration; 3, high leukocyte infiltration, moderate fibrosis, high vascular density, thickening of the colon wall, moderate goblet cell loss, and focal loss of crypts; 4, transmural infiltration, massive loss of goblet cells, extensive fibrosis, and diffuse loss of crypts.
Cytokine Analysis
The levels of related proinflammatory cytokines (interferon-γ (IFN-γ), interleukin-1β (IL-1β), IL-6, IL-10, and IL-12) in the colonic mucosa were evaluated by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Myeloperoxidase (MPO) activity in the colonic mucosa was analyzed by commercially available kits (Jiancheng Biotech, Nanjing, China) according to the manufacturer's instructions.
Statistics
All images of Western blots and qRT-PCR assays are representative of at least three independent experiments. qRT-PCR and luciferase reporter assays were performed in triplicate. The data shown are presented as the means ± S.E. of three or more independent experiments. A two-tailed Student's t test was used for evaluating statistical significance between groups. A p value <0.05 was considered significant.
Study Approval
All of the patients and normal control individuals gave informed consent before the procedure. The study was approved by the Institutional Review Board of Ruijin Hospital, Shanghai Jiaotong University School of Medicine. All animal experiments were performed in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Nanjing University (Nanjing, China).
Results
Up-regulation of PepT1 and Down-regulation of miR-193a-3p in Active UC Tissues
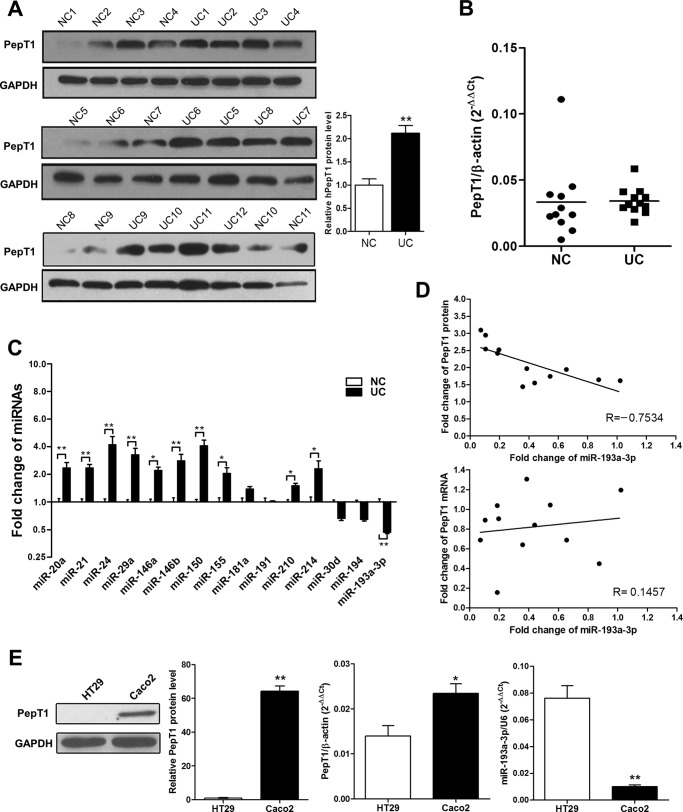
We first assessed PepT1 protein expression by Western blotting in 12 cases of active UC tissue samples compared with 11 cases of normal controls and found that PepT1 was markedly up-regulated (Fig. 1A). However, unlike PepT1 protein expression, there was no significant difference in PepT1 mRNA levels between UC tissues and normal controls (Fig. 1B). Given the disparity between PepT1 mRNA and protein levels in UC tissues, it is likely that a post-transcriptional regulatory mechanism exists. Because miRNA is an important post-transcriptional regulator of gene expression, we hypothesized that miRNAs may be involved in the regulation of PepT1 expression. Based on our previous studies (15, 16, 19, 20) and the present understanding of miRNA expression in UC, we investigated whether a suite of inflammation-relevant miRNAs are differentially expressed in UC compared with normal controls by qRT-PCR. As shown in Fig. 1C, although several miRNAs including miR-212, miR-24, miR-29a, miR-146a, miR-146b, and miR-150 were significantly increased in active UC (-fold change >2 and p < 0.01), only miR-193a-3p was significantly decreased (by 45%, p < 0.01). The inverse relationship between miR-193a-3p and PepT1 protein expression and the disparity between miR-193a-3p and PepT1 mRNA levels were further illustrated using Pearson correlation scatter plots (Fig. 1D).
FIGURE 1.
The inverse correlation between PepT1 and miR-193a-3p. A, Western blotting of PepT1 levels in active UC and normal control (NC) colon tissues: representative Western blot (left panels) and quantitative analysis of PepT1 levels (right panels). B, The relative expression of PepT1 mRNA transcripts in active UC and NC tissues by qRT-PCR. C, the relative miRNA expression ratios of active UC to NC tissues. D, Pearson's correlation scatter plots of the -fold changes of miR-193a-3p and PepT1 mRNA and PepT1 protein. E, PepT1 protein expression by Western blotting and PepT1 mRNA and miR-193a-3p expression by qRT-PCR in Caco2 and HT29 cells. *, p < 0.05; **, p < 0.01.
Furthermore, we assessed whether PepT1 expression levels were inversely correlated with miR-193a-3p levels in Caco2 and HT29 cells, as previous studies have employed these cell lines as enterocyte models to investigate the functions of PepT1 (4, 7). Low endogenous miR-193a-3p expression levels and high levels of PepT1 protein were detected in Caco2 cells, whereas highly expressed miR-193a-3p but low levels of PepT1 protein were detected in HT29 cells (Fig. 1E). The inverse relationship between endogenous miR-193a-3p and PepT1 protein was not observed between miR-193a-3p and PepT1 mRNA. Taken together, these results implied that there is miR-193a-3p-mediated post-transcriptional regulation of PepT1 expression in active UC tissues.
Validation of Human Colonic PepT1 as a Direct Target of miR-193a-3p
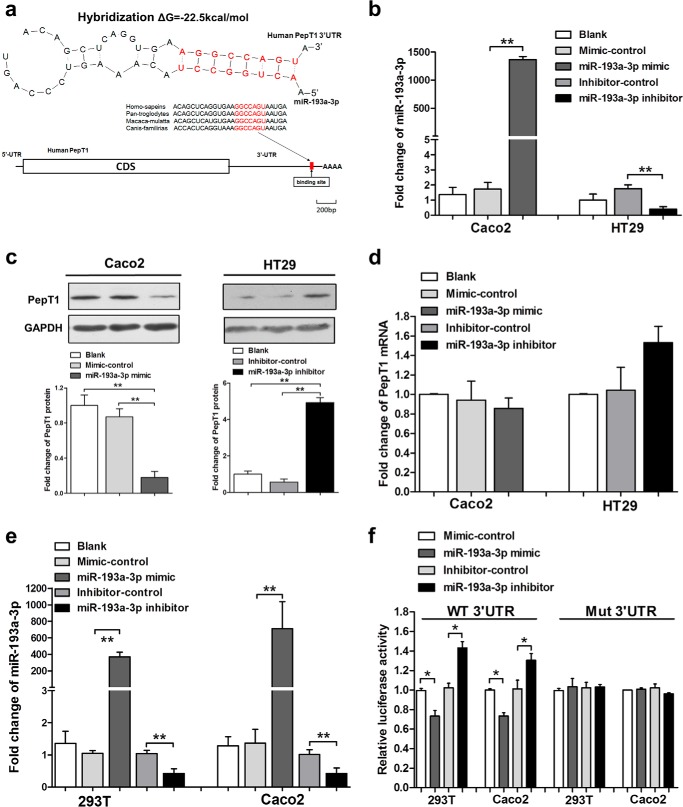
Given the inverse correlation between miR-193a-3p and PepT1, we then used three algorithms (TargetScan, miRanda, and PicTar) in combination to calculate whether human PepT1 is a putative target of miR-193a-3p. As shown in Fig. 2A, a hybrid between the PepT1 3′-UTR and miR-193a-3p was observed. The free energy value of this hybrid was −22.6 kcal/mol, which was well within the range of genuine miRNA target pairs. Moreover, there was perfect base-pairing between the “seeds” (the core sequence that encompasses the first 2–8 bases of the mature miRNA) and cognate targets. Overexpression of miR-193a-3p was achieved by transfecting Caco2 cells with a miR-193a-3p mimic, whereas knockdown of miR-193a-3p was achieved by transfecting HT29 cells with a miR-193a-3p inhibitor (Fig. 2B). As shown in Fig. 2C, overexpression of miR-193a-3p in Caco2 cells strongly decreased PepT1 levels, whereas inhibition of miR-193a-3p in HT29 cells increased PepT1 levels. Although the miR-193a-3p intracellular level was altered significantly after mimic or inhibitor treatment, overexpression or knockdown of miR-193a-3p did not affect PepT1 mRNA stability significantly (Fig. 2D).
FIGURE 2.
Identification of PepT1 as a target of miR-193a-3p. A, schematic description of the conserved binding site for miR-193a-3p and human PepT1. B, qRT-PCR analysis of miR-193a-3p expression levels after transfection with the miR-193a-3p mimic, mimic-control in Caco2, the miR-193a-3p inhibitor, and inhibitor-control in HT29 cells normalized to U6. C, Western blotting of PepT1 protein expression levels after above transfection in Caco2 cells and HT29 cells. D, qRT-PCR analysis of PepT1 mRNA levels after above transfection in Caco2 and HT29 cells. E, qRT-PCR analysis of miR-193a-3p expression levels after transfection with the miR-193a-3p mimic, mimic-control, the miR-193a-3p inhibitor, and inhibitor-control in 293T and Caco2 cells. F, luciferase reporter activity after expression of the above transfections in 293T cells. Luciferase reporters carrying the wild-type (WT) or mutant (Mut) PepT1 3′-UTR were cotransfected into 293T and Caco2 cells along with the indicated oligonucleotides. *, p < 0.05; **, p < 0.01.
To examine whether miR-193a-3p directly targets the 3′-UTR of human PepT1 mRNA, the full-length segment of the PepT1 3′-UTR containing a possible miR-193a-3p complementary site was cloned into a luciferase reporter plasmid. As shown in Fig. 2E, transfection of the miR-193a-3p mimic and inhibitor significantly increased and decreased miR-193a-3p levels, respectively, in 293T and Caco2 cells. After normalization to β-gal activity, luciferase reporter activity was suppressed after overexpression of miR-193a-3p in 293T and Caco2 cells. Meanwhile, transfection of the miR-193a-3p inhibitor alone resulted in an increase in luciferase reporter activity compared with the inhibitor-control. Mutating the nucleotides of the seeding sequence in the human PepT1 3′-UTR largely abolished the inhibitory effect of the miR-193a-3p mimic and the positive effect of the miR-193a-3p inhibitor on luciferase reporter activity (Fig. 2F). Taken together, the results demonstrated that miR-193a-3p directly recognizes the 3′-UTR of PepT1 transcripts and regulates its expression at the post-transcriptional level. Besides miR-193a-3p target PepT1, we have examined several other miRNAs that were possibly involved in the regulation of PepT1 predicted by the algorithms, and only miR-193a-3p was significantly down-regulated in active IBD patients compared with normal controls (data not shown).
miR-193a-3p Reduces PepT1 Transport Ability and Suppresses the Bacterial Peptide-induced Inflammatory Response in Vitro
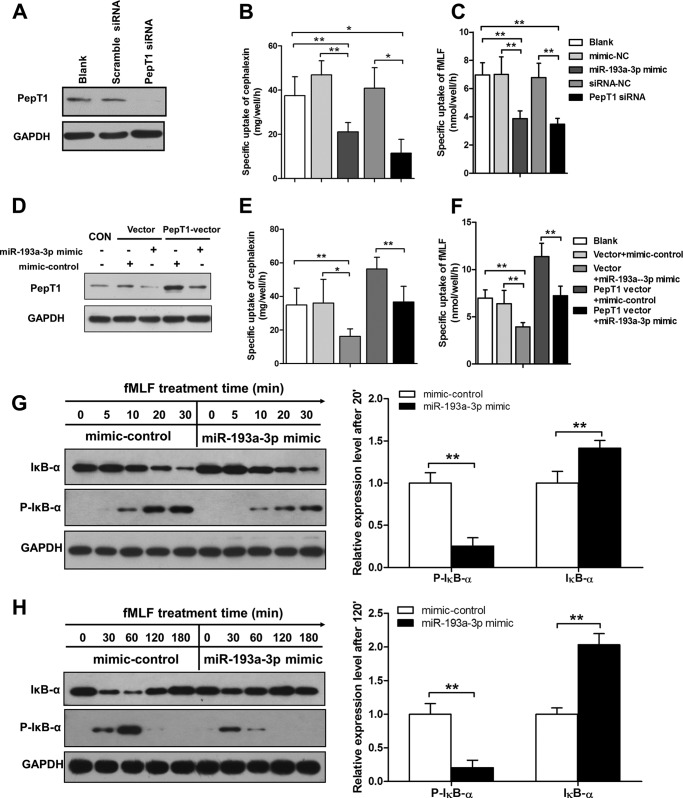
Because the main function of PepT1 is to facilitate transport of bacterial products into colonic epithelial cells, we investigated the effects of miR-193a-3p on the uptake of cephalexin, a specific substrate of PepT1, and fMLF, a kind of bacterial product, in human intestinal enterocyte-like Caco2 cells. siRNA against PepT1 was transfected into Caco2 cells to serve as a positive control (Fig. 3A). As expected, overexpression of miR-193a-3p hampered the uptake of cephalexin and fMLF, compared with mimic-control-transfected cells or untreated cells (Fig. 3, B and C). By contrast, transfection with PepT1 overexpressing-vector significantly increased the PepT1 protein and transport activity of cephalexin and fMLF, whereas co-transfection with miR-193a-3p mimic suppressed PepT1 expression and transport activity to baseline (Fig. 3, D–F).
FIGURE 3.
MiR-193a-3p reduces PepT1 transport ability and suppresses the bacterial peptide induced inflammatory response in vitro. A, PepT1 siRNA compared with the scrambled siRNA in Caco2 cells. B and C, PepT1 transport activity 24 h after transfection with the miR-193a-3p mimic or PepT1 siRNA expressed by measuring the specific uptake of cephalexin and fMLF. D, Western blotting of PepT1 protein expression levels after cotransfection of PepT1 overexpressing-vector and miR-193a-3p mimic or mimic-control. E and F, PepT1 transport activity 24 h after the cotransfection above expressed by measuring the specific uptake of cephalexin and fMLF. G and H, Caco2 cells pretransfected with the miR-193a-3p mimic or mimic control were stimulated with fMLP (100 nm) for the indicated times, and levels of phosphor IκB-α and IκB-α were measured by Western blotting. Bar graphs represent the densitometric quantification at the 30- and 120-min time points. *, p < 0.05; **, p < 0.01.
According to previous reports, PepT1 transports the bacterial peptide product fMLF into intestinal epithelial cells and induces inflammatory responses (6, 21). We then sought to explore whether miR-193a-3p can suppress fMLF-induced intestinal inflammation via inhibiting PepT1. Because that NF-κB is a well known transcription factor activated in response to immune and proinflammatory signals (22–24), we assessed the activation of NF-κB in Caco2 cells after fMLF stimulation. Caco2 cells pretransfected with the miR-193a-3p mimic or mimic-control were treated with fMLF (100 nm) for the indicated times, and NF-κB activation was assessed by Western blotting of phosphorylated IκB-α and IκB-α levels. Notably, phosphorylated IκB-α in response to fMLF occurred later and was slightly increased in the presence of the miR-193a-3p mimic when compared with mimic-control 30 min after fMLF stimulation (Fig. 3G). We also found that degradation of IκB-α was reduced in the presence of fMLF, whereas a high level of IκB-α degradation was observed after the treatment of mimic-control (Fig. 3G). Furthermore, the miR-193a-3p restoration facilitated IκB-α returning to baseline after fMLF stimulation (Fig. 3H).
We also performed fMLF stimulation in HT29 cells after transfection with miR-193a-3p mimic or mimic-control and assessed NF-κB activation. Altered miR-193a-3p expression did not affect the downstream NF-κB activation in HT29 cells, which were absent of PepT1 expression (data not shown).
Cellular Localization of miR-193a-3p after miR-193a-3p Mimic Administration
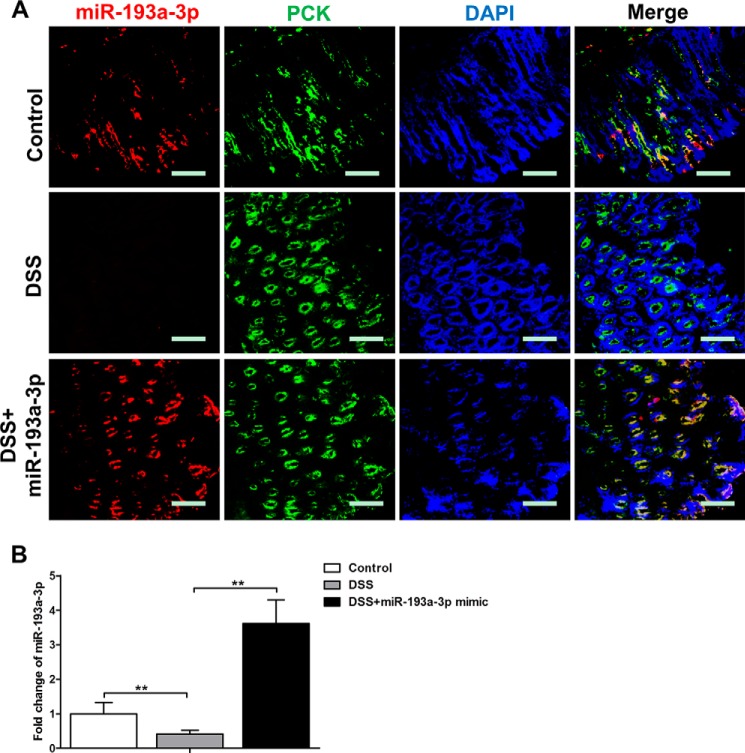
To identify the miRNA mimic uptake by CECs, frozen sections prepared from the colons of Cy3-labeled miRNA mimic-treated mice were stained with epithelial cell markers. In situ hybridization and immunofluorescent staining demonstrated that the intraluminal administration of miR-193a-3p mimic significantly increased miR-193a-3p levels in the CECs (pan-cytokeratin+ [PCK+] cells) of DSS-colitic colons (Fig. 4A), whereas miR-193a-3p was not taken up by the CECs of normal colons (data not shown). To further quantify the cellular level of miR-193a-3p, qRT-PCR was performed on colonic tissue 24 h after the intraluminal administration of miR-193a-3p mimics. Intraluminal administration of miR-193a-3p mimics significantly up-regulated miR-193a-3p levels (Fig. 4B) in DSS-treated colonic tissue.
FIGURE 4.
Cellular location of miR-193a-3p after miR-193a-3p mimic administration in vivo. A, immunofluorescent staining with CEC marker (pan-cytokeratin (PCK)) and in situ hybridization for miR-193a-3p were performed on colon sections from DSS colitis after miR-193a-3p mimic administration (red, miR-193a-3p; green, PCK; blue, DPAI nuclear staining). B, the levels of miR-193a-3p from DSS colitic colon after miR-193a-3p mimic administration by qRT-PCR. The values are expressed as the means ± S.E.; n = 5–6 mice per group. **, p < 0.01. Bars = 50 μm.
miR-193a-3p Reduces the Intestinal Inflammatory Response in DSS-induced Colitis
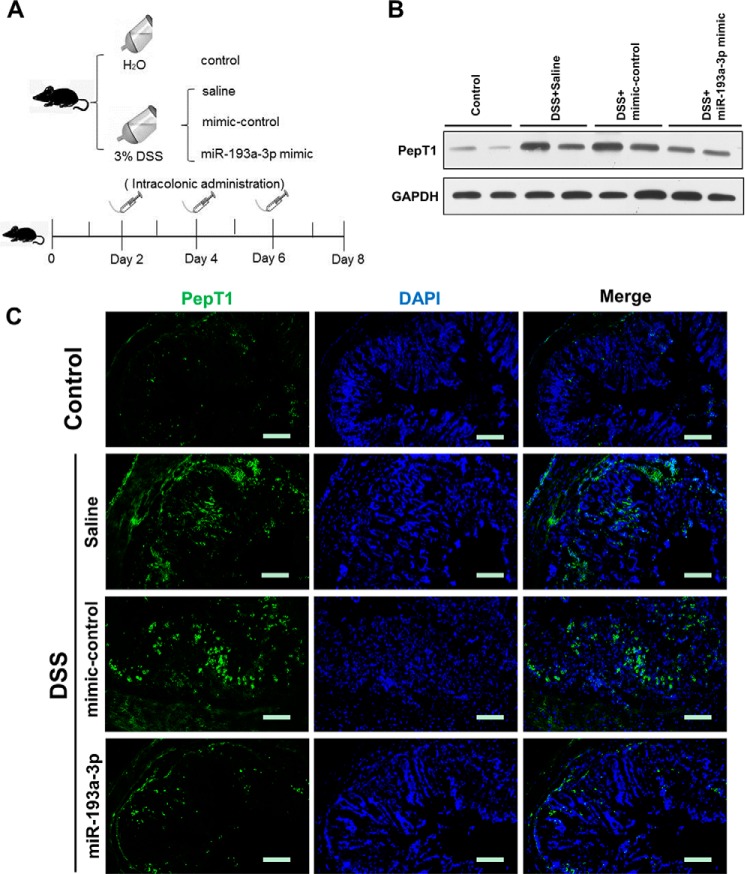
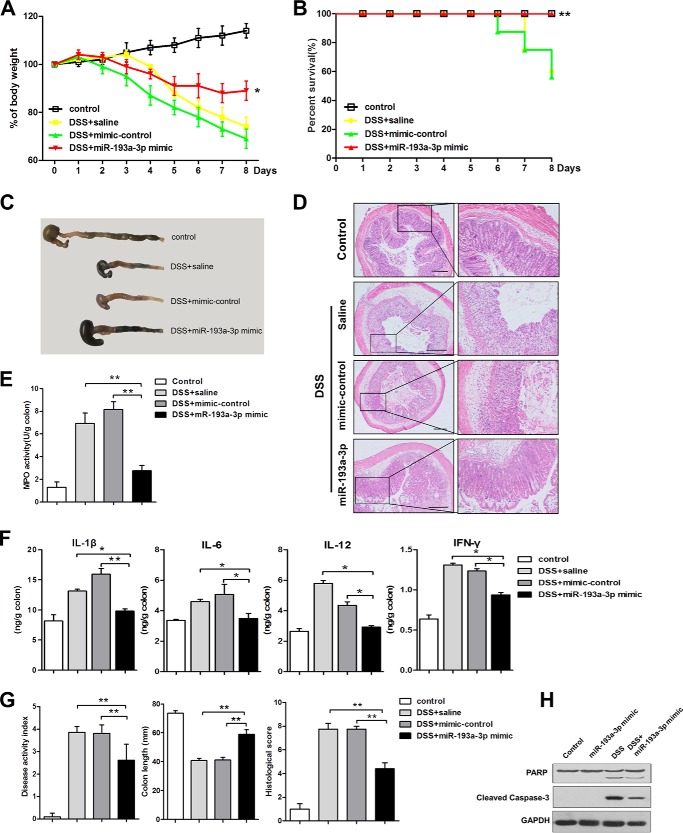
We employed a DSS-induced colitis model in mice, an experimental model of human colonic inflammation, to examine PepT1 expression after intra-colonic administration of the miR-193a-3p mimic, mimic-control, or saline (Fig. 5A). In agreement with our in vitro experimental results, PepT1 expression in colon tissue was increased in mice with colitis compared with control mice and was suppressed in the mice treated with miR-193a-3p mimic compared with mice treated with mimic-control or saline after induction of colitis (Fig. 5, B and C). Intra-colonic treatment of the miR-193a-3p mimic significantly reduced weight loss induced by DSS and resulted in 100% survival rates (Fig. 6, A and B). After miR-193a-3p mimic treatment, the colons of mice showed fewer signs of macroscopic inflammation, such as hyperemia, inflammation, and necrosis, compared with the colons of mice treated with mimic control or saline (Fig. 6C). The anti-inflammatory effect of the miR-193a-3p mimic was confirmed at the histological level using H&E-stained colonic sections. DSS treatment induced cell wall damage, interstitial edema, and a general increase in the number of inflammatory cells in the lamina propria. Mice treated with the miR-193a-3p mimic after induction of colitis showed markedly reduced intestinal inflammation and improved histological signs (Fig. 6D). Colonic MPO activity was measured as an indicator of the extent of neutrophil infiltration. DSS-induced MPO activity was significantly decreased, by ∼50%, after treatment with the miR-193a-3p mimic (Fig. 6E). The expression of proinflammatory cytokines was also evaluated by ELISA. DSS treatment increased expressions of various proinflammatory cytokines (e.g. IL-1β, IL-6, IL-12, and IFN-γ) in the mouse colon, whereas miR-193a-3p mimic treatment reduced elevated proinflammatory cytokines induced by DSS (Fig. 6F). The miR-193a-3p mimic also prevented other inflammatory changes such as increases in the DAI and decreases in colon length (Fig. 6G).
FIGURE 5.
miR-193a-3p suppresses mucosal PepT1 in vivo in DSS-induced colitis. A, experimental design. Mice were given water alone, 3% DSS, and treated with saline, 3% DSS, and treated with mimic normal control (NC) or 3% DSS, and treated with miR-193a-3p mimic every 2 days (7 mice/group). After intracolonic treatment with the different above mentioned conditions (B), colonic PepT1 expression was determined by Western blotting and immunofluorescent staining (C). Scale bars = 50 μm.
FIGURE 6.
miR-193a-3p mimic treatment reduces the intestinal inflammatory response in DSS-induced colitis. After intracolonic treatment of mice with miR-193a-3p mimic, body weight was assessed during treatment in each group (A). Results are expressed as percent weight loss over time, and survival was monitored (B). On day 8, the mice were sacrificed, and the colons were removed for macroscopic observation (C), H&E staining of colonic sections (D), colonic myeloperoxidase (MPO) determination (E), expression of colonic cytokines (IL-1β, IL-6, IL-12, and IFN-γ), determination by ELISA (F), and DAI, colon length, and histological score, monitored daily (G). H, Western blotting for PARP cleavage and caspase-3 activation after miR-193a-3p mimic treatment with or without DSS induction. *, p < 0.05; **, p < 0.01 compared with 3% DSS mice treated with saline or mimic-control. Bars = 100 μm.
Recent studies have revealed that miR-193a-3p is associated with apoptosis and proliferation in cancer cells (25, 26). We examined two markers of apoptosis, PARP and caspase-3, to investigate the alteration in apoptosis after the introduction of miR-193a-3p in DSS-induced colitis. As shown in Fig. 6H, PARP cleavage and caspase-3 activation verified that apoptosis of epithelial cells in the miR-193a-3p-treated group was reduced compared with the control group.
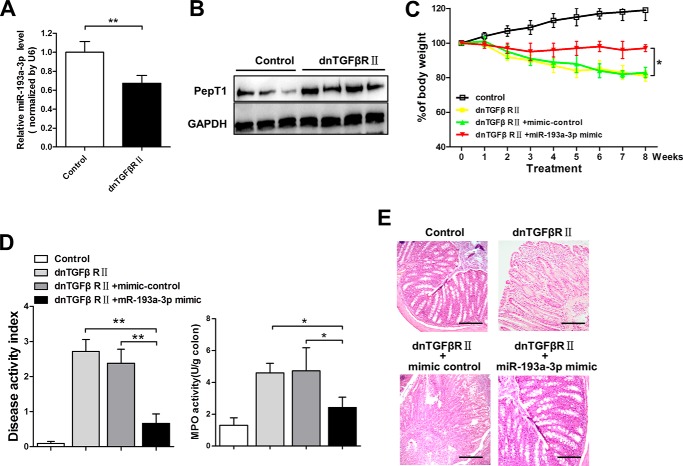
Mice transgenic for directed expression of dnTGFbRII, under the control of the CD4 promoter lacking the CD8 silencer, spontaneously develop IBD and reveal inflammatory infiltration of the large intestine (27, 28). As shown in Fig. 7, A and B, an inverse relationship between miR-193a-3p and PepT1 was also observed in the inflamed colon of dnTGFβRII mice. We further evaluated the therapeutic effects of miR-193a-3p for the spontaneously developed colitis in dnTGFβRII mice. Weight changes, colonic MPO activity, DAI, and macroscopic observation (Fig. 7, C–E) demonstrated that miR-193a-3p-treated dnTGFβRII mice developed less serve colitis.
FIGURE 7.
The therapeutic effect of miR-193a-3p in the spontaneously developed colitis of dnTGFβRII mice. qRT-PCR analysis of miR-193a-3p (A) and Western blotting of PepT1 of the inflamed colon in dnTGFβRII mice (B) are shown. C, body weight changes were monitored weekly after miR-193a mimic treatment. At the age of 20 weeks, the mice were sacrificed, and the colons were removed for the determination of DAI and colonic MPO (D) and for H&E staining of colonic sections (E). *, p < 0.05; **, p < 0.01.
The Therapeutic Effect of miR-193a-3p Mimic on DSS-induced Colitis Is Mediated by PepT1 Suppression
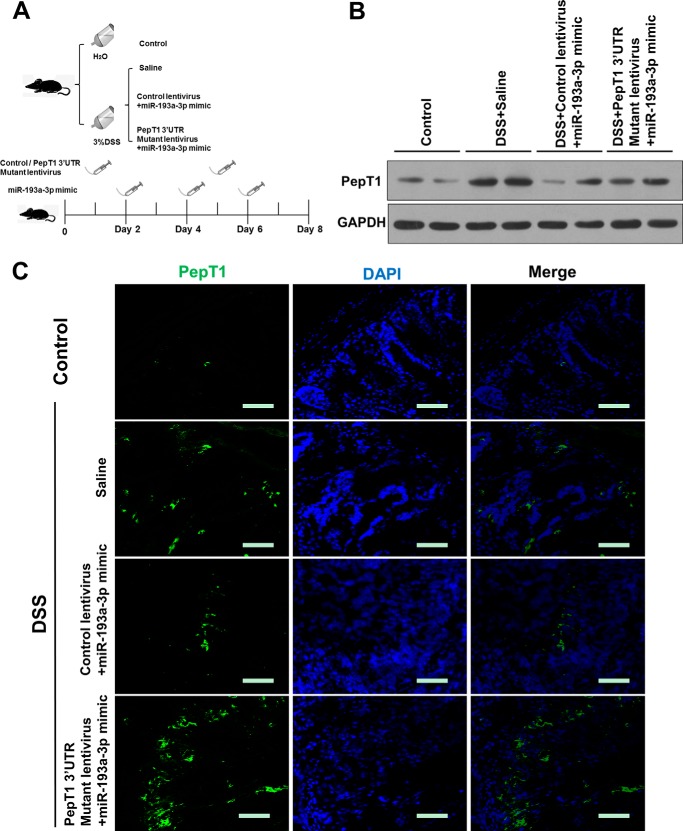
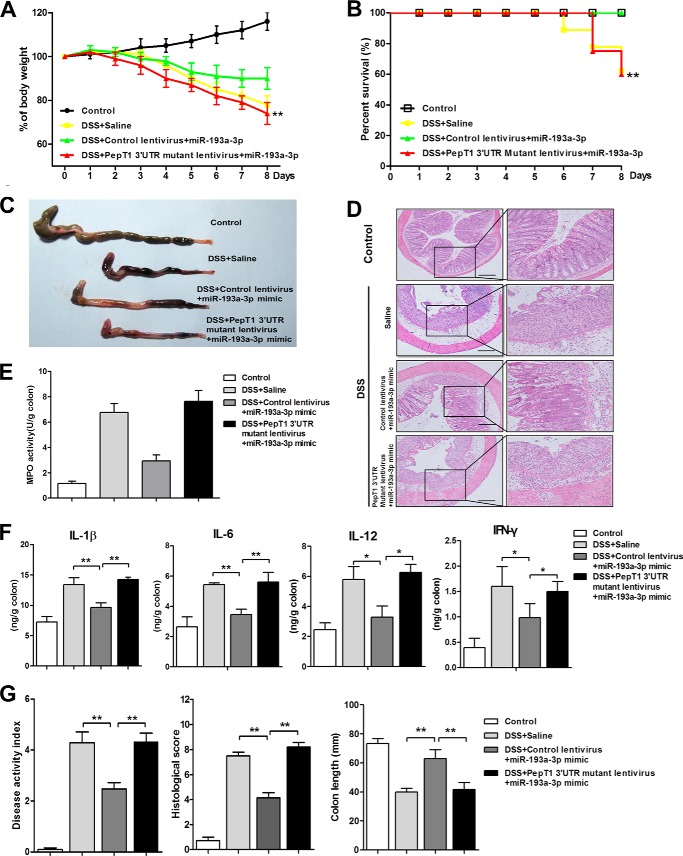
Given that administration of miR-193a-3p mimic to mice with DSS-induced colitis leads to down-regulation of PepT1, we further examined whether miR-193a-3p mimic alleviated experimental colitis through the inhibition of PepT1. A PepT1 3′-UTR-mutant lentivirus was intracolonically administered before the induction of DSS (Fig. 8A). The lentivirus significantly improved colonic PepT1 expression when compared with DSS mice with control lentivirus treatment or without any treatment (Fig. 8, B and C). The overexpression of colonic PepT1 with this lentivirus vector abolished the anti-inflammatory effect of miR-193a-3p mimic on DSS-induced colitis (Fig. 9, A–G). Taken together, these results demonstrated that the intracolonically delivered miR-193a-3p mimic regulated the expression of colonic PepT1 and subsequently improved the severity of colitis in mice.
FIGURE 8.
Colonic overexpression of PepT1 by PepT1 3′-UTR mutant lentivirus intracolonic administration in DSS colitic mice. A, experimental design. Mice were given water alone, 3% DSS and treated with saline, 3% DSS and treated with control lentivirus and miR-193a-3p mimic or 3% DSS and treated with PepT1 3′-UTR mutant lentivirus and miR-193a-3p mimic (7 mice/group). After intracolonic treatment with the different above-mentioned conditions, colonic PepT1 expression was determined by Western blotting (B) and immunofluorescent staining (C). Bars = 50 μm.
FIGURE 9.
The therapeutic effect of miR-193a-3p mimic on DSS-induced colitis is mediated by PepT1 suppression. After intracolonic treatment of the PepT1 3′-UTR mutant lentivirus and miR-193a-3p mimic in mice, body weight was assessed during treatment in each group (A). Results are expressed as percent weight loss over time. B, survival was monitored. On day 8 the mice were sacrificed, and the colons were removed for macroscopic observation (C), colonic sections were H&E-stained (D) colonic MPO was determined (E), colonic cytokines (IL-1β, IL-6, IL-12, and IFN-γ) expression was determined by ELISA (F) and DAI, colon length, and histological score were monitored daily (G). *, p < 0.05; **, p < 0.01 compared with 3% DSS mice treated with saline or control lentivirus. Bars = 100 μm.
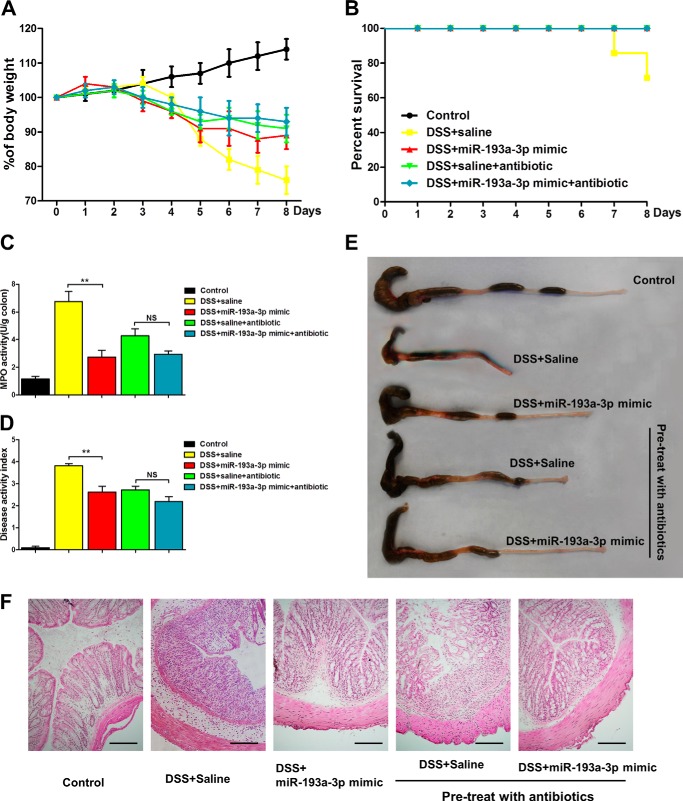
Antibiotic Treatment Eliminated the Difference in the Severity of DSS-induced Colitis with or without miR-193a-3p Administration
To further demonstrate that the anti-inflammatory effect of miR-193a-3p was driven by its repression of PepT1 and response to GI microbiota, we treated mice with broad-spectrum antibiotics to deplete colonic microbial communities for 4 weeks before introducing DSS as previously described (29). As shown in Fig. 10, A and B, DSS treatment produced a much milder impact in both mice treated with antibiotics and mice administrated with miR-193a-3p, reducing body weight <10%, compared with that in untreated mice. Similar results were also observed in inflammatory activity and at the macroscopic and histological levels (Fig. 10, C–F). Antibiotic treatment reduced the DSS-induced inflammation and eliminated the difference in the severity of DSS-induced colitis between the presence and absence of miR-193a-3p treatment. These results suggest that bacteria and/or bacterial products are crucial for aggravation of DSS-induced colitis, and miR-193a-3p exerts its anti-inflammatory effect though mediating the uptake of bacterial products by targeting colonic PepT1.
FIGURE 10.
Broad-spectrum antibiotic pretreatment reduces the severity of DSS-induced colitis and eliminates the difference between the groups of miR-193a mimic or saline. Body weight changes (A) and survival (B) were monitored daily after miR-193a mimic treatment. On day 8 the mice were sacrificed, and the colons were removed for determination of colonic MPO (C), DAI (D), macroscopic observation (E), and colonic sections H&E-staining (F). *, p < 0.05; **, p < 0.01; NS, no significant change. Bars = 50 μm.
Discussion
Among multiple mechanisms involved in the pathogenesis of IBD, the complex interactions between genetics, environment, and gut microbiota are integral to the pathogenesis and regulation of IBD (30). In this study we uncovered the role of miR-193a-3p in manipulating the uptake of the properties of colonic microbiota via modulation of PepT1 expression in colonic inflammation and extended the understanding of miRNA as a critical gene regulator involved in mediating the activation of host responses to gut microbiota.
Accumulating evidences and closer attention illustrate the importance of post-transcriptional regulation by miRNAs on multiple aspects of IBD pathogenesis. Notably, host intestinal miR-10a expression was found to be negatively regulated by gut microbiota through regulating the dendritic cell expression of IL-12/IL-23p40 (31). Similarly, commensal bacteria and proinflammatory cytokines could regulate dendritic cell and macrophage expression of IL-23p19 by inhibiting miR-107 through the interaction of TLR-TLR ligands (32). Furthermore, miR-10a was recently demonstrated to regulate chronic intestinal inflammation and maintain intestinal homeostasis by inhibiting the expression of IL-12/IL-23p40 and NOD2 as well as Th1 and Th17 cell function in IBD (12). Our data showed the differential expression of miRNAs and an inverse correlation between miR-193a-3p and PepT1 protein expression in active UC as well as among the colonic epithelial cell lines. Therefore, it is reasonable to hypothesize that miR-193a-3p is a potential post-transcriptional regulator for PepT1 expression during the colonic inflammatory process, which was well proven by the luciferase assay in this study.
It has been well established that PepT1 is expressed in CECs and immune cells in DSS-induced colitis and IBD patients and regulates proinflammatory cytokine secretion, which is dependent on bacteria and/or bacterial products (7, 33, 34). In a recent study, overexpression of colonic PepT1 was found to alter the profile of local miRNA expression/secretion in both epithelial and non-epithelial cells such as intestinal macrophages in a tissue-specific manner (35). Our observation of the dysregulated PepT1 in the colon tissues from active IBD patients is consistent with the previous studies of PepT1 involvement in aggravation of intestinal inflammation. Moreover, we demonstrated that restoration of miR-193a-3p reduced PepT1 expression and transport activity, which subsequently decreased the activation of the NF-κB signaling pathway and led to the suppression of fMLF triggered inflammation in Caco2 cells. Several studies have indicated that fMLF could stimulate a time-dependent increase of IκB-α phosphorylation in the intestinal epithelial cells, and the NF-κB pathway may play a role in the synergistic activation of intestinal immune response induced by fMLF (36, 37). Activation of the NF-κB pathway could be induced by IκB kinase and ubiquitination of the NF-κB essential modulator, which subsequently activates NOD2 by muramyl-dipeptide, which derives from peptidoglycan of bacteria (38). On the other hand, the overexpression of miR-193a-3p did not affect the activation of the NF-κB pathway in HT29 cells, which were absent of PepT1 expression. This observation supported that the impaction of the NF-κB pathway of miR-193a-3p was through the regulation of PepT1. Collectively, our data indicated that the inhibition of PepT1 by miR-193a-3p could contribute to blocking the gateway for gut microbial products to trigger the innate immune responses.
PepT1 overexpression increased intestinal inflammation in a NOD2-dependent manner and resulted in an aggravation of DSS-induced colitis in mice (33). Interestingly, intestinal epithelial cell-specific hPepT1 overexpression in villin-hPepT1 transgenic mice increased the severity of inflammation induced by DSS, but not 2,4,6-trinitrobenzenesulfonic acid (TNBS), suggesting PepT1 expression specifically in the colon does not contribute to TNBS-induced colitis. This observation might be partially explained by the different mechanisms of these two chemically induced models of intestinal inflammation in which DSS seems to be directly toxic to CECs of the basal crypts, whereas TNBS is believed to induce a T cell-mediated response against hapten-modified autologous proteins and luminal antigens (39). Based on our in vitro results, the regulation of PepT1 by miR-193a-3p and the resulting impact on the development of colitis were further investigated in vivo. Intracolonic administration of miR-193a-3p mimics effectively reduced weight loss, colon shortening, and colonic MPO activity in DSS-induced colitis in mice, whereas colonic PepT1 overexpression via lentivirus abolished the therapeutic effects of miR-193a-3p in DSS-induced colitis. Indeed, DSS-induced colitis may not be an ideal model for studying the effects of subtle changes in epithelial inflammatory signaling and the interactions between bacterial products and immune cells within the lamina propria. Confirmation of the efficacy of miR-193a-3p in reducing colitis in dnTGFβRII mice strengthened the relevance of miR-193a-3p-mediated regulation of PepT1 to IBD pathogenesis. Similarly, Huang et al. (40) up-regulation of miR-141 regulated leukocyte infiltration and alleviated experimental colitis both in the TNBS-induced and IL-10 KO colitic colon. Importantly, we also showed that antibiotics reduced the DSS-induced inflammation in both the presence and absence of miR-193a-3p, and the difference between the two groups was eliminated. These results suggest that bacteria and/or bacterial products play an important role in the exacerbation of DSS-induced colitis in mice and further suggest that miR-193a-3p is crucial for regulating PepT1-mediated bacterial products in inducing intestinal inflammation. These data are consistent with a recent work demonstrating that knocking down the PepT1 expression decreases chemotaxis of immune cells recruited during intestinal inflammation, which is dependent on bacteria and/or bacterial products (34).
miRNAs can modulate cellular activities through the regulation of extensive gene networks and may exert different functions that depend on the cellular context (41). Recent studies have revealed that miR-193a-3p is associated with apoptosis and proliferation of human malignancies, such as hepatocellular carcinoma and glioblastoma. Kwon et al. (26) presented a novel miR-193a-3p-dependent mechanism for regulating Mcl-1 expression and inducing apoptosis, whereas Salvi et al. (25) found that miR-193a-3p was dysregulated in tumor tissues from patients, and transfection of miR-193a-3p decreased proliferation and increased apoptosis in HCC cell lines. We also analyzed PARP cleavage and caspase-3 activation to investigate the alteration in apoptosis after miR-193a-3p was introduced in DSS-induced colitis. As shown in Fig. 6H, PARP cleavage and caspase-3 activation verified that apoptosis of epithelial cells in the miR-193a-3p-treated group was reduced compared with the control group. Our results supported that increased apoptosis and decreased proliferation of the epithelium takes place in the acute phase of DSS colitis (42). These results indicated that miR-193a-3p plays an important role in mediating epithelial cell survival in vivo by targeting PepT1 in our model, although miR-193a-3p was observed to induce apoptosis in cancer cell lines. In our study the apoptosis of epithelial cells in the miR-193a-3p-treated group was hampered compared with controls, which might be explained by that increased apoptosis and decreased proliferation of CECs take the dominant place during the development of DSS colitis (42). The survival of CECs might be attributed to the reduced intestinal inflammatory response by miR-193a-3p-mediated PepT1 regulation, which needs more work in depth for better understanding its role in epithelial cells.
As previously reported, PepT1 expression could be regulated by miR-92b, with subsequent reduced PepT1 transport activity and bacterial peptide-induced proinflammatory responses, in Caco2-BBE cells (43). Hence, it is plausible that there are multiple mechanisms underlying the regulation of PepT1. Our study cannot rule out to the possibility that other miRNAs are involved in the regulation of colonic PepT1 here due to the limitation of sample size and miRNAs involved, and future studies in larger populations and may be helpful to address this issue. The potential of multiple-regulation of PepT1 indeed points to an essential role for host defense against microbiota in IBD. In this regard, the current results focus ever-closer attention on the molecular mediation of the host immune response to gut microbes during the process of intestinal inflammation.
In summary, our results indicated that miR-193a-3p is involved in the pathogenesis of IBD, at least in part by targeting colonic PepT1, thus inhibiting the uptake of bacterial products and reducing the activation of host immune responses via the NF-κB pathway. Intracolonic delivery of miR-193a-3p to the colon ameliorated the severity of DSS-induced colitis and spontaneously developed colitis in mice, suggesting the promise of miR-193a-3p as an anti-inflammatory agent.
Acknowledgments
We thank Professor Xiong Ma from Shanghai Institute of Digestive Disease for his generous gift of dnTGFβRII mice. We also thank Professors Zhiping Li and James Potter from the Johns Hopkins Medical Institutions for insightful discussions and editorial assistance.
This work was supported by National Natural Science Foundation of China Grants 81200281, 81101330, 31271378, 81250044, and J1103512 and Natural Science Foundation of Jiangsu Province Grants BK2011013 and BK2012014. The authors declare that they have no conflicts of interest with the contents of this article.
- IBD
- inflammatory bowel disease
- CD
- Crohn's disease
- UC
- ulcerative colitis
- GI
- gastrointestinal
- microRNA
- miRNA
- fMLF
- N-formylmethionylleucyl-phenylalanine
- DSS
- dextran sodium sulfate
- CEC
- colonic epithelial cell
- qRT-PCR
- quantitative RT-PCR
- PEI
- polyethylenimine
- TRITC
- tetramethylrhodamine isothiocyanate
- DAI
- disease activity index
- MPO
- myeloperoxidase
- PARP
- poly(ADP-ribose) polymerase
- TNBS
- 2,4,6-trinitrobenzenesulfonic acid.
References
- 1. Saleh M., Elson C. O. (2011) Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity 34, 293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jostins L., Ripke S., Weersma R. K., Duerr R. H., McGovern D. P., Hui K. Y., Lee J. C., Schumm L. P., Sharma Y., Anderson C. A., Essers J., Mitrovic M., Ning K., Cleynen I., Theatre E., Spain S. L., Raychaudhuri S., Goyette P., Wei Z., Abraham C., Achkar J. P., Ahmad T., Amininejad L., Ananthakrishnan A. N., Andersen V., Andrews J. M.., Baidoo L., Balschun T., Bampton P. A., Bitton A., Boucher G., Brand S., Büning C., Cohain A., Cichon S., D'Amato M., De Jong D., Devaney K. L., Dubinsky M., Edwards C., Ellinghaus D., Ferguson L. R., Franchimont D., Fransen K., Gearry R., Georges M., Gieger C., Glas J., Haritunians T., Hart A., Hawkey C. (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adibi S. A. (1997) The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology 113, 332–340 [DOI] [PubMed] [Google Scholar]
- 4. Merlin D., Si-Tahar M., Sitaraman S. V., Eastburn K., Williams I., Liu X., Hediger M. A., Madara J. L. (2001) Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology 120, 1666–1679 [DOI] [PubMed] [Google Scholar]
- 5. Adibi S. A. (2003) Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G779–G788 [DOI] [PubMed] [Google Scholar]
- 6. Buyse M., Tsocas A., Walker F., Merlin D., Bado A. (2002) PepT1-mediated fMLP transport induces intestinal inflammation in vivo. Am. J. Physiol. Cell Physiol. 283, C1795–C1800 [DOI] [PubMed] [Google Scholar]
- 7. Dalmasso G., Nguyen H. T., Charrier-Hisamuddin L., Yan Y., Laroui H., Demoulin B., Sitaraman S. V., Merlin D. (2010) PepT1 mediates transport of the proinflammatory bacterial tripeptide l-Ala-γ-d-Glu-meso-DAP in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G687–G696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalmasso G., Charrier-Hisamuddin L., Nguyen H. T., Yan Y., Sitaraman S., Merlin D. (2008) PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology 134, 166–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pasquinelli A. E. (2012) MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 13, 271–282 [DOI] [PubMed] [Google Scholar]
- 10. Iborra M., Bernuzzi F., Invernizzi P., Danese S. (2012) MicroRNAs in autoimmunity and inflammatory bowel disease: crucial regulators in immune response. Autoimmun. Rev. 11, 305–314 [DOI] [PubMed] [Google Scholar]
- 11. Dai R., Ahmed S. A. (2011) MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl. Res. 157, 163–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu W., He C., Liu C., Cao A. T., Xue X., Evans-Marin H. L., Sun M., Fang L., Yao S., Pinchuk I. V., Powell D. W., Liu Z., Cong Y. (2014) miR-10a inhibits dendritic cell activation and Th1/Th17 cell immune responses in IBD. Gut 10.1136/gutjnl-2014-307980 [DOI] [PubMed] [Google Scholar]
- 13. Koukos G., Polytarchou C., Kaplan J. L., Morley-Fletcher A., Gras-Miralles B., Kokkotou E., Baril-Dore M., Pothoulakis C., Winter H. S., Iliopoulos D. (2013) MicroRNA-124 regulates STAT3 expression and is down-regulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology 145, 842–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y., Wang C., Liu Y., Tang L., Zheng M., Xu C., Song J., Meng X. (2013) miR-122 targets NOD2 to decrease intestinal epithelial cell injury in Crohn's disease. Biochem. Biophys. Res. Commun. 438, 133–139 [DOI] [PubMed] [Google Scholar]
- 15. Bian Z., Li L., Cui J., Zhang H., Liu Y., Zhang C. Y., Zen K. (2011) Role of miR-150-targeting c-Myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. J. Pathol. 225, 544–553 [DOI] [PubMed] [Google Scholar]
- 16. Li L. M., Hou D. X., Guo Y. L., Yang J. W., Liu Y., Zhang C. Y., Zen K. (2011) Role of microRNA-214-targeting phosphatase and tensin homolog in advanced glycation end product-induced apoptosis delay in monocytes. J. Immunol. 186, 2552–2560 [DOI] [PubMed] [Google Scholar]
- 17. Hindlet P., Bado A., Farinotti R., Buyse M. (2007) Long-term effect of leptin on H+-coupled peptide cotransporter 1 activity and expression in vivo: evidence in leptin-deficient mice. J. Pharmacol. Exp. Ther. 323, 192–201 [DOI] [PubMed] [Google Scholar]
- 18. Hindlet P., Bado A., Kamenicky P., Deloménie C., Bourasset F., Nazaret C., Farinotti R., Buyse M. (2009) Reduced intestinal absorption of dipeptides via PepT1 in mice with diet-induced obesity is associated with leptin receptor down-regulation. J. Biol. Chem. 284, 6801–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y., Liu D., Chen X., Li J., Li L., Bian Z., Sun F., Lu J., Yin Y., Cai X., Sun Q., Wang K., Ba Y., Wang Q., Wang D., Yang J., Liu P., Xu T., Yan Q., Zhang J., Zen K., Zhang C. Y. (2010) Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell 39, 133–144 [DOI] [PubMed] [Google Scholar]
- 20. Chen X., Ba Y., Ma L., Cai X., Yin Y., Wang K., Guo J., Zhang Y., Chen J., Guo X., Li Q., Li X., Wang W., Zhang Y., Wang J., Jiang X., Xiang Y., Xu C., Zheng P., Zhang J., Li R., Zhang H., Shang X., Gong T., Ning G., Wang J., Zen K., Zhang J., Zhang C. Y. (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 18, 997–1006 [DOI] [PubMed] [Google Scholar]
- 21. Shi B., Song D., Xue H., Li N., Li J. (2006) PepT1 mediates colon damage by transporting fMLP in rats with bowel resection. J. Surg. Res. 136, 38–44 [DOI] [PubMed] [Google Scholar]
- 22. Atreya I., Atreya R., Neurath M. F. (2008) NF-κB in inflammatory bowel disease. J. Intern. Med. 263, 591–596 [DOI] [PubMed] [Google Scholar]
- 23. Andersen V., Christensen J., Ernst A., Jacobsen B. A., Tjønneland A., Krarup H. B., Vogel U. (2011) Polymorphisms in NF-κB, PXR, LXR, PPARγ and risk of inflammatory bowel disease. World J. Gastroenterol. 17, 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bantel H., Domschke W., Schulze-Osthoff K., Kaskas B., Gregor M. (2000) Abnormal activation of transcription factor NF-κB involved in steroid resistance in chronic inflammatory bowel disease. Am. J. Gastroenterol. 95, 1845–1846 [DOI] [PubMed] [Google Scholar]
- 25. Salvi A., Conde I., Abeni E., Arici B., Grossi I., Specchia C., Portolani N., Barlati S., De Petro G. (2013) Effects of miR-193a and sorafenib on hepatocellular carcinoma cells. Mol. Cancer 12, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kwon J. E., Kim B. Y., Kwak S. Y., Bae I. H., Han Y. H. (2013) Ionizing radiation-inducible microRNA miR-193a-3p induces apoptosis by directly targeting Mcl-1. Apoptosis 18, 896–909 [DOI] [PubMed] [Google Scholar]
- 27. Gorelik L., Flavell R. A. (2000) Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12, 171–181 [DOI] [PubMed] [Google Scholar]
- 28. Ando Y., Yang G. X., Tsuda M., Kawata K., Zhang W., Nakajima T., Tsuneyama K., Leung P., Lian Z. X., Okazaki K., Ridgway W. M., Norman G. L., Ansari A. A., He X. S., Coppel R. L., Gershwin M. E. (2012) The immunobiology of colitis and cholangitis in interleukin-23p19 and interleukin-17A deleted dominant negative form of transforming growth factor β receptor type II mice. Hepatology 56, 1418–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garrett W. S., Lord G. M., Punit S., Lugo-Villarino G., Mazmanian S. K., Ito S., Glickman J. N., Glimcher L. H. (2007) Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 131, 33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dalal S. R., Chang E. B. (2014) The microbial basis of inflammatory bowel diseases. J. Clin. Invest. 124, 4190–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xue X., Feng T., Yao S., Wolf K. J., Liu C. G., Liu X., Elson C. O., Cong Y. (2011) Microbiota down-regulates dendritic cell expression of miR-10a, which targets IL-12/IL-23p40. J. Immunol. 187, 5879–5886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xue X., Cao A. T., Cao X., Yao S., Carlsen E. D., Soong L., Liu C. G., Liu X., Liu Z., Duck L. W., Elson C. O., Cong Y. (2014) Down-regulation of microRNA-107 in intestinal CD11c(+) myeloid cells in response to microbiota and proinflammatory cytokines increases IL-23p19 expression. Eur. J. Immunol. 44, 673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dalmasso G., Nguyen H. T., Ingersoll S. A., Ayyadurai S., Laroui H., Charania M. A., Yan Y., Sitaraman S. V., Merlin D. (2011) The PepT1-NOD2 signaling pathway aggravates induced colitis in mice. Gastroenterology 141, 1334–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ayyadurai S., Charania M. A., Xiao B., Viennois E., Merlin D. (2013) PepT1 expressed in immune cells has an important role in promoting the immune response during experimentally induced colitis. Lab. Invest 93, 888–899 [DOI] [PubMed] [Google Scholar]
- 35. Ayyadurai S., Charania M. A., Xiao B., Viennois E., Zhang Y., Merlin D. (2014) Colonic miRNA expression/secretion, regulated by intestinal epithelial PepT1, plays an important role in cell-to-cell communication during colitis. PloS ONE 9, e87614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carlson R. M., Vavricka S. R., Eloranta J. J., Musch M. W., Arvans D. L., Kles K. A., Walsh-Reitz M. M., Kullak-Ublick G. A., Chang E. B. (2007) fMLP induces Hsp27 expression, attenuates NF-κB activation, and confers intestinal epithelial cell protection. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1070–G1078 [DOI] [PubMed] [Google Scholar]
- 37. Chen L. Y., Pan W. W., Chen M., Li J. D., Liu W., Chen G., Huang S., Papadimos T. J., Pan Z. K. (2009) Synergistic induction of inflammation by bacterial products lipopolysaccharide and fMLP: an important microbial pathogenic mechanism. J. Immunol. 182, 2518–2524 [DOI] [PubMed] [Google Scholar]
- 38. Burns K. A., Martinon F. (2004) Inflammatory diseases: is ubiquitinated NEMO at the hub? Curr. Biol. 14, R1040–R1042 [DOI] [PubMed] [Google Scholar]
- 39. Wirtz S., Neufert C., Weigmann B., Neurath M. F. (2007) Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2, 541–546 [DOI] [PubMed] [Google Scholar]
- 40. Huang Z., Shi T., Zhou Q., Shi S., Zhao R., Shi H., Dong L., Zhang C., Zeng K., Chen J., Zhang J. (2014) miR-141 Regulates colonic leukocytic trafficking by targeting CXCL12β during murine colitis and human Crohn's disease. Gut 63, 1247–1257 [DOI] [PubMed] [Google Scholar]
- 41. Kasinski A. L., Slack F. J. (2011) Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev. Cancer 11, 849–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Araki Y., Mukaisyo K., Sugihara H., Fujiyama Y., Hattori T. (2010) Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol. Rep. 24, 869–874 [DOI] [PubMed] [Google Scholar]
- 43. Dalmasso G., Nguyen H. T., Yan Y., Laroui H., Charania M. A., Obertone T. S., Sitaraman S. V., Merlin D. (2011) MicroRNA-92b regulates expression of the oligopeptide transporter PepT1 in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G52–G59 [DOI] [PMC free article] [PubMed] [Google Scholar]