Background: The identity of calcium channels in the thyroid is undefined.
Results: TRPC1 functions as a major regulator of S1P and VEGF receptors via a calcium-dependent mechanism. This is important for cell migration.
Conclusion: We have defined a novel physiological role for the TRPC1 channel.
Significance: This study explains how TRPC1 regulates receptor expression and migration in thyroid cancer cells.
Keywords: cancer, receptor, sphingosine-1-phosphate (S1P), thyroid, transient receptor potential channels (TRP channels), vascular endothelial growth factor (VEGF)
Abstract
The identity of calcium channels in the thyroid is unclear. In human follicular thyroid ML-1 cancer cells, sphingolipid sphingosine 1-phosphate (S1P), through S1P receptors 1 and 3 (S1P1/S1P3), and VEGF receptor 2 (VEGFR2) stimulates migration. We show that human thyroid cells express several forms of transient receptor potential canonical (TRPC) channels, including TRPC1. In TRPC1 knockdown (TRPC1-KD) ML-1 cells, the basal and S1P-evoked invasion and migration was attenuated. Furthermore, the expression of S1P3 and VEGFR2 was significantly down-regulated. Transfecting wild-type ML-1 cells with a nonconducting TRPC1 mutant decreased S1P3 and VEGFR2 expression. In TRPC1-KD cells, receptor-operated calcium entry was decreased. To investigate whether the decreased receptor expression was due to attenuated calcium entry, cells were incubated with the calcium chelator BAPTA-AM (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid). In these cells, and in cells where calmodulin and calmodulin-dependent kinase were blocked pharmacologically, S1P3 and VEGFR2 expression was decreased. In TRPC1-KD cells, both hypoxia-inducible factor 1α expression and the secretion and activity of MMP2 and MMP9 were attenuated, and proliferation was decreased in TRPC1-KD cells. This was due to a prolonged G1 phase of the cell cycle, a significant increase in the expression of the cyclin-dependent kinase inhibitors p21 and p27, and a decrease in the expression of cyclin D2, cyclin D3, and CDK6. Transfecting TRPC1 to TRPC1-KD cells rescued receptor expression, migration, and proliferation. Thus, the expression of S1P3 and VEGFR2 is mediated by a calcium-dependent mechanism. TRPC1 has a crucial role in this process. This regulation is important for the invasion, migration, and proliferation of thyroid cancer cells.
Introduction
Of all the endocrine cancers, thyroid cancer is the most common. According to the National Cancer Institute of the United States, the number of estimated new cases of thyroid cancer in the country was 62,980 in 2014 (1). Human follicular thyroid cancer is an aneuploid form of cancer (2) with less prevalence and usually good prognosis. However, in some cases where these cells may dedifferentiate, or because of Ras and PAX-8-peroxisome proliferator-activated receptor γ mutations (3), very aggressive tumors may arise, metastasizing to bones and lungs. The treatment of these aggressive forms of thyroid cancers has proven difficult, thus warranting novel approaches to understand the mechanisms.
In several forms of cancer, calcium signaling plays an important role in mediating the proliferative, invasive and migratory responses. In these cancer cells, different calcium channels belonging to the transient receptor potential superfamily of cation channels have proven important in this process (4). The transient receptor canonical (TRPC)2 subfamily comprises six members in man (TRPC1 and TRPC3–TRPC7). These channels participate in both receptor-operated calcium entry, as well as store-operated calcium entry (together with the STIM1 and Orai1 proteins). Of the TRPC channels, the TRPC1 channels are widely expressed in human tissues and are involved in regulation of many cellular processes, including cancer cell invasion, migration, and proliferation (5–8). The structure of TRPC1 enables it to interact with other TRPC channels to form complexes and with adaptor proteins and signaling proteins like G-coupled receptor proteins, phospholipase C, and calcium regulatory proteins. The role of TRPC1 in regulation of cellular calcium homeostasis in both normal and cancer cells is well known (9). Sphingosine 1-phosphate (S1P) is a bioactive lipid that activates the S1P receptors 1–5 (S1P1–5) and downstream signaling cascades to regulate a multitude of physiological and pathophysiological processes in cells, including cell invasion and migration (10–12). We have previously shown that S1P enhances migration of thyroid follicular ML-1 cancer cells through activation of the pro-migratory S1P1 and S1P3 (13, 14). Furthermore, we have shown that activation of the vascular endothelial growth factor receptor 2 (VEGFR2) is important for activation of migration of these cells and that VEGFR2 forms a signaling complex with S1P1 and S1P3 that is necessary for migration (15).
In the present study, we investigated the expression of TRPC channels in thyroid cells and their importance in regulating S1P-evoked invasion and migration. We report for the first time that in normal human thyroid cells, as well as in follicular thyroid cancer cells, several forms of TRPC channels are expressed, including TRPC1. We showed that in thyroid follicular ML-1 cancer cells, receptor-operated calcium entry, but not store-operated calcium entry, was significantly decreased in stable TRPC1 knockdown cells, compared with control mock transfected ML-1 cells. Furthermore, the basal invasion and migration was decreased, whereas the S1P-evoked invasion and migration was abolished in TRPC1 knockdown cells. We also showed that in TRPC1 knockdown cells, the expression of S1P3, VEGFR2, and H1F-1α was decreased, as well as the secretion and activity of MMP2 and MMP9. In addition, the proliferation was significantly decreased in TRPC1 knockdown cells, compared with control cells. We conclude that the expression of S1P3 and VEGFR2 is mediated by a calcium-dependent mechanism and that TRPC1 has a crucial role in this process. This regulation is important for ML-1 cancer cell invasion, migration, and proliferation.
Experimental Procedures
DMEM, BSA, fatty acid-free BSA, ethidium bromide, poly-l-lysine, hexadimethrine bromide, puromycin, and JumpStart Taq DNA polymerase were purchased from Sigma-Aldrich. RPMI 1640 medium (without l-glutamine) was from Lonza (Basel, Switzerland). FBS, 2.5% trypsin, l-glutamine, penicillin/streptomycin (P/S), OptiMEM, and F-12 (Ham's nutrient medium) were from Gibco Life Technologies. S1P was from Biomol (Plymouth, PA). Primary antibodies against S1P1, S1P2, S1P3, TRPC1, PKC isoforms, and Hsc70 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). HRP-conjugated goat anti-rabbit IgG and the Aurum total RNA isolation kit were from Bio-Rad. Primary antibodies against HIF-1α,VEGFR2, p21waf1/cip1, p27kip1, cyclin D1, cyclin D2, cyclin D3, cdk2, cdk4, cdk6, MMP2, ERK1/2, and HRP-conjugated anti-rat and anti-mouse IgG were from Cell Signaling Technology (Denver, MA). Primary antibody against MMP9 was purchased from Abcam (Cambridge, MA). Cell culture plastic ware and human collagen type IV were from Becton Dickinson, and Transwell inserts for migration assays were from Corning, Inc. (Corning, NY). The bicinchoninic acid protein assay reagent kit was from Pierce. All the chemicals and reagents used were of molecular biology and reagent grades. Fura-2 AM was from Molecular Probes (Eugene, OR). Thapsigargin was from Alexis Corporation (San Diego, CA). GsMTx-4 was purchased from Peptides International, Inc. (Louisville, KY). KN-93 and W-13 hydrochloride were purchased from Tocris Bioscience R&D Systems Company (Abingdon, UK). RevertAid reverse transcriptase, RiboLock RNase inhibitor, random hexamer primers, dNTPs, and GeneRuler 100-bp Plus DNA Ladder were from Thermo Fisher Scientific (Waltham, MA). KAPA Probe Fast Master Mix was from Kapa Biosystems (Boston, MA), and the Universal Probe Library probes were from Roche.
Cell Culture
The human ML-1 follicular thyroid cancer cells were cultured in DMEM supplemented with 10% FBS, 1% l-glutamine, and 1% P/S. FTC-133 thyroid follicular cancer cells were obtained from Banca Biologica e Cell Factory, National Institute of Cancer Research (Genova, Italy). The cells were grown in DMEM and F-12 (Ham's) medium (1:1) supplemented with 10% FBS and 2 mm l-glutamine and 1% P/S. Wild-type HEK-293 cells were cultured in DMEM supplemented with 10% FBS, 1% P/S, and 1% l-glutamine. The human immortal keratinocyte HaCat cells were grown in DMEM supplemented with 10% FBS, 1% P/S, and 1% l-glutamine. Normal human follicular thyroid cell line N-Thy-ori-3–1 was purchased from Health Protection Agency Culture Collection (Salisbury, UK). The cells were grown in RPMI 1640 supplemented with 10% FBS, 1% P/S, and 1% l-glutamine. Rat thyroid FRTL-5 cells were grown in Coon's modified Ham's F-12 medium supplemented with 5% calf serum, 50 units/ml penicillin and 50 μg/ml streptomycin and six hormones including 10 μg/ml insulin, 0.3 milliunits/ml TSH, 10 nm hydrocortisone, 10 ng/ml somatostatin, 5 μg/ml transferrin, and 10 ng/ml tripeptide Gly-His-Lys. The stable FRTL-5 TRPC2 knockdown cells (FRTL-5 TRPC2-KD) and the shRNA control transfected cells (MOCK) were generated by using shRNA plasmids and grown as described elsewhere (16). The cell cultures were maintained in a water-saturated atmosphere of 5% CO2 and 95% air at 37 °C in the incubators.
Viral Transduction and Generation of Stable Cell Lines
Cells were plated on 12-well plates. The transduction was performed according to the manufacturer's instructions using nontargeting shRNA lentivirus particles, and TRPC1-targeting lentiviral particles (Sigma). The sequences are shown in Table 1. After 48 h, the medium was changed to the medium containing 0.5 μg/ml puromycin. The cells were cultured with this medium hereafter. The knockdown of TRPC1 was measured on mRNA by quantitative PCR and at the protein level by Western blotting.
TABLE 1.
The sequence of control (Tcf) and TRPC1-targeting shRNA
| Symbol | TRC ID | Gene ID | Nucleotide sequence |
|---|---|---|---|
| Tcf25 | TRCN0000190494 | 66855 | 5′-CGGGCCTCTGTCTCCCAAATGTTACTCGAGTAACATTTGGGAGACAGAGGCTTTTTTG-3′ |
| TRPC1 | TRCN0000043998 | 7220 | 5′-CCGGGCCCACCTGTAAGAAGATAATCTCGAGATTATCTTCTTACAGGTGGGCTTTTTG-3′ |
Transient Transfections
Approximately 4 million cells were pelleted and resuspended in 400 μl OptiMEM together with 20 μg of the control plasmid, the shRNA TRPC1 plasmid, the pore-mutated TRPC1 plasmid, or the HA-TRPC1 plasmid (pTRPC1). The cells were electroporated at 975 microfarads and 240 V and were grown in respective media for 48 h before the start of the experiments.
Qualitative End Point PCR and Quantitative Real Time PCR
RNA was extracted with Aurum Total RNA mini kit. The human normal thyroid RNA and the human papillary thyroid cancer RNA were obtained from BioChain Institute, Inc. The human follicular thyroid cancer RNA was purchased from OriGene Technologies, Inc. (Rockville, MD). RNA integrity was checked by gel electrophoresis. RNA purity and concentrations were determined spectrophotometrically. cDNA was prepared with RevertAid reverse transcriptase from equal amounts of RNA. Reaction mixtures lacking either reverse transcriptase or RNA were used as negative controls. Primer information is in Table 2. Qualitative end point PCR was performed with JumpStart Taq DNA polymerase and a PTC-200 Thermal cycler (MJ Research, Waltham, MA). PCR products were run on ethidium bromide-containing agarose gels and visualized with UV light. GeneRuler 100-bp Plus DNA Ladder was used as a DNA size marker, HEK-293 and HaCat were used samples as positive controls, and β-actin was used as a reference gene. Quantitative real time PCR assays were designed with the Universal Probe Library assay design center. Universal Probe Library probe #10 was used for TRPC1 and probe #18 for the reference gene PBGD. RT-PCR was performed with KAPA Probe Fast Master Mix and the StepOnePlus real time PCR system (Applied Biosystems) using the relative standard curve method.
TABLE 2.
Primer Information
| Gene | Primer sequences | Annealing T °C | Amplicon bp | |
|---|---|---|---|---|
| End point PCR | TRPC1 | Forward 5′-TCCTTAGCGCATGTGGCAAT-3′ | 61.3 | 506 |
| Reverse 5′-AGATCTTGGCGCAGTTCGTT-3′ | ||||
| TRPC3 | Forward 5′-CCTCAGACAGGTTCGAAGGC-3′ | 61.6 | 469 | |
| Reverse 5′-TTTGCAGGGAGGATGTACGC-3′ | ||||
| TRPC4 | Forward 5′-TTGCCTCTCAGCACATCGAC-3′ | 57.0 | 943 | |
| Reverse 5′-GCTCGCCTCCCTATTGTTCC-3′ | ||||
| TRPC5 | Forward 5′-CGCTCCTTCATGGGTCCTTC-3′ | 63.2 | 774 | |
| Reverse 5′-CGGCAACAAGACCAGCAAAG-3′ | ||||
| TRPC6 | Forward 5′-AAGATGGGACACGGTTCTCC-3′ | 59.3 | 526 | |
| Reverse 5′-TGCTGTTGGCAGTTTGGATG-3′ | ||||
| TRPC7 | Forward 5′-AAGCTAGGACGAACCCTGAG-3′ | 55.0 | 713 | |
| Reverse 5′-AACGCTGGGTTGTATTTGGC-3′ | ||||
| β-Actin | Forward 5′-CACACTGTGCCCATCTACGA-3′ | 63.0 | ||
| Reverse 5′-CCATCTCTTGCTCGAAGTCC-3′ | ||||
| RT-PCR | TRPC1 | Forward 5′-GAGAGCATTTGAACTTAGTGCTGA-3′ | 60.0 | 93 |
| Reverse 5′-TTACATTGCCGGGCTAGTTC-3′ | ||||
| PBGD | Forward 5′-CGCATCTGGAGTTCAGGAGTA-3′ | 60.0 | 90 | |
| Reverse 5′-CCAGGATGATGGCACTGA-3′ |
Measurements of [Ca2+]i in Single Cells
Cells were processed and analyzed as described elsewhere (16). A HAMAMATSU digital camera C10600 ORCA-R2 with controller (Photonics K.K.) was used to capture fluorescence images at 1–3 s to avoid bleaching. Images were processed using the Axon Imaging Workbench 6 software (INDEC BioSystems, Santa Clara, CA). The F340/F380 ratio was used as a measure of intracellular free calcium concentrations.
Measurements of [Ca2+]i in Cell Suspension
The medium was aspirated, and the FRTL-5 cells were harvested with Hanks' balanced salt solution buffer lacking Ca2+ but containing 0.02% EDTA and 0.1% trypsin. The cells were washed three times by pelleting and then incubated with 1 μm Fura 2-AM for 30 min at 37 °C. The cells were then washed twice with Hanks' balanced salt solution buffer, incubated for at least 10 min at room temperature, and washed once more. Fluorescence was measured at 37 °C with a Hitachi F2000 fluorimeter using excitation wavelengths of 340 and 380 nm and detecting emission at 510 nm. The signal was calibrated by the addition of 1 mm CaCl2 and Triton X-100 (maximum fluorescence) and then chelation of extracellular Ca2+ with 5 mm EGTA and the addition of Tris base to elevate the pH above 8.3 (minimum fluorescence). [Ca2+]i was calculated as described by (17), using a computer program designed for the fluorimeter with a Kd value of 224 nm for Fura 2.
Migration and Invasion Assays
Migration and invasion assays were performed as described previously (18). In some experiments, the cells were preincubated with 10 μm KN-93, 100 μm W-13 hydrochloride, or 2 μm GsMTx-4 for 1 h, respectively, at 37 °C. The inhibitors were present in both the upper and lower chambers. The cells were allowed to migrate for 8 or 16 h.
Zymography
ML-1 MOCK and TRPC1-KD cells were grown on 35-mm plates up to 80% confluency. On the day of the experiment, the medium was changed to fresh medium (1 ml on each plate). After 6 h, the medium was collected. Equal volumes of medium were mixed with loading buffer (0.1 m Tris-phosphate buffer, pH 6.8, containing 20% glycerol, 6% SDS, and 0.04% bromphenol blue). The samples were electrophoresed with the 10% SDS gels containing gelatin (2.65 mg/ml). Next, the gels were incubated in Zymo buffer (50 mm Tris-HCl containing 2.5% Tween 80 and 0.02 NaN3, pH 7.5) for 30 min. The gels were then incubated in Zymo buffer containing 1 μm ZnCl2 and 5 mm CaCl2 for 30 min. For gelatinolytic activity gels were incubated at 37 °C for overnight in buffer containing 50 mm Tris-HCl, 5 mm CaCl2, 1 μm ZnCl2, and 0.02% NaN3 (pH 7.5). The gelatin degradation was visualized under UV light, and after that, the gels were stained with Coomassie Blue R250 for 1 h. The gelatinolytic activity was visualized as clear bands against blue background on stained gels. The clear bands after destaining, as shown in Fig. 7 (C and D), were quantified by the program ImageJ. The data were normalized with the respective total protein concentrations of the respective cells in the culture plates.
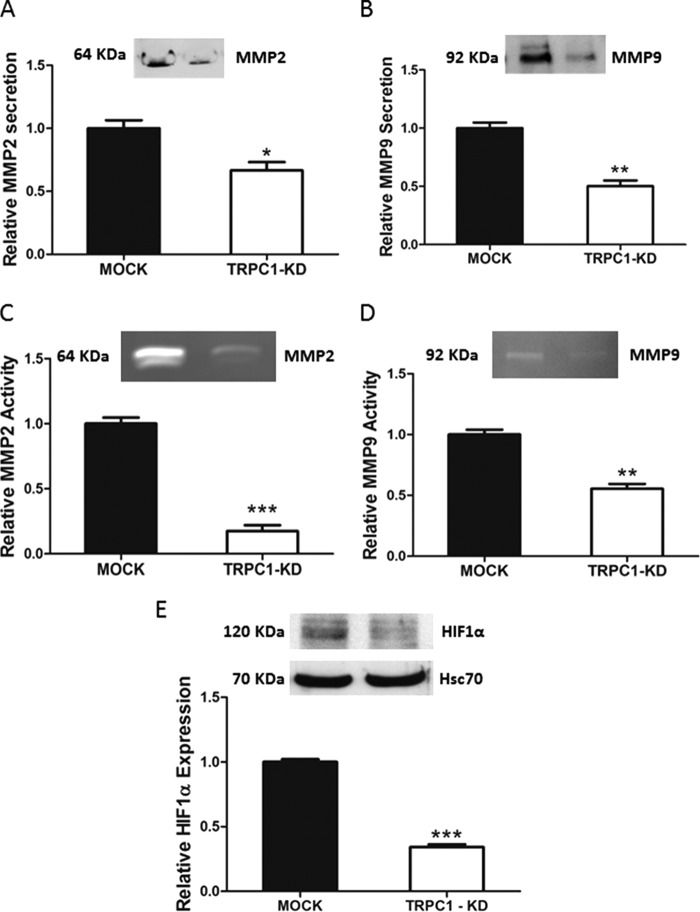
FIGURE 7.
TRPC1 knockdown decreased the secretion and activity of MMP2 and MMP9 and the expression of HIF-1α in ML-1 cells. A and B, the secretion of MMP2 and MMP9 was significantly decreased in TRPC1-KD cells compared with MOCK cells. Representative Western blots are shown. The bar diagrams show the means ± S.E. (n = 3). *, p < 0.05; **, p < 0.01. C and D, the gelatinolytic activity of MMP2 and MMP9 was significantly decreased in TRPC1-KD cells compared with MOCK cells. Representative blots are shown. The bar diagrams show the means ± S.E. (n = 3). **, p < 0.01; ***, p < 0.001. E, the expression of HIF-1α was significantly decreased in TRPC1-KD cells compared with MOCK cells. A representative Western blot is shown. The bar diagram shows the means ± S.E. (n = 3). ***, p < 0.001.
Proliferation Assays
The [3H]thymidine incorporation method was used to study the proliferation of the cells. 100,000 transfected cells were seeded on 35-mm plates and allowed to grow for 24, 48, or 72 h. Four hours prior to the end of each experiment, 0.4 μCi/ml [3H]thymidine was added. The cells were washed three times with PBS, incubated for 10 min with 5% trichloric acetic acid, and then incubated for 10 min with 0.1 m NaOH. The radioactivity was measured using a Wallac 1410 liquid scintillation counter.
FACS Analysis
500,000 ML-1 Mock and TRPC1-KD cells were grown overnight on 35-mm plates. The cells were detached with EDTA-trypsin solution and pelleted. The cell pellets were suspended in 500 μl of propidium iodide solution (0.05 mg/ml propidium iodide, 3.8 μm sodium citrate, 0.1% Triton X-100 in PBS) and incubated for 15 min at room temperature. The samples were then analyzed by flow cytometry using FACSCalibur and CellQuest Pro software (BD Biosciences, San Jose, CA). The percentage of cells in each phase of cell cycle was calculated by using ModFit LT 4.1 software.
Western Blotting
The protocol used for making whole cell lysates and for the Western blotting has been described elsewhere (14). Densitometric analysis was performed using the ImageJ program for image analysis (National Institutes of Health, Bethesda, MD), and the results were corrected for protein loading by normalization with Hsc70 expression. In experiments for measuring the matrix metalloproteinase 2 and 9 secretion, the cells were grown on 35-mm plates to 80% confluency. On the day of experiment, 1 ml of fresh medium was added to the plates. After 6 h, the medium was collected, and the cells were lysed in lysis buffer (10 mm Tris, pH 7.7, 150 mm NaCl, 7 mm EDTA, 0.5% Nonidet P-40). Cell lysates were centrifuged at 13000 rpm for 15 min at 4 °C. The supernatants were collected, and the protein concentrations were measured for each sample. The Laemmli sample buffer was added to each sample. Equal volumes of samples (30 μl) were subjected to SDS-PAGE, and Western blotting was performed as described (14). The primary antibodies used were anti-MMP2 and anti-MMP9 (1:1000 in 5% BSA in TBS containing 150 mm NaCl, 20 mm Tris base, pH 7.5, and 0.1% Tween 20 (TBST)). The secondary antibodies used were peroxide-conjugated goat anti-rabbit antibodies (1:3000 in 5% milk in 0.1% TBST). The amounts of secreted MMP2 and MMP9 in Fig. 7 (C and D) were normalized against total protein concentration of each cell culture plate.
Statistics
The results are presented as means ± S.E. from at least three independent measurements. The data were normalized to make a comparison of different experiments feasible. Student's t test was used when two means were compared. One-way analysis of variance and Bonferroni's post hoc test was applied when three or more means were compared. GraphPad Prism 5 program (GraphPad Software Inc., San Diego, CA) was used for the statistical analyses. p values less than 0.05 were considered statistically significant.
Results
Expression of TRPC Channels in Human Thyroid Tissues and Cell Lines
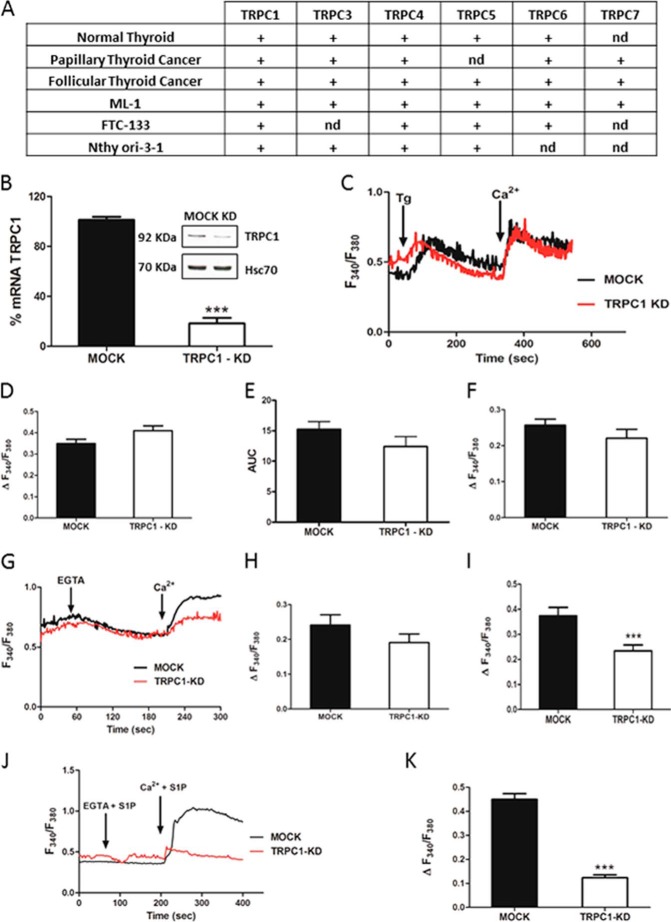
Although the importance of calcium for thyroid function is indisputable, the identity of calcium channels in thyroid cells is not well defined. In the present study, we show for the first time that human normal thyroid tissue and a normal thyroid cell line, follicular thyroid cancer tissue and cell lines, and papillary thyroid cancer tissue express several (but not necessary all) ion channels of the TRPC superfamily(Fig. 1A).
FIGURE 1.
Expression of TRPC channels in thyroid cells and the effects of TRPC1 knockdown on calcium entry in thyroid cancer ML-1 cells. A, expression profiles of TRPC channel isoforms in normal human thyroid, papillary thyroid cancer and follicular thyroid cancer tissue, and cell lines determined by qualitative end point PCR. (+) denotes expressed. nd, not detected. B, expression of TRPC1 in shRNA expressing (MOCK) and shRNA TRPC1 expressing (TRPC1-KD) cells on mRNA and protein levels by RT-PCR and Western blotting, respectively. The bars show the means ± S.E. (n = 3). ***, p < 0.001. C, representative traces showing Tg-evoked (final concentration, 1 μm) changes in [Ca2+]i in MOCK and TRPC1-KD cells in calcium-free buffer and the effect of readdition of 1 mm calcium. D, bar diagram showing the magnitude of the Tg-evoked peak in [Ca2+]i. The bars show the means ± S.E. of at least 50 cells. E, bar diagram showing the area under the Tg curve (time, 60–380 s). The bars show the means ± S.E. of at least 50 cells. F, bar diagram showing the magnitude of the change in [Ca2+]i after readdition of calcium (final concentration, 1 mm) to Tg-treated cells. The bars show the means ± S.E. of at least 50 cells. G, representative traces showing the decrease in [Ca2+]i after addition of EGTA (final concentration, 1 mm) to MOCK and TRPC1-KD cells in a calcium-containing buffer and the effect of readdition of calcium (final concentration, 2 mm). H, bar diagram showing the magnitude of EGTA-evoked decrease in [Ca2+]i. The bars show the means ± S.E. of at least 50 cells. I, bar diagram showing the magnitude of the change in [Ca2+]i after readdition of calcium (final concentration, 2 mm) to EGTA-treated cells. The bars show the means ± S.E. of at least 50 cells. ***, p < 0.001. J, representative traces showing S1P-evoked (final concentration, 1 μm) changes in [Ca2+]i in MOCK and TRPC1-KD cells in calcium-free EGTA, containing buffer and the effect of readdition of calcium (final concentration, 1 mm). K, bar diagram showing the increase in [Ca2+]i when S1P and calcium was readded to TRPC1-KD cells and to MOCK cells. The bars show the means ± S.E. of at least 60 cells. ***, p < 0.001.
Previous studies have shown that TRPC1 is ubiquitously expressed in humans and regulates many cellular processes, including cell proliferation and migration in both normal and cancer cells (5, 6, 8, 9). To investigate the importance of the TRPC1 channel in thyroid cancer cells, we thus generated stable TRPC1 knockdown cells. For this purpose, we used follicular ML-1 thyroid cancer cells, a cell line previously studied in our laboratory (14, 15, 19–21). Our results showed that the TRPC1 expression in TRPC-KD cells was down-regulated by 80 and 50% on mRNA and protein level, respectively (Fig. 1B).
TRPC1 Knockdown Attenuates S1P-induced Calcium Entry
Previous studies have shown that TRPC1 is of crucial importance in store-operated calcium entry (SOCE) (22–25). Thus, we first investigated whether TRPC1 was involved in SOCE in ML-1 cells. However, we could not detect a significant difference in the thapsigargin (Tg)-evoked calcium transient (Fig. 1C) or in the entry of calcium when calcium was readded to Tg-treated TRPC1-KD cells, compared with control MOCK cells (Fig. 1, D and F). There was no significant difference observed by calculating area under the Tg-evoked calcium curve as shown in Fig. 1E. In another experiment, we observed a significantly decreased calcium entry when calcium was readded to the TRPC1-KD cells in an EGTA-containing, calcium-free buffer, compared with MOCK cells (Fig. 1, G–I).
In addition to SOCE, TRPC1 has been shown to participate in receptor-operated calcium entry (9, 26). To investigate this possibility, we stimulated TRPC1-KD cells and MOCK cells with S1P. As shown in Fig. 1 (J and K), S1P could not induce a change in intracellular calcium in a calcium-free environment, either in MOCK cells or TRPC1-KD cells. Interestingly, in a calcium-containing buffer the S1P-evoked calcium response was substantially attenuated in TRPC1-KD cells, compare with the response seen in MOCK cells. Thus, in ML-1 cells, TRPC1 seems to function predominantly as a receptor-operated calcium channel. Furthermore, to evaluate the importance of TRPC channels for S1P-evoked calcium signaling, we used rat thyroid FRTL-5 cells, where only TRPC2 is expressed (27). In TRPC2 knockdown FRTL-5 cells (FRTL-KD cells), the S1P-evoked increase in intracellular free calcium was almost totally abolished (Fig. 2C).
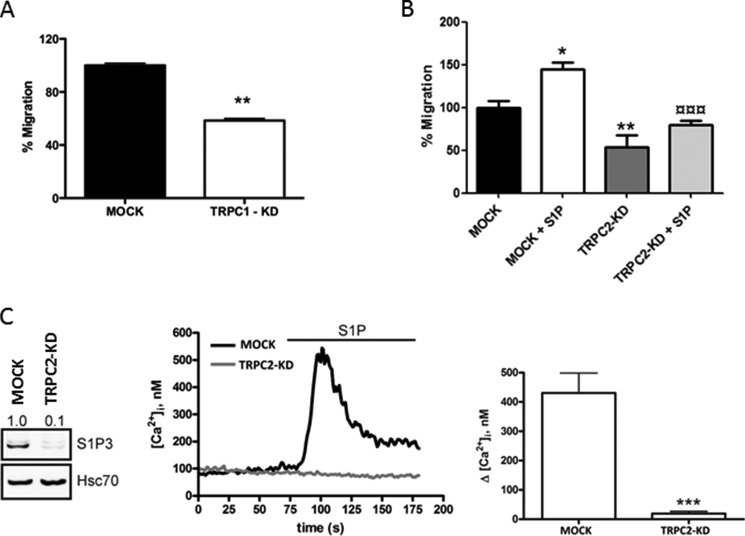
FIGURE 2.
TRPC1-KD and TRPC2-KD in human and rat thyroid cells, respectively, affect migration, calcium entry, and pro-migratory S1P3 receptor expression. A, human follicular thyroid cancer FTC-133 cells were electroporated with shRNA control and shTRPC1 to generate MOCK and FTC-133 TRPC1-KD cells. After 48 h of transduction, 50,000 of each cells were allowed to migrate toward serum-free medium for 20 h. The normalized results in the graphs are the means ± S.E. (n = 3). **, p < 0.01). B, TRPC2 knockdown decreased the basal and S1P-evoked migration in rat thyroid FRTL-5 cells. 100,000 cells in serum-free medium were allowed to migrate toward 5% LS-FBS for 16 h. The bar diagrams show the means ± S.E. (n = 3). *, p < 0.05; **, p < 0.01; ¤¤¤, p < 0.001). *, statistically significant differences in migration compared with control; ¤, statistically significant differences in migration of MOCK +S1P compared with TRPC2-KD + S1P (n = 3). *, p < 0.05; **, p < 0.01; ¤¤¤, p < 0.001. C, TRPC2 knockdown decreased the expression of S1P3 and the S1P-evoked increase in [Ca2+]i in rat thyroid FRTL-5 cells. A representative blot and representative calcium traces are shown. The bar diagram shows the means ± S.E. (n = 3). ***, p < 0.001.
Knockdown of TRPC1 Decreases Invasion, Migration, and Receptor Expression in Thyroid Cancer Cells
TRPC channels have been suggested to enhance migration of several types of cancer cells (7, 28). Furthermore, S1P is a potent activator of cancer cell migration, including the ML-1 thyroid cancer cells (14). The pro-migratory effect of S1P is mediated through S1P1 and S1P3, whereas activation of S1P2 usually has an anti-migratory effect. Thus, we investigated the migratory response of TRPC1-KD cells. As can be seen in Fig. 3 (A and B), both the basal invasion and migration of TRPC1-KD cells was attenuated, compared with MOCK cells. Furthermore, in the TRPC1-KD cells, S1P was without any effects on either invasion or migration, compared with MOCK cells.
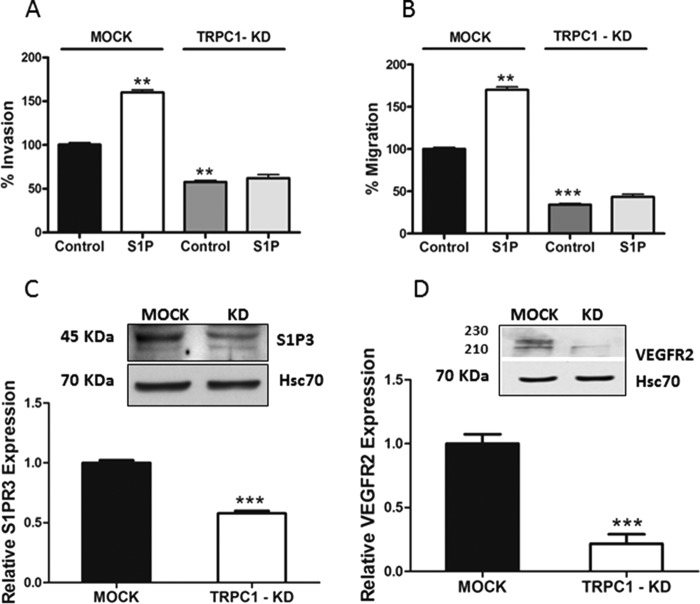
FIGURE 3.
TRPC1 knockdown attenuates invasion, migration, and receptor expression in thyroid cancer ML-1 cells. A and B, TRPC1 knockdown decreased the basal and the S1P-evoked (final concentration, 100 nm) invasion and migration in ML-1 cells. The bars show the means ± S.E. (n = 3). **, p < 0.01; ***, p < 0.001. C, TRPC1 knockdown decreased the relative expression of S1P3. A representative Western blot is shown. The bar diagram shows the decreased expression of S1P3 in TRPC1-KD cells compared with MOCK cells (n = 3). ***, p < 0.001. D, TRPC1 knockdown decreased the relative expression of VEGFR2. A representative Western blot is shown. The bar diagram shows the decreased expression of VEGFR2 in TRPC1-KD cells compared with MOCK cells (n = 3). ***, p < 0.001.
Corroborative results were obtained using the follicular FTC-133 thyroid cancer cell line. Knockdown of TRPC1 in these cells attenuated the basal migration, compared with MOCK FTC-133 cells (Fig. 2A). In addition, in the TRPC2 knockdown rat thyroid FRTL-KD cells, a substantial decrease in both basal and S1P-evoked migration was observed (Fig. 2B), further highlighting the importance of TRPC channels for basal and S1P-evoked migration.
The abolished effect of S1P on invasion could be due to a decreased expression of the pro-migratory S1P1 and S1P3 receptors or an increase in the anti-migratory S1P2 receptor. Thus, we next investigated the expression of S1P receptors in TRPC1-KD cells. The results showed that S1PR3 was significantly down-regulated in TRPC1-KD cells (Fig. 3C), whereas there was no effect on the expression of S1PR1 and S1PR2 in these cells, compared with MOCK cells (data not shown). There was no significant change on S1PR3 mRNA expression in TRPC1-KD cells compared with MOCK cells (data not shown). We also showed that in FRTL-5 TRPC2-KD cells, the expression of S1P3 was almost totally abolished (Fig. 2C).
Previously, we have shown that S1P receptors and VEGFR2 interact and form a complex and that this complex is important for S1P-evoked ML-1 cell migration (15, 19). Furthermore, S1P regulates VEGFR2 expression by a mechanism dependent on S1P3, PKCα, and ERK1/2 (19). We thus investigated the expression of VEGFR2 in TRPC1-KD cells and observed that VEGFR2 was markedly down-regulated in these cells, compared with MOCK cells (Fig. 3D).
Importance of Calcium in Regulating Migration and the Expression of S1P3 and VEGFR2
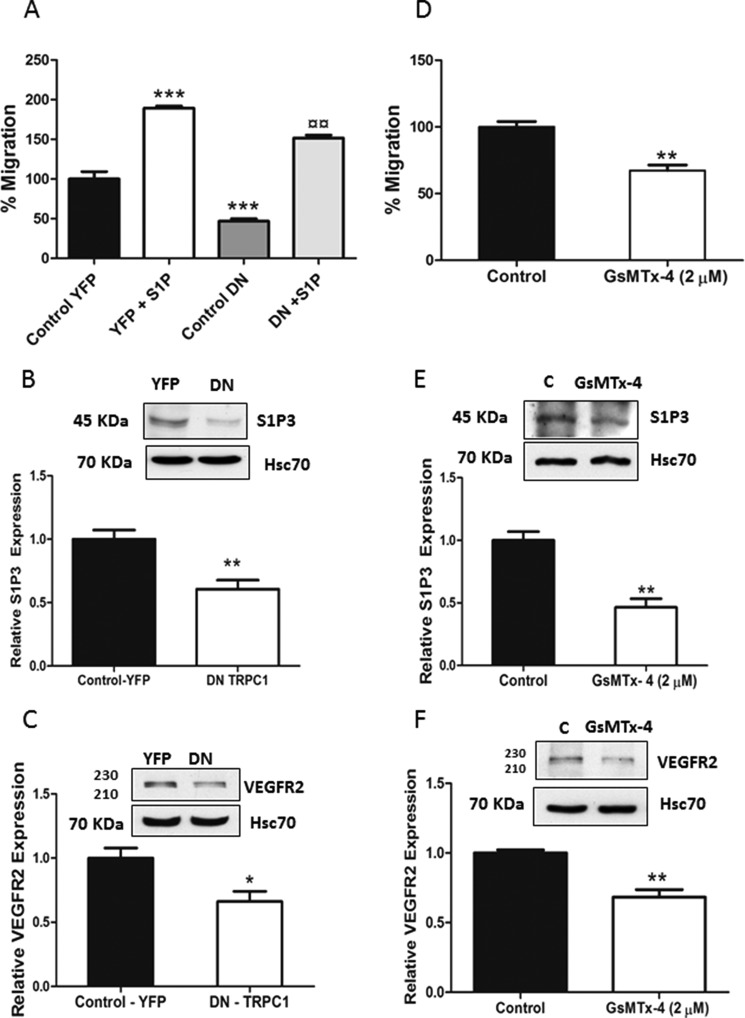
Calcium signaling is important in regulating the pathophysiology of cancer cells (29, 30). We next investigated the possible involvement of calcium in the expression of S1P3 and VEGFR2. For this purpose, wild-type ML-1 cells were transfected with a dominant negative, nonconducting, pore-mutated TRPC1 channel. In these cells, the invasion, migration, and expression of both S1P3 and VEGFR2 were significantly decreased, compared with mock transfected cells (Fig. 4, A–C). To further corroborate our results, we incubated wild-type ML-1 cells with the spider toxin GsMTx-4 (2 μm for 24 h), a potent inhibitor of TRPC1 channels (7, 31, 32). In these experiments, the basal migration, as well as the expression of S1P3 and VEGFR2, was significantly decreased (Fig. 4, D–F).
FIGURE 4.
Expression of a dominant negative form of TRPC1 or pretreatment with a channel inhibitor decreases migration and expression of S1P3 and VEGFR2 in wild-type ML-1 cells. A, expression of the dominant negative pore-mutated form of TRPC1 (TRPC1-DN) in wild-type ML-1 cells decreased both basal and S1P-evoked migration. The normalized results in the graphs are the means ± S.E. *, statistically significant differences in migration compared with YFP control; ¤, statistically significant differences in migration of DN + S1P compared with YFP + S1P. (n = 3). ¤¤, p < 0.01; ***, p < 0.001. B and C, the expression of TRPC1-DN decreased the expression of S1P3 and VEGFR2 in ML-1 cells. Representative Western blots are shown. The normalized results in the graphs are the means ± S.E. (n = 3). *, p < 0.05; **, p < 0.01. D, cells were preincubated with 2 μm GsMTx-4 for 1 h and were allowed to migrate for 16 h. The normalized results in the graphs are the means ± S.E. (n = 3). **, p < 0.01. E and F, preincubating cells with 2 μm GsMTx-4 decreased the expression of S1P3 and VEGFR2. Representative Western blots are shown. The normalized results in the graphs are the means ± S.E. (n = 3). **, p < 0.01.
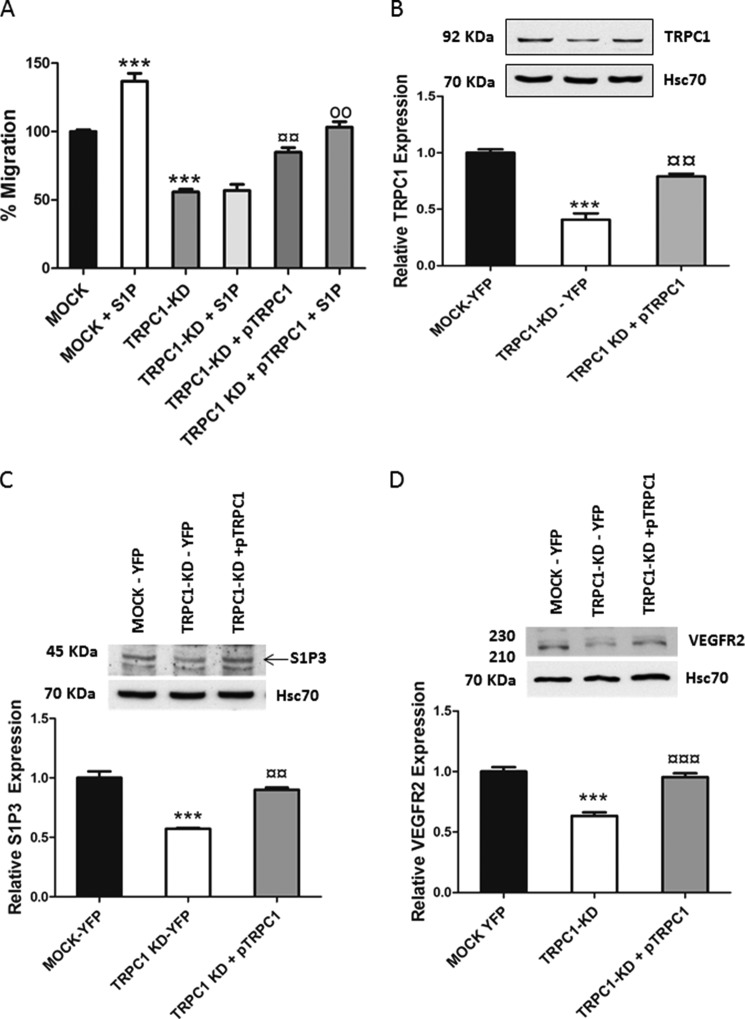
To further prove that TRPC1 is regulating migration and receptor expression, we transfected back the TRPC1 into the TRPC1-KD cells by using the HA-TRPC1/pRK5 plasmid (pTRPC1). These rescue experiments clearly showed that restoring TRPC1 in the TRPC1-KD cells enhanced both basal and S1P-evoked migration and increased S1P3 and VEGFR2 expression (Fig. 5, A–D). Thus, TRPC1 has a profound role in regulating thyroid ML-1 cancer cell migration and the expressional level of S1P3 and VEGFR2.
FIGURE 5.
Effects seen by TRPC1 knockdown are reversible. A, expressing TRPC1 back (pTRPC1) to TRPC1-KD cells rescues both basal and S1P-evoked (final concentration, 100 nm) migration. *, statistically significant differences in migration compared with MOCK cells; ¤, statistically significant differences in migration of TRPC1-KD compared with TRPC1-KD + pTRPC1; o, statistically significant differences in migration of TRPC1-KD + pTRPC1 compared with TRPC1-KD + pTRPC1 + S1P. The normalized results in the graphs are the means ± S.E. (n = 3). ***, p < 0.001; ¤¤, p < 0.01; oo, p < 0.01. B, bar diagram showing the TRPC1 levels in mock cells, TRPC1-KD cells transfected with YFP, and TRPC1-KD cells transfected with pTRPC1. Each bar shows the means ± S.E. (n = 3). ***, p < 0.001 in comparison with MOCK-YFP cells; ¤¤, p < 0.01 in comparison with TRPC1-KD-YFP cells. A representative Western blot is shown. C and D, expression of TRPC1 back to TRPC1-KD cells restores the expressional level of S1P3 and VEGFR2. Representative Western blots are shown. The bar diagrams show the means ± S.E. (n = 3). ***, p < 0.001 comparison with MOCK-YFP cells; ¤¤, p < 0.01; ¤¤¤, p < 0.001 in comparison with TRPC1-KD-YFP cells.
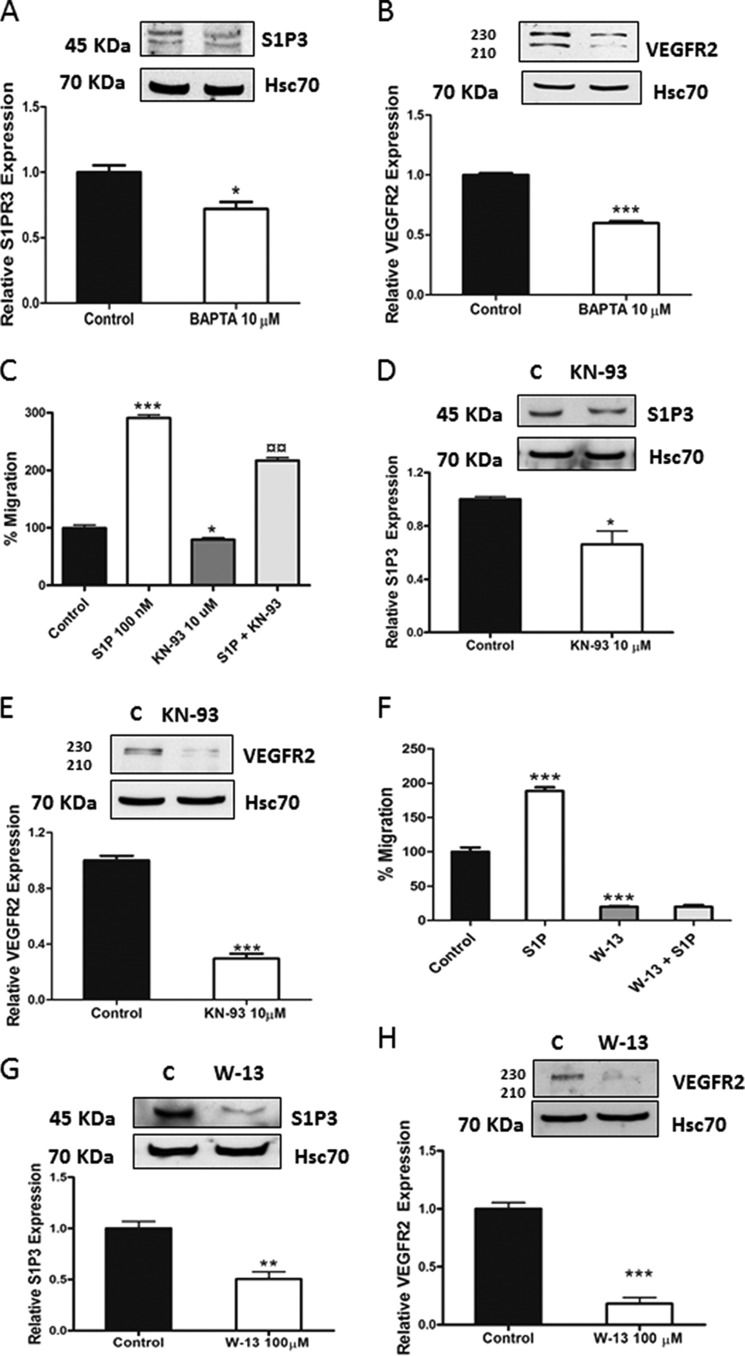
We next took a pharmacological approach to block calcium signaling in wild-type ML-1 cells. By incubating the cells with the intracellular calcium chelator BATA-AM (10 μm for 24 h), the expression of S1P3 and VEGFR2 was significantly decreased (Fig. 6, A and B). In ML-1 cells incubated with the calmodulin kinase inhibitor KN-93 (10 μm for 24 h), the migration and the expression of S1P3 and VEGFR2 were significantly decreased (Fig. 6, C–E). Furthermore, incubating wild-type ML-1 cells with the calmodulin antagonist W-13 (100 μm for 6 h) also decreased both the receptor expression and migration (Fig. 6, F–H). In all these experiments, the expressional level of TRPC1 remained unchanged (data not shown). Thus, our overall conclusion is that TRPC1 and calcium signaling has a profound role in regulating thyroid ML-1 cancer cell migration and the expressional levels of S1P3 and VEGFR2.
FIGURE 6.
Calcium is regulating migration and the expression of S1P3 and VEGFR2 in ML-1 cells. A and B, incubating wild-type ML-1 cells with 10 μm BAPTA-AM (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) for 24 h decreased the expression of S1P3 and VEGFR2. Representative Western blots are shown. The bar diagrams show the means ± S.E. (n = 3). *, p < 0.05; ***, p < 0.001. C, in wild-type ML-1 cells preincubated with 10 μm KN-93 for 1 h and allowed to migrate for 16 h; the basal and S1P-evoked migration was decreased. The normalized results in the graph are the means ± S.E. *, statistically significant differences in migration compared with control; ¤, statistically significant difference in migration compared with S1P-evoked migration (n = 3). *, p < 0.05; ***, p < 0.001; ¤¤, p < 0.01. D and E, incubating wild-type ML-1 cells with 10 μm KN-93 for 24 h decreased the expression of S1P3 and VEGFR2. Representative Western blots are shown. The bar diagrams show the means ± S.E. (n = 3). *, p < 0.05; ***, p < 0.001. F, in wild-type ML-1 cells preincubated with 100 μm W-13 for 1 h and allowed to migrate for 8 h; the basal and S1P-evoked migration was significantly decreased. The normalized results in the graph are the means ± S.E. (n = 3). *, statistically significant differences in migration compared with control (n = 3). ***, p < 0.001. G and H, incubating wild-type ML-1 cells with 100 μm W-13 for 6 h decreased the expression of S1P3 and VEGFR2. Representative Western blots are shown. The bar diagrams show the means ± S.E. (n = 3). **, p < 0.01; ***, p < 0.001.
TRPC1 Knockdown Attenuates the Expression of Matrix Metalloproteinases 2 and 9 and HIF-1α
Recently, we have shown that S1P regulates the secretion and activity of matrix metalloproteinase 2 and 9 through S1P1,3 (33). Thus, we investigated the secretion and activity of MMP2 and MMP9 in MOCK and TRPC1-KD cells. The results showed that both the secretion and activity of MMP2 and MMP9 were significantly decreased in TRPC1-KD cells, compared with MOCK cells (Fig. 7, A–D).
Previously, we have reported that S1P through S1P3 regulates the expression and the activity of HIF-1α in normoxic conditions in ML-1 cells (21). Next, we investigated the expression of HIF-1α in TRPC1-KD cells. Our results showed that the expression of HIF-1α was significantly decreased in ML-1 TRPC1-KD cells, compared with MOCK cells (Fig. 7E).
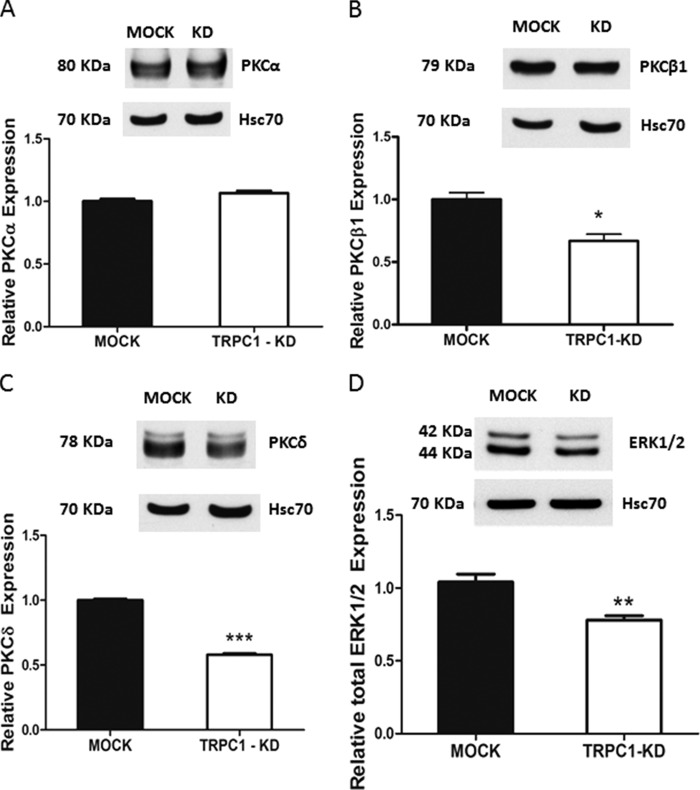
Knockdown of TRPC1 Affects the Expression of PKC Isoforms and ERK1/2 in ML-1 Cells
PKC and ERK1/2 are involved in mediating the S1P3-evoked expression of both VEGFR2 (42) and HIF-1α (21). In TRPC2-down-regulated FRTL-5 cells, TRPC2 regulates the expression of PKC isoforms (27). Therefore, we investigated whether PKC isoforms or ERK1/2 were down-regulated in TRPC1-KD cells. As can be seen in Fig. 8 (A–C), both PKCβ and PKCδ, but not PKCα, were down-regulated in TRPC1-KD cells. In addition, ERK1/2 was also down-regulated (Fig. 8D).
FIGURE 8.
Knockdown of TRPC1 affects the expression of PKC isoforms and ERK1/2 in ML-1 cells. A, expression of PKCα in ML-1 MOCK and TRPC1-KD cells. A representative Western blot is shown. The bar diagram shows no significant difference in expression of PKCα in TRPC1-KD cells compared with MOCK cells. B, expression of PKCβ1 in ML-1 MOCK and TRPC1-KD cells. A representative Western blot is shown. The bar diagram shows the decreased expression of PKCβ1 in TRPC1-KD cells compared with MOCK cells (n = 3). *, p < 0.05. C, expression of PKCδ in ML-1 MOCK and TRPC1-KD cells. A representative Western blot is shown. The bar diagram shows the decreased expression of PKCδ in TRPC1-KD cells compared with MOCK cells (n = 3). ***, p < 0.001. D, a representative Western blot is shown. The bar diagram shows the decreased expression of total ERK1/2 in TRPC1-KD cells compared with MOCK cells (n = 3). **, p < 0.01.
Knockdown of TRPC1 Affects ML-1 Cell Proliferation
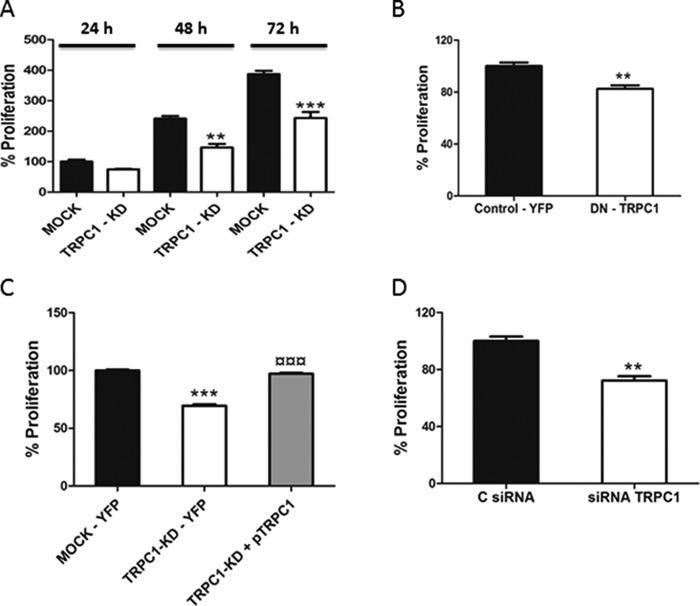
Several investigations have shown that TRPC1 is involved in the regulation of normal as well as cancer cell proliferation (8, 9). We next investigated the effect of TRPC1 knockdown on ML-1 cell proliferation and observed that the proliferation was significantly attenuated in TRPC1 knockdown cells, compared with MOCK cells (Fig. 9A).
FIGURE 9.
TRPC1 regulates proliferation of thyroid cancer ML-1 cells. A, TRPC1 knockdown decreased proliferation after 48 and 72 h. The bar diagram shows the means ± S.E. (n = 3). **, p < 0.01; ***, p < 0.001. B, the expression of TRPC1-DN decreased proliferation after 24 h. The bar diagram shows the means ± S.E. (n = 3). **, p < 0.01. C, expression of TRPC1 in TRPC1-KD cells restores proliferation after 48 h. The bar diagram shows the means ± S.E. *, the statistically significant differences in proliferation compared with control; ¤, the statistically significant differences in proliferation of TRPC1-KD cells compared with TRPC1-KD + pTRPC1 (n = 3). ***, p < 0.001; ¤¤¤, p < 0.001. D, TRPC1 knockdown by siRNA decreased the proliferation of FTC-133 cells after 48 h. The bar diagram shows the means ± S.E. (n = 3). **, p < 0.01.
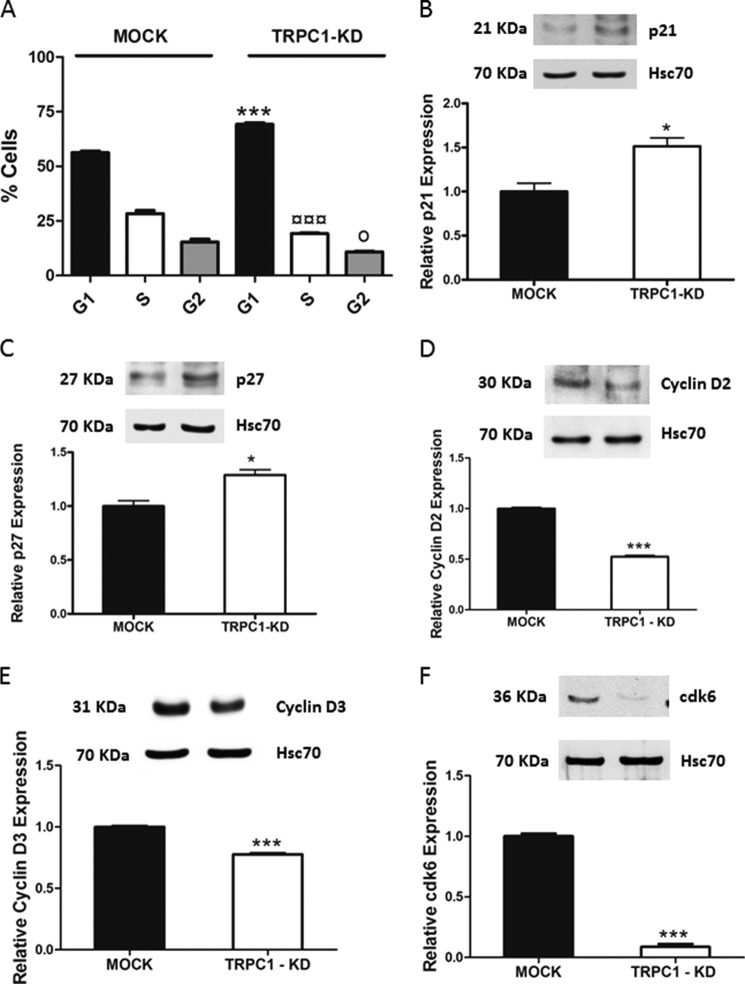
To confirm the importance of TRPC1 in regulating ML-1 cell proliferation, wild-type ML-1 cells were transfected with the dominant negative, nonconducting, pore-mutated TRPC1 channel. The results showed a significant decrease in proliferation, thus confirming that TRPC1 is involved in regulating ML-1 cell proliferation (Fig. 9B). To prove that TRPC1 is involved in regulating the proliferation in ML-1 cells, we transfected back TRPC1 in TRPC1-KD cells by using the HA-TRPC1/pRK5 plasmid (pTRPC1). These TRPC1 rescue experiments showed that the proliferation was significantly restored in these cells as compared with MOCK cells (Fig. 9C). Furthermore, in FTC-133 cells transfected with a TRPC1 siRNA, a decrease in proliferation was observed (Fig. 9D). Thus, TRPC1 is important in regulating thyroid cancer cell proliferation. We next investigated the mechanism by which TRPC1 knockdown induced a decrease in proliferation. We first analyzed the cell cycle by FACS analysis. In TRPC1-KD cells, there was a significant increase in cell population in the G1 phase and a significant decrease in the S and G2 phases of the cell cycle, compared with the MOCK cells (Fig. 10A). Interestingly, we detected a small apoptotic peak of cells (3–5%) in the TRPC-KD cells. We also investigated the expression of the cell cycle regulatory proteins p21kip1and p27waf1/cip1 and observed that these proteins were significantly increased in TRPC1-KD cells (Fig. 10, B and C). Furthermore, cyclin D2, cyclin D3, and cdk6 were significantly decreased in TRPC1-KD cells, compared with MOCK cells (Fig. 10, D–F). We did not detect any significant changes in the expression of cyclin D1, cdk2, and cdk4 (data not shown).
FIGURE 10.
Mechanism by which knockdown of TRPC1 decreased proliferation in ML-1 cells. A, knockdown of TRPC1 decreased proliferation by prolonging the G1 phase and decreasing the S and G2 phases of the cell cycle. ***, p < 0.001; ¤¤¤, p < 0.001; o, p < 0.05 n = 3. *, ¤, and o, statistically significant differences between the G1, S, and G2 phases of cell cycle, respectively, in TRPC1-KD cells compared with the MOCK cells. B and C, up-regulation of the p21 and p27 cell cycle regulatory proteins in TRPC1-KD cells. Representative Western blots are shown. The bar diagrams show the means ± S.E. (n = 3). *, p < 0.05. D and E, down-regulation of cyclin D2 and cyclin D3 in TRPC1-KD cells. Representative Western blots are shown. The bar diagrams show the means ± S.E. (n = 3). ***, p < 0.001. F, the expression of cdk6 is decreased in TRPC1-KD cells. A representative blot is shown. The bar diagram shows the means ± S.E. (n = 3). ***, p < 0.001.
Discussion
In the present report, we have characterized the expression of TRPC ion channels in human thyroid cells. Our results show that both normal human thyroid tissue and a normal thyroid cell line, as well as human thyroid cancer cells and tissues, express several members of the TRPC family of ion channels, i.e. TRPC1, TRPC3, TRPC4, TRPC5, TRPC6, and TRPC7. This is an important observation, because calcium signaling is of crucial importance in thyroid cells, but the channel profile is unknown (34, 35). Previously TRPC ion channels have been identified in rat thyroid FTRL-5 cells only. These cells exclusively express TRPC2, which is of profound importance in regulating TSH receptor expression, calcium signaling, iodide homeostasis, proliferation, and migration (16, 27, 28, 36). However, a certain caution must be expressed, because the tissue samples might contain different cell types as well, and these cells might express TRPC channels that are not expressed in the thyroid cells, thus giving false positive results.
We decided to further investigate the significance of TRPC1. We based this decision on the fact that previous studies have unambigously shown that TRPC1 is ubiquitously expressed in humans and is of physiological significance in regulating many cellular processes, including calcium signaling, cell proliferation, and migration, in both normal and cancer cells (5, 6, 8, 9, 37). Surprisingly, we observed that knockdown of TRPC1 did not affect SOCE but rather receptor-operated calcium entry. This is in sharp contrast to a multitude of observations, rendering TRPC1 an important player in SOCE (25). However, in some cell types and in response to receptor-evoked stimuli, TRPC1 can, in fact, evoke receptor-operated calcium entry (9, 26). The situation is similar to what we have observed in TRPC2 knockdown thyroid FRTL-5 cells. In these cells, TRPC2 seems to function solely as a receptor-operated channel (27). We also show that in both ML-1 cells, and in FRTL-5 cells, S1P potently activates calcium entry through TRPC1 and TRPC2, respectively. A similar S1P-evoked, TRPC1-dependent calcium entry has been observed in retinal amacrine cells (38). Furthermore, S1P enhanced calcium entry through stretch-activated TRPC1 channels in skeletal muscle cells (39). In vascular smooth muscles cells, S1P activates TRPC5 via a receptor-mediated mechanism (40).
A previous investigation has shown that TRPC1 can be found in complex with other TRPC channels, and in these complexes TRPC1 attenuates calcium entry (26). When TRPC1 was down-regulated, calcium entry increased (26). Such an effect was clearly not observed in our ML-1 cells, suggesting that TRPC1 is not in complex with other channels or may not interfere with the gating of other channels. However, this possibility was not further investigated.
Several TRPC channels, including TRPC1, are of importance in regulating cancer cell migration (28, 41). We have previously shown that S1P, through activation of S1P1 and S1P3 and through cross-communication with VEGFR2, potently stimulates thyroid cancer cell migration (14, 15, 42). The importance of ion channels for the S1P-evoked migration was not investigated in these studies. Although the effects of S1P on ion channels in relationship to migration is not well studied, we have shown that S1P enhances calcium entry both in human and rat thyroid cells (14, 34, 43).
We observed that both basal and especially S1P-evoked invasion and migration was potently hampered in TRPC1 knockdown ML-1 cells and that the basal and S1P-induced migration was attenuated in TRPC2 knockdown FRTL-5 cells. Similar findings regarding the importance of TRPC1 on migration have been found in, for example, skeletal myoblasts, immortalized GnRH neurons, and MDCK-F cells (7, 44, 45). For a recent review, see Ref. 9. Furthermore, in smooth muscle cells, S1P-induced migration is dependent on activation of TRPC5 (40). In light of our observation and the significance of S1P in enhancing the migration of several cancer cells, it would thus be of interest to more closely investigate the relationships between S1P and TRPC-dependent calcium entry also in other cancer cells.
A most interesting finding was that in the TRPC1 knockdown cells, the pro-migratory S1P3 and VEGFR2 receptors were significantly down-regulated. To corroborate our finding, we transfected wild-type ML-1 cells either with siRNA against TRPC1 or with a dominant negative pore-mutated TRPC1 or treated the cells with a TRPC1 specific toxin GsMTx-4. All these treatments decreased the expression of S1P3 and VEGFR2. Furthermore, transfecting back TRPC1 to TRPC1 knockdown cells restored both S1P3 and VEGFR2 levels. This regulation of receptor expression is a totally novel effect coupled to TRPC channel activity. The effect seems to be strictly coupled to calcium signaling, because BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) treatment, as well as pharmacological blocking of calmodulin and calmodulin-dependent kinases, also resulted in down-regulation of both S1P3 and VEGFR2.
The precise mechanisms for the down-regulation are presently not known, but we have previously shown that S1P3 regulates the expression of VEGFR2 (42). The present investigation highlights how S1P3 is regulated and shows that one physiologically important effect of down-regulating TRPC1 is probably the decreased expression of S1P3. The mechanism for the down-regulation of S1P3 is (except for its dependence on calcium) presently not known but is not on the transcriptional level, because the S1P3 mRNA level was not altered in TRPC1 knockdown cells compared with MOCK cells. Interestingly, not only VEGFR2 is regulated by S1P3, but also HIF-1α, MMP2, and MMP9 (21, 33). Both MMP2 and MMP9, as well as HIF-1α, are important for thyroid cell migration (21, 46, 47). Knockdown of TRPC1 resulted in decreased expression of HIF-1α and also decreased secretion of MMP2 and MMP9, resulting in decreased gelatinolytic activity. In rat thyroid FRTL-5 cells, down-regulation of TRPC2 also resulted in decreased MMP2 secretion and migration (28), in addition to a substantial down-regulation of S1P3 and an attenuated S1P-evoked migration (this work).
Presently we do not know whether S1P3 regulates VEGFR2 expression via the same signaling pathways by which HIF-1α, MMP2, and MMP9 are regulated. Because TRPC2 down-regulation in the FRTL-5 cells decreases Rac activity (28) and because S1P3 regulates MMP2 and MMP9 via Rac (33), Rac could be a good candidate for regulating the MMPs in our study. We did, however, observe that in TRPC1-KD cells, both PKCβ and PKCδ were down-regulated, as well as ERK1/2. These kinases are involved in mediating the S1P3-evoked expression of both VEGFR2 (42) and HIF-1α (21) in ML-1 cells. Furthermore, in TRPC2-down-regulated FRTL-5 cells, TRPC2 regulates the expression of PKC isoforms (27). It seems obvious that PKCs and ERK1/2 are common signaling intermediates downstream from S1P3, regulating at least VEGFR2 and HIF-1α expression. By regulating the expression of S1P3, TRPC1 clearly has a profound role in regulating thyroid cancer cell invasion and migration.
In addition to having a crucial importance in migration, TRPC channels also regulate proliferation in several cell types (6, 48). It was thus of interest to investigate this aspect, and we observed that down-regulation of TRPC1 or transfection with the pore-mutated nonconducting TRPC1 construct decreased proliferation. In accord with this, we observed an increase in the cyclin-dependent kinase inhibitors p21 and p27, an increase of cells in the G1 phase, and a concomitant decrease in both the S and G2 phases of the cells cycle in TRPC1 knockdown cells. Similar results were observed in TRPC1 down-regulated small cell lung carcinoma cells (49) and hippocampal neural progenitor cells (50). Furthermore, down-regulation of TRPC2 in thyroid FRTL-5 cells (28) and TRPC3 in skeletal myoblasts (51) resulted in an attenuation of proliferation and an increase in the G1 phase of the cell cycle.
In conclusion, we have shown that both normal and cancerous human thyroid cells express several TRPC isoforms. Furthermore, we show that TRPC1 functions as a receptor-operated channel and that down-regulation of TRPC1 has a profound attenuating effect on invasion, migration, and proliferation in human thyroid cancer cells. This occurs, in part, by down-regulating the expression of S1P3 and VEGFR2, and signaling entities downstream from these receptors. Our findings can thus be of significance when planning novel strategies for treating human thyroid cancer and possibly other cancers as well.
Acknowledgments
We thank Dr. Brij Singh (University of North Dakota, Grand Forks, ND) for sharing the nonconducting/pore-mutated TRPC1 and HA-TRPC1/pRK5 plasmids. The human ML-1 follicular thyroid cancer cells were provided as a generous gift by Dr. Johann Schönberger (University of Regensburg, Regensburg, Germany), and the human immortal keratinocyte HaCat cells were a generous gift from Dr. Veli-Matti Kähäri (University of Turku, Turku, Finland).
This work was supported by the Sigrid Juselius Foundation and the Center of Excellence in Cell Stress and Molecular Ageing of Åbo Akademi University. The authors declare that they have no conflicts of interest with the contents of this article.
- TRPC
- transient receptor potential canonical
- VEGFR
- vascular endothelial growth factor receptor
- S1P
- sphingosine 1-phosphate
- SOCE
- store-operated calcium entry
- HIF
- hypoxia-inducible factor
- P/S
- penicillin/streptomycin
- Tg
- thapsigargin.
References
- 1. Siegel R., Ma J., Zou Z., Jemal A. (2014) Cancer statistics, 2014. CA Cancer J. Clin. 64, 9–29 [DOI] [PubMed] [Google Scholar]
- 2. Joensuu H., Klemi P., Eerola E. (1986) DNA aneuploidy in follicular adenomas of the thyroid gland. Am. J. Pathol. 124, 373–376 [PMC free article] [PubMed] [Google Scholar]
- 3. Nikiforova M. N., Lynch R. A., Biddinger P. W., Alexander E. K., Dorn G. W., 2nd, Tallini G., Kroll T. G., Nikiforov Y. E. (2003) RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J. Clin. Endocrinol. Metab. 88, 2318–2326 [DOI] [PubMed] [Google Scholar]
- 4. Prevarskaya N., Skryma R., Shuba Y. (2011) Calcium in tumour metastasis: new roles for known actors. Nat. Rev. Cancer 11, 609–618 [DOI] [PubMed] [Google Scholar]
- 5. Riccio A., Medhurst A. D., Mattei C., Kelsell R. E., Calver A. R., Randall A. D., Benham C. D., Pangalos M. N. (2002) mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res. Mol. Brain Res. 109, 95–104 [DOI] [PubMed] [Google Scholar]
- 6. Abramowitz J., Birnbaumer L. (2009) Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 23, 297–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Louis M., Zanou N., Van Schoor M., Gailly P. (2008) TRPC1 regulates skeletal myoblast migration and differentiation. J. Cell Sci. 121, 3951–3959 [DOI] [PubMed] [Google Scholar]
- 8. Kuang C. Y., Yu Y., Wang K., Qian D. H., Den M. Y., Huang L. (2012) Knockdown of transient receptor potential canonical-1 reduces the proliferation and migration of endothelial progenitor cells. Stem Cells Dev. 21, 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nesin V., Tsiokas L. (2014) TRPC1. Handb. Exp. Pharmacol. 222, 15–51 [DOI] [PubMed] [Google Scholar]
- 10. Maceyka M., Harikumar K. B., Milstien S., Spiegel S. (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22, 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pyne S., Pyne N. J. (2013) New perspectives on the role of sphingosine 1-phosphate in cancer. Handb. Exp. Pharmacol. 216, 55–71 [DOI] [PubMed] [Google Scholar]
- 12. Blaho V. A., Hla T. (2014) An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 55, 1596–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pyne N. J., Pyne S. (2010) Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 10, 489–503 [DOI] [PubMed] [Google Scholar]
- 14. Balthasar S., Samulin J., Ahlgren H., Bergelin N., Lundqvist M., Toescu E. C., Eggo M. C., Törnquist K. (2006) Sphingosine 1-phosphate receptor expression profile and regulation of migration in human thyroid cancer cells. Biochem. J. 398, 547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balthasar S., Bergelin N., Löf C., Vainio M., Andersson S., Törnquist K. (2008) Interactions between sphingosine-1-phosphate and vascular endothelial growth factor signalling in ML-1 follicular thyroid carcinoma cells. Endocr. Relat. Cancer 15, 521–534 [DOI] [PubMed] [Google Scholar]
- 16. Löf C., Sukumaran P., Viitanen T., Vainio M., Kemppainen K., Pulli I., Näsman J., Kukkonen J. P., Törnquist K. (2012) Communication between the calcium and cAMP pathways regulate the expression of the TSH receptor: TRPC2 in the center of action. Mol. Endocrinol. 26, 2046–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grynkiewicz G., Poenie M., Tsien R. Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 18. Asghar M. Y., Viitanen T., Kemppainen K., Törnquist K. (2012) Sphingosine 1-phosphate and human ether-à-go-go-related gene potassium channels modulate migration in human anaplastic thyroid cancer cells. Endocr. Relat. Cancer 19, 667–680 [DOI] [PubMed] [Google Scholar]
- 19. Bergelin N., Blom T., Heikkilä J., Löf C., Alam C., Balthasar S., Slotte J. P., Hinkkanen A., Törnquist K. (2009) Sphingosine kinase as an oncogene: autocrine sphingosine 1-phosphate modulates ML-1 thyroid carcinoma cell migration by a mechanism dependent on protein kinase Cα and ERK1/2. Endocrinology 150, 2055–2063 [DOI] [PubMed] [Google Scholar]
- 20. Blom T., Bergelin N., Meinander A., Löf C., Slotte J. P., Eriksson J. E., Törnquist K. (2010) An autocrine sphingosine-1-phosphate signaling loop enhances NF-κB-activation and survival. BMC Cell Biol. 11, 45–2121-11–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalhori V., Kemppainen K., Asghar M. Y., Bergelin N., Jaakkola P., Törnquist K. (2013) Sphingosine-1-phosphate as a regulator of hypoxia-induced factor-1α in thyroid follicular carcinoma cells. PLoS One 8, e66189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wes P. D., Chevesich J., Jeromin A., Rosenberg C., Stetten G., Montell C. (1995) TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. U.S.A. 92, 9652–9656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ambudkar I. S. (2007) TRPC1: a core component of store-operated calcium channels. Biochem. Soc. Trans. 35, 96–100 [DOI] [PubMed] [Google Scholar]
- 24. Ambudkar I. S., Ong H. L., Liu X., Bandyopadhyay B. C., Cheng K. T. (2007) TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium 42, 213–223 [DOI] [PubMed] [Google Scholar]
- 25. Cheng K. T., Ong H. L., Liu X., Ambudkar I. S. (2013) Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr. Top. Membr. 71, 149–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Storch U., Forst A. L., Philipp M., Gudermann T., Mederos y Schnitzler M. (2012) Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J. Biol. Chem. 287, 3530–3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sukumaran P., Löf C., Kemppainen K., Kankaanpää P., Pulli I., Näsman J., Viitanen T., Törnquist K. (2012) Canonical transient receptor potential channel 2 (TRPC2) as a major regulator of calcium homeostasis in rat thyroid FRTL-5 cells: importance of protein kinase Cδ (PKCδ) and stromal interaction molecule 2 (STIM2). J. Biol. Chem. 287, 44345–44360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sukumaran P., Löf C., Pulli I., Kemppainen K., Viitanen T., Törnquist K. (2013) Significance of the transient receptor potential canonical 2 (TRPC2) channel in the regulation of rat thyroid FRTL-5 cell proliferation, migration, adhesion and invasion. Mol. Cell. Endocrinol. 374, 10–21 [DOI] [PubMed] [Google Scholar]
- 29. Cheng H. P., Wei S., Wei L. P., Verkhratsky A. (2006) Calcium signaling in physiology and pathophysiology. Acta Pharmacol. Sin. 27, 767–772 [DOI] [PubMed] [Google Scholar]
- 30. Monteith G. R., McAndrew D., Faddy H. M., Roberts-Thomson S. J. (2007) Calcium and cancer: targeting Ca2+ transport. Nat. Rev. Cancer 7, 519–530 [DOI] [PubMed] [Google Scholar]
- 31. Suchyna T. M., Johnson J. H., Hamer K., Leykam J. F., Gage D. A., Clemo H. F., Baumgarten C. M., Sachs F. (2000) Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J. Gen. Physiol. 115, 583–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suchyna T. M., Tape S. E., Koeppe R. E., 2nd, Andersen O. S., Sachs F., Gottlieb P. A. (2004) Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 430, 235–240 [DOI] [PubMed] [Google Scholar]
- 33. Kalhori V., Törnquist K. (2015) MMP2 and MMP9 participate in S1P-induced invasion of follicular ML-1 thyroid cancer cells. Mol. Cell. Endocrinol. 404, 113–122 [DOI] [PubMed] [Google Scholar]
- 34. Törnquist K., Saarinen P., Vainio M., Ahlström M. (1997) Sphingosine 1-phosphate mobilizes sequestered calcium, activates calcium entry, and stimulates deoxyribonucleic acid synthesis in thyroid FRTL-5 cells. Endocrinology. 138, 4049–4057 [DOI] [PubMed] [Google Scholar]
- 35. Törnquist K., Sukumaran P., Kemppainen K., Löf C., Viitanen T. (2014) Canonical transient receptor potential channel 2 (TRPC2): old name-new games. Importance in regulating of rat thyroid cell physiology. Pflugers Arch. 466, 2025–2034 [DOI] [PubMed] [Google Scholar]
- 36. Viitanen T. M., Sukumaran P., Löf C., Törnquist K. (2013) Functional coupling of TRPC2 cation channels and the calcium-activated anion channels in rat thyroid cells: implications for iodide homeostasis. J. Cell. Physiol. 228, 814–823 [DOI] [PubMed] [Google Scholar]
- 37. Ong H. L., de Souza L. B., Cheng K. T., Ambudkar I. S. (2014) Physiological functions and regulation of TRPC channels. Handb. Exp. Pharmacol. 223, 1005–1034 [DOI] [PubMed] [Google Scholar]
- 38. Crousillac S., Colonna J., McMains E., Dewey J. S., Gleason E. (2009) Sphingosine-1-phosphate elicits receptor-dependent calcium signaling in retinal amacrine cells. J. Neurophysiol. 102, 3295–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Formigli L., Sassoli C., Squecco R., Bini F., Martinesi M., Chellini F., Luciani G., Sbrana F., Zecchi-Orlandini S., Francini F., Meacci E. (2009) Regulation of transient receptor potential canonical channel 1 (TRPC1) by sphingosine 1-phosphate in C2C12 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation. J. Cell Sci. 122, 1322–1333 [DOI] [PubMed] [Google Scholar]
- 40. Xu S. Z., Muraki K., Zeng F., Li J., Sukumar P., Shah S., Dedman A. M., Flemming P. K., McHugh D., Naylor J., Cheong A., Bateson A. N., Munsch C. M., Porter K. E., Beech D. J. (2006) A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ. Res. 98, 1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwab A., Fabian A., Hanley P. J., Stock C. (2012) Role of ion channels and transporters in cell migration. Physiol. Rev. 92, 1865–1913 [DOI] [PubMed] [Google Scholar]
- 42. Bergelin N., Löf C., Balthasar S., Kalhori V., Törnquist K. (2010) S1P1 and VEGFR-2 form a signaling complex with extracellularly regulated kinase 1/2 and protein kinase Cα regulating ML-1 thyroid carcinoma cell migration. Endocrinology 151, 2994–3005 [DOI] [PubMed] [Google Scholar]
- 43. Gratschev D., Löf C., Heikkilä J., Björkbom A., Sukumaran P., Hinkkanen A., Slotte J. P., Törnquist K. (2009) Sphingosine kinase as a regulator of calcium entry through autocrine sphingosine 1-phosphate signaling in thyroid FRTL-5 cells. Endocrinology 150, 5125–5134 [DOI] [PubMed] [Google Scholar]
- 44. Ariano P., Dalmazzo S., Owsianik G., Nilius B., Lovisolo D. (2011) TRPC channels are involved in calcium-dependent migration and proliferation in immortalized GnRH neurons. Cell Calcium 49, 387–394 [DOI] [PubMed] [Google Scholar]
- 45. Fabian A., Fortmann T., Bulk E., Bomben V. C., Sontheimer H., Schwab A. (2011) Chemotaxis of MDCK-F cells toward fibroblast growth factor-2 depends on transient receptor potential canonical channel 1. Pflugers Arch. 461, 295–306 [DOI] [PubMed] [Google Scholar]
- 46. Shi Y., Zou M. (2004) Matrix metalloproteinases in thyroid cancer. Cancer Treat. Res. 122, 179–190 [DOI] [PubMed] [Google Scholar]
- 47. Baldini E., Toller M., Graziano F. M., Russo F. P., Pepe M., Biordi L., Marchioni E., Curcio F., Ulisse S., Ambesi-Impiombato F. S., D'Armiento M. (2004) Expression of matrix metalloproteinases and their specific inhibitors in normal and different human thyroid tumor cell lines. Thyroid 14, 881–888 [DOI] [PubMed] [Google Scholar]
- 48. Birnbaumer L. (2009) The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu. Rev. Pharmacol. Toxicol. 49, 395–426 [DOI] [PubMed] [Google Scholar]
- 49. Tajeddine N., Gailly P. (2012) TRPC1 protein channel is major regulator of epidermal growth factor receptor signaling. J. Biol. Chem. 287, 16146–16157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li M., Chen C., Zhou Z., Xu S., Yu Z. (2012) A TRPC1-mediated increase in store-operated Ca2+ entry is required for the proliferation of adult hippocampal neural progenitor cells. Cell Calcium 51, 486–496 [DOI] [PubMed] [Google Scholar]
- 51. Woo J. S., Cho C. H., Kim do H., Lee E. H. (2010) TRPC3 cation channel plays an important role in proliferation and differentiation of skeletal muscle myoblasts. Exp. Mol. Med. 42, 614–627 [DOI] [PMC free article] [PubMed] [Google Scholar]