Background: The B cell survival factors B cell activation factor (BAFF) and a proliferation-inducing ligand (APRIL) can heteromerize.
Results: BAFF-APRIL2 and APRIL-BAFF2 heteromers have distinct receptor-binding specificities and activities.
Conclusion: BAFF-APRIL2 resembles APRIL, and APRIL-BAFF2 resembles BAFF but poorly activates the BAFF receptor.
Significance: Heteromers should be taken into account when evaluating the physiology or pharmacological inhibition of BAFF and APRIL.
Keywords: cytokine, immunology, protein engineering, receptor, signaling, APRIL, BAFF, heteromers
Abstract
The closely related TNF family ligands B cell activation factor (BAFF) and a proliferation-inducing ligand (APRIL) serve in the generation and maintenance of mature B-lymphocytes. Both BAFF and APRIL assemble as homotrimers that bind and activate several receptors that they partially share. However, heteromers of BAFF and APRIL that occur in patients with autoimmune diseases are incompletely characterized. The N and C termini of adjacent BAFF or APRIL monomers are spatially close and can be linked to create single-chain homo- or hetero-ligands of defined stoichiometry. Similar to APRIL, heteromers consisting of one BAFF and two APRILs (BAA) bind to the receptors B cell maturation antigen (BCMA), transmembrane activator and CAML interactor (TACI) but not to the BAFF receptor (BAFFR). Heteromers consisting of one APRIL and two BAFF (ABB) bind to TACI and BCMA and weakly to BAFFR in accordance with the analysis of the receptor interaction sites in the crystallographic structure of ABB. Receptor binding correlated with activity in reporter cell line assays specific for BAFFR, TACI, or BCMA. Single-chain BAFF (BBB) and to a lesser extent single-chain ABB, but not APRIL or single-chain BAA, rescued BAFFR-dependent B cell maturation in BAFF-deficient mice. In conclusion, BAFF-APRIL heteromers of different stoichiometries have distinct receptor-binding properties and activities. Based on the observation that heteromers are less active than BAFF, we speculate that their physiological role might be to down-regulate BAFF activity.
Introduction
BAFF,3 also known as BLyS (B lymphocyte stimulator), is a TNF family ligand primarily expressed by myeloid cells and radiation-resistant stromal cells of secondary lymphoid organs. Together with its close relative APRIL, BAFF acts on peripheral B cells at various stages of differentiation through three receptors as follows: BAFFR that responds to BAFF only, and TACI and BCMA that bind to both BAFF and APRIL (1). BAFFR controls the survival and metabolic fitness of peripheral B cells, with the exception of memory cells, plasma cells, and B1 B cells (2–4). BAFFR is highly expressed in naive B cells but decreases and finally completely disappears during activation and differentiation of B cells to plasma cells. In contrast, TACI is transiently expressed in B cells upon activation, for example in response to Toll-like receptor ligands (5). Its expression is particularly high in marginal zone B cells, but, as for BAFFR, it is not maintained in plasma cells. The role of TACI is complex. It is required for efficient antibody production in response to type II T-independent antigens, but at the same time it exerts a negative role on B cells. Indeed, TACI-deficient mice display higher numbers of B cells than their wild type counterparts (6, 7). In marginal zone B cells, simultaneous engagement of TACI and Toll-like receptors induces expression of both Fas and FasL to help contract this innate-like type of response by apoptotic cell death at a later stage (8). Finally, BCMA is expressed late during B cell differentiation and is probably the prime receptor mediating the action of BAFF and APRIL on plasma cells (2, 9). The blockade of BAFF with a monoclonal antibody has proven to be moderately effective in the autoimmune disease systemic lupus erythematosus, and several other reagents blocking either BAFF alone or BAFF and APRIL are presently in clinical development (10).
BAFF exists in membrane-bound (11) or -soluble (12) forms, and the latter can crystallize as 3- or 60-mers (13, 14). Although APRIL is mainly released in a soluble form (15), it can bind to proteoglycans and thus create niches for antibody-secreting cells (16, 17). Heteromers of BAFF and APRIL are produced in autoimmune diseases and are sometimes also detected in healthy subjects (18, 19). Because of the intrinsic difficulty of producing and purifying BAFF-APRIL heteromers, these ligands have remained incompletely characterized. Here, we produced single-chain heteromers of BAFF and APRIL with defined compositions; we crystallized one of them and characterized their receptor-binding profile, their activities, and their susceptibility to BAFF or BAFF and APRIL inhibitors.
Experimental Procedures
Animals
C57BL/6 WT and BAFF-KO were as described (20). Mice were handled according to guidelines and under the authorization of the Swiss Federal Food Safety and Veterinary Office (authorization 1370.6 to P. S.).
Plasmids and Recombinant Proteins
Expression plasmids used in this study were constructed using standard molecular biology techniques. Proteins they code for are described in Table 1. Atacicept was provided by Merck, KGaA. Belimumab (registered trade name Benlysta) was purchased from the Pharmacy of Lausanne University Hospital. Other recombinant proteins were produced essentially as described (21, 22).
TABLE 1.
Plasmids used in this study
aa is amino acid. HA signal = MAIIYLILLFTAVRG Ig signal = MNFGFSLIFLVLVLKG CamLinker = PQPQPKPQPKPEPEGS PreSci = LEVLFQGP TEV = ENLYFQ.
| Plasmid | Designation | Protein encoded | Vector |
|---|---|---|---|
| ps193 | FLAG-hTNF | HA signal-FLAG-GPGQVQLQ-hTNF (aa 85–263) | PCR3 |
| ps420 | hTNFR1-Fc | hTNFR1 (aa 1–217)-VD-hIgG1 (aa 245–470) | PCR3 |
| ps515 | EGFP | Enhanced green fluorescent protein | PCR3 |
| ps645 | FLAG-hLTa | HA signal-FLAG-GPGQVQLQVD-hTNF (aa 43–205) | PCR3 |
| ps739 | hBCMA-Fc | Ig signal-EVKLVPRGS-hBCMA (aa 2–54)-VD-hIgG1 (aa 245–470) | PCR3 |
| ps897 | hTACI-GPI | HA signal-LD-hTACI (aa 2–160)-VD-hTRAILR3 (aa 157–269) | PCR3 |
| ps1196 | Fc-hBAFF | HA signal-LD-hIgG1 (aa 245–470)-RS-CamLinker-PreSci-GSLQ-hBAFF (aa 137–285) | PCR3 |
| ps1377 | pMSCV-puro | Modified pMSCV-puro (<zco;clontech>Clontech) with HindIII-BglII-EcoRI-NotI-XhoI-HpaI-ApaI cloning sites | ps1377 |
| ps1406 | Fc-hLTb | HA signal-LD-hIgG1 (aa 245–470)-RS-CamLinker-PreSci-GSLQ-hLTb (aa 66–244) | PCR3 |
| ps1467 | hBCMA-GPI | Ig signal-VQCEVKLVPRGS-hBCMA (aa 2–54)-VD-hTRAILR3 (aa 157–269) | PCR3 |
| ps1589 | hBAFFR-GPI | HA signal-LE-hBAFFR (aa 2–71)-EFGSVD-hTRAILR3 (aa 157–269) | PCR3 |
| ps1799 | mLTbR-Fc | mLTbR (aa 1–220)-VD-PreSci-hIgG1 (aa 245–470) | PCR3 |
| ps2297 | mBAFFR-Fc | HA signal-LD-mBAFFR (aa 2–70)-VD-hIgG1 (aa 245–470) | PCR3 |
| ps2308 | hBAFFR:Fas | HA signal-LE-hBAFFR (aa 2–71)-EFGSVD-hFas (aa 169–355) | ps1377 |
| ps2309 | hBCMA:Fas | Ig signal-VQCEVKLVPRGS-hBCMA (aa 2–54)-VD-hFas (aa 169–335) | ps1377 |
| ps2455 | hTACI:Fas | HA signal-L-hTACI (aa 1–118)-VD-hFas (aa 169–335) | ps1377 |
| ps2642 | FLAG-hLT-ααα | HA signal-FLAG-GPGQVQLQVD-hLTa (aa 58–205)-GGGGS-hLTa (aa 58–205)-GGGGS-hLTa (aa 58–205) | PCR3 |
| ps2643 | FLAG-hLT-ααβ | HA signal-FLAG-GPGQVQLQVD-hLTa (aa 58–205)-GGGGS-hLTa (aa 58–205)-GGGGS-hLTb (aa 83–244) | PCR3 |
| ps2644 | FLAG-hLT-ββα | HA signal-FLAG-GPGQVQLQ-hLTb (aa 83–244)-GGGGS-hLTa hLTb (aa 83–244)-GGGGS-hLTa (aa 58–205) | PCR3 |
| ps2645 | FLAG-hLT-βββ | HA signal-FLAG-GPGQVQLQ-hLTb (aa 83–244)-GGGGS-hLTa hLTb (aa 83–244)-GGGGS-hLTb (aa 83–244) | PCR3 |
| ps2680 | Fc-BBB | HA signal-LD-hIgG1 (aa 245–470)-RS-CamLinker-LQ-hBAFF (aa 140–285)-GGGGS-hBAFF (aa 140–285)-GGGGS-hBAFF (aa 140–285) | PCR3 |
| ps2826 | Fc-hAPRIL | HA signal-LD-hIgG1 (aa 245–470)-RS-CamLinker-GSLQ-hAPRIL (aa 115–250) | PCR3 |
| ps2876 | Fc-AAA | HA signal-LD-hIgG1 (aa 245–470)-RS-CamLinker-LQ-hAPRIL (aa 112–250)-GGGGS-hAPRIL (aa 112–250)-GGGGS-hAPRIL (aa 112–250) IRES GFP | PCR3 |
| ps2877 | Fc-BAA | HA signal-LD-hIgG1 (aa 245–470)-RS-CamLinker-LQ-hBAFF (aa 140–285)-GGGGS-hAPRIL (aa 112–250)-GGGGS-hAPRIL (aa 112–250) IRES GFP | PCR3 |
| ps2879 | FLAG-AAA | HA signal-FLAG-GPGQVQLQ-hAPRIL (aa 112–250)-GGGGS-hAPRIL (aa 112–250)-GGGGS-hAPRIL (aa 112–250) IRES GFP | PCR3 |
| ps2883 | Fc-ABB | HA signal-LD-hIgG1 (aa 245–470)-RS-CamLinker-LQ-hAPRIL (aa 112–250)-GGGGS-hBAFF (aa 140–285)-GGGGS-hBAFF (aa 140–285) IRES GFP | PCR3 |
| ps2886 | FLAG-ABB | HA signal-FLAG-GPGQVQLQ-hAPRIL (aa 112–250)-GGGGS-hBAFF (aa 140–285)-GGGGS-hBAFF (aa 140–285) IRES GFP | PCR3 |
| ps2889 | FLAG-BAA | HA signal-FLAG-GPGQVQLQ-hBAFF (aa 140–285)-GGGGS-hAPRIL (aa 112–250)-GGGGS-hAPRIL (aa 112–250) IRES GFP | PCR3 |
| ps2890 | FLAG-BBB | HA signal-FLAG-GPGQVQLQ-hBAFF (aa 140–285)-GGGGS-hBAFF (aa 140–285)-GGGGS-hBAFF (aa 140–285) IRES GFP | PCR3 |
| ps3413 | hTACI-Fc | Modified Ig signal (METDTLLLWVLLLWVPGVHG)-hTACI (aa 31–110) hIgG1 (aa 245–470; L258E, A353S, P354S) | PCR3 |
| pcDNA3.1_AAB | AAB | Ig signal-His6-FLAG-TEV-GS-hAPRIL (aa 111–250; T126A)-GGGGS-hAPRIL (aa 111–250; T126A)-GGGGS-hBAFF (aa 140–285) | pcDNA3.1 |
| pcDNA3.1_ABA | ABA | Ig signal-His6-FLAG-TEV-GS-hAPRIL (aa 111–250; T126A)-GGGGS-hBAFF (aa 140–285)-GGGGS-hAPRIL (aa 111–250; T126A) | pcDNA3.1 |
| pcDNA3.1_BAA | BAA | Ig signal-His6-FLAG-TEV-GS-hBAFF (aa 140–285)-GGGGS-hAPRIL (aa 111–250; T126A)-GGGGS-hAPRIL (aa 111–250; T126A) | pcDNA3.1 |
| pcDNA3.1_BBA | BBA | Ig signal-His6-FLAG-TEV-GS-hBAFF (aa 140–285)-GGGGS-hBAFF (aa 140–285)-GGGGS-hAPRIL (aa 111–250; T126A) | pcDNA3.1 |
| pcDNA3.1_BAB | BAB | Ig signal-His6-FLAG-TEV-GS-hBAFF (aa 140–285)-GGGGS-hAPRIL (aa 111–250; T126A)-GGGGS-hBAFF (aa 140–285) | pcDNA3.1 |
| pcDNA3.1_ABB | ABB | Ig signal-His6-FLAG-TEV-GS-hAPRIL (aa 111–250; T126A)-GGGGS-hBAFF (aa 140–285)-GGGGS-hBAFF (aa 140–285) | pcDNA3.1 |
Transfections
HEK 293T cells were grown in DMEM, 10% fetal calf serum (FCS) and transfected by the calcium phosphate or polyethyleneimide methods. Cells were grown for 7 days in serum-free Opti-MEM medium (Invitrogen) for the production of single-chain lymphotoxins or for 48 h in complete medium for surface expression of receptors:TRAILR3 fusion proteins.
CHO cells (Sigma) grown in DMEM/F-12 (1:1) supplemented with 2% FCS were transfected with polyethylenimide or Polyfect (Qiagen) and selected 48 h later in medium containing 500 μg/ml G418 sulfate (Calbiochem). Cells were cloned by limiting dilution or by two or three rounds of FACS sorting when constructs of interest were linked to green fluorescent protein expression via an internal ribosome entry site sequence. The best producing clones were selected.
Protein Production and Purification
Conditioned supernatants were affinity-purified on anti-FLAG M2-agarose (Sigma) or protein A-Sepharose (GE Healthcare) for FLAG- and Fc-tagged ligands, respectively, essentially as described (21). Single-chain ligands were subsequently fractionated by size exclusion chromatography on a Superdex 200 column (GE Healthcare) equilibrated in PBS. Protein concentrations were inferred from the extinction coefficient calculated from the theoretical amino acid sequence. Untagged single-chain heteromers were obtained by secretion from HEK 293 cells with N-terminal His6-FLAG tags, affinity chromatography on nickel-nitrilotriacetic acid, tag cleavage with tobacco etch virus protease, and size exclusion chromatography on a Superdex 200 column. The final purified proteins were prepared in 20 mm Hepes/NaOH, pH 7.5, 150 mm NaCl and were concentrated on 30-kDa ultrafiltration devices (Vivascience).
SDS-PAGE and Western Blot
SDS-PAGE and Western blot were performed according to standard procedures. Proteins were revealed directly with horseradish peroxidase-coupled anti-human IgG antibody or in two steps with anti-FLAG M2 at 1 μg/ml, anti-hBAFF Buffy-2 rat IgM at 1 μg/ml, or anti-hAPRIL Aprily-2 mouse IgG1 at 1 μg/ml, followed by appropriate horseradish peroxidase-coupled secondary reagents. Coomassie blue staining was performed with a semidry iD Stain System (Eurogentec).
Receptor-Ligand Interaction ELISA
The direct binding of FLAG-tagged ligands to immobilized receptor-Fcs, either adsorbed to or captured via anti-human antibodies in 96-well immunoplates, was revealed with biotinylated anti-FLAG M2 antibody (Sigma) and horseradish-coupled streptavidin as described previously in detail (22). For the competition ELISA, titrated amounts of untagged ligands were added to immobilized receptor-Fcs for 30 min and followed, without intermediate washing step, by an empirically determined fixed and nonsaturating concentration of FLAG-tagged ligand sufficient to generate ∼80% of the maximal signal in the absence of competitor (22).
Cytotoxic Assays with Receptor:Fas Reporter Cell Lines
Jurkat BCMA:Fas (cl13) and Jurkat JOM2 TACI:Fas (cl112.3) have been described (20, 23). Similarly, Jurkat JOM2 BAFFR:Fas cell lines (cl21) were generated as described in detail elsewhere by retrovirus-mediated transduction of a BAFFR:Fas chimeric receptor, puromycin selection, and screening of clones for their selective ability to respond to Fc-BAFF by apoptotic cell death (22). Reporter cells were incubated for 16 h with the indicated concentrations of ligands, after which time cell viability was measured by the phenazine methosulfate/3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay essentially as described (22). FLAG-tagged ligands were used in the presence of 1 μg/ml anti-FLAG M2 antibody.
Crystallography
Crystals of the single-chain APRIL-BAFF-BAFF heterotrimer (His6-FLAG-TEV-GS-hAPRIL (aa 111–250, T126A)-GGGGS-hBAFF(140–285)-GGGGS-hBAFF(140–285), with cleaved tags) were obtained by mixing 0.5 μl of protein solution (6 mg/ml in 20 mm Hepes/NaOH, pH 7.5, 150 mm NaCl) with 0.5 μl of reservoir solution (0.1 m Tris/HCl, pH 8.75, 14% PEG6000 (w/v), and 1 m LiCl) using the hanging drop vapor diffusion method at 293 K. Before flash-freezing in liquid nitrogen, crystals were cryo-protected by reservoir solution supplemented with 10% (v/v) 2,3-butanediol.
Diffraction data were collected at 100 K at beamline X06SA (Swiss Light Source, Villigen, Switzerland). Data were processed to 2.43 Å resolution using the programs XDS and XSCALE (24). The crystals belong to space group C 2 2 21 containing two heterotrimers per asymmetric unit. Initial phases were obtained by molecular replacement using Phaser and the published structures of APRIL (Protein Data Bank code 1Q5X) and BAFF (Protein Data Bank code 1KD7) as search models (25). Multiple rounds of manual model building in COOT and bulk solvent correction, positional, B-factor, and TLS refinement using REFMAC and BUSTER yielded the final model (26–29). Data collection and model statistics are shown in Table 2. The atomic coordinates and structure factors have been deposited in the Protein Data Bank, Protein Data Bank code 4ZCH. Images were generated with the PyMOL Molecular Graphics System, Schrödinger, LLC.
TABLE 2.
Crystallographic data and model refinement statistics
Administration of Proteins to BAFF-deficient and Wild Type Animals
Fc-BBB, Fc-ABB, Fc-BAA, Fc-APRIL, and PBS were administered intraperitoneally to BAFF-KO mice at 1 mg/kg on days 0, 4, 7, 11, and 14. Untreated wild type mice were used as controls. Mice were sacrificed at day 18, and spleens and lymph nodes (inguinal, axillary, and brachial) were collected, homogenized, incubated in ACK lysis buffer (150 mm NH4Cl, 10 mm KHCO3, 10 μm Na2-EDTA) for 5 min on ice to lyse red blood cells, washed in PBS 2% FCS, and filtered on a nylon mesh.
FACS Analyses
293T cells co-transfected with EGFP and receptor-GPI expression plasmids were stained with titrated amounts of FLAG- or Fc-tagged ligands, whose binding was revealed with appropriate phycoerythrin-coupled secondary reagents, as described (22, 30). Cells were analyzed using FACScan or Accuri 6 flow cytometers (BD Biosciences) and FlowJo software (TreeStar, Ashland, OR).
Splenocytes or lymph node cells were incubated with anti-CD16/32 (Fc-block, clone 93) and stained with a mix of anti-CD19-PE.Cy7 (clone eBio1D3, 1:200), anti-CD93-biot (mAb493, 1:100, a kind gift of Antonius Rolink, University of Basel, Switzerland), and anti-CD3-APC (clone 17A2, 1:100) (all from eBiosciences) for 20 min on ice followed by phycoerythrin Cy5.5-coupled streptavidin (1:100) and analysis with a FACS Canto (BD Biosciences). Data were analyzed with the FlowJo software.
Results
Production of Functional Single-chain Heteromers of LTα and -β with Defined Stoichiometry
TNF family members share a common structural fold, the TNF homology domain, that trimerizes to form receptor-binding sites at the interface of ligand subunits. In the structure of TNF and of several other TNF family ligands, the N- and C-terminal regions of monomeric ligand subunits are only 5–11 Å away from each other (14, 31, 32), making it possible to link three individual monomers within a trimer by replacing the Stop codon of the first and second monomers by short linker peptides (sequence GGGGS) to produce single-chain ligands (Fig. 1A). TNF is known to remain active as a single-chain ligand (33). We first validated the single-chain ligand approach using lymphotoxin-α and -β (LTα and LTβ). LTα and LTβ form both homo- and heterotrimers of physiologic relevance that differ in their receptor-binding profile: LTα binds to TNFR1 and LTα1β2 binds to LTβR (34). Moreover, single-chain LTα1β2 remains active (35).
FIGURE 1.
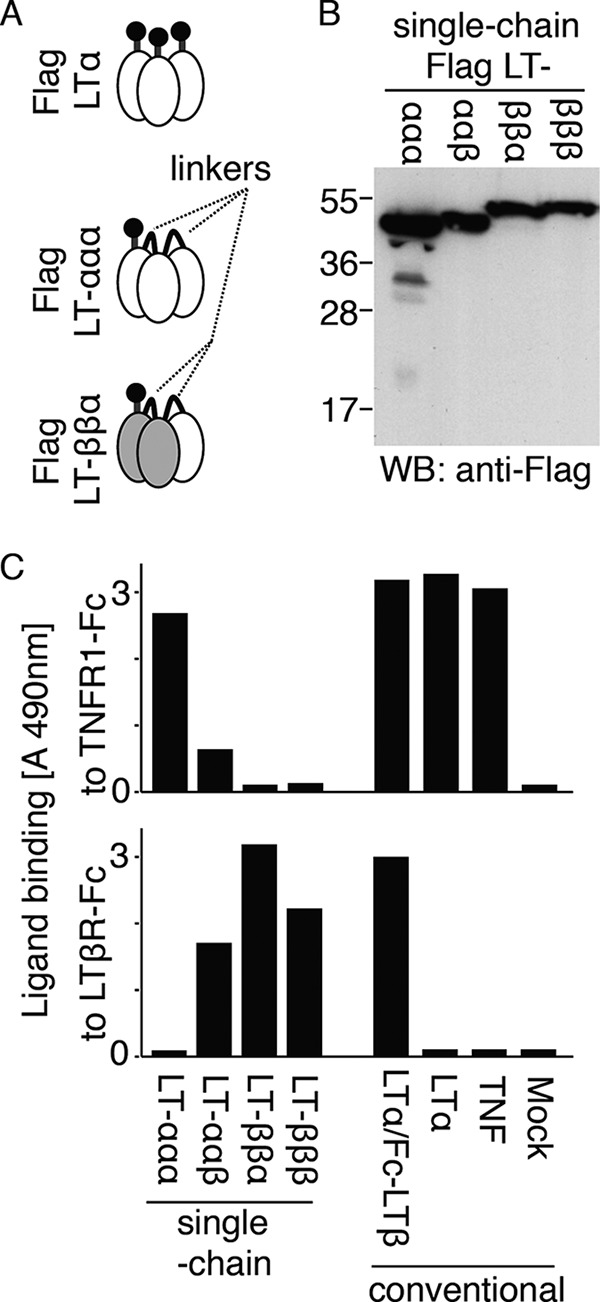
Receptor-binding specificity of lymphotoxin-αβ heteromers of defined stoichiometries. A, schematic representation of “conventional” (FLAG LTα) and single-chain ligands (FLAG single-chain LTα-LTα-LTα, FLAG LT-ββα) in which individual monomers are covalently linked by a flexible linker of five amino acid residues. B, anti-FLAG Western blot (WB) of the indicated single-chain FLAG-tagged lymphotoxins. C, binding of the indicated FLAG-tagged conventional or single-chain ligands to immobilized TNFR1-Fc (upper panel) or LTβR-Fc (lower panel), as measured in an ELISA-based assay. LTα/Fc-LTβ is FLAG-LTα co-expressed with Fc-LTβ.
Single-chain FLAG-tagged constructs expressing LTα and LTβ in various combinations (ααα, ααβ, ββα, and βββ, theoretical molecular weight of 51.5, 52.4, 53.0, and 53.9) were all successfully expressed and secreted by 293T cells (Fig. 1B). Conventional FLAG-TNF, FLAG-LTα, or FLAG-LTα co-expressed with Fc-LTβ were used as controls. As expected, FLAG-TNF and FLAG-LTα bound TNFR1, but not LTβR, in an ELISA-based assay, and the same was true for single-chain LTα-LTα-LTα (Fig. 1C). LT-ββα, the bona fide ligand for LTβR, and single-chain LTβ-LTβ-LTβ bound to LTβR but not to TNFR1, whereas single-chain LTα-LTα-LTβ showed an intermediate specificity with some binding to both TNFR1 and LTβR (Fig. 1C). Taken together, these results indicate that both LTα and LTβ retain the ability to interact with cognate receptors when modified at their C termini and expressed as single-chain ligands. The observation that LT-ββα did not bind to TNFR1 strongly suggests that, as expected, the stoichiometry is fixed in single-chain ligands. Indeed, if α subunits from different single-chain heteromers had the capacity to assemble into pure LTα trimers, binding to TNFR1 should have been detected, which was not the case.
Expression and Purification of Single-chain BAFF, APRIL, and BAFF-APRIL Heteromers
Affinity-purified FLAG- and Fc-tagged single-chain ligands produced in CHO cells migrated by SDS-PAGE with sizes of about 60 and 100 kDa, respectively, corresponding roughly to the predicted molecular masses of the glycosylated proteins (Fig. 2A and Table 3). A Western blot analysis confirmed the presence of both BAFF and APRIL in the single-chain heteromers and of either BAFF or APRIL in the homomers (Fig. 2A). Despite several expression attempts, single-chain APRIL was only obtained in low yield, especially when Fc-tagged.
FIGURE 2.
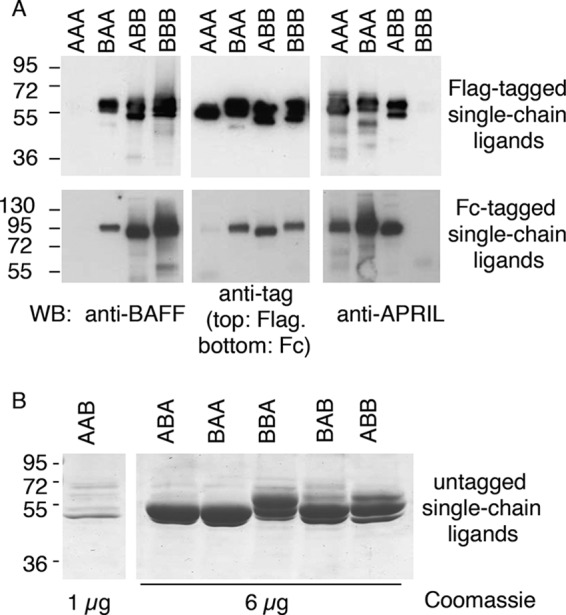
SDS-PAGE analysis of single-chain BAFF-APRIL heteromers. A, Western blot analysis with 200 ng/lane of FLAG-tagged or 100 ng/lane of Fc-tagged single-chain BAFF-APRIL heteromers (except for Fc-AAA, ∼20 ng) revealed as indicated with either anti-BAFF Buffy-2, anti-FLAG M2, anti-APRIL Aprily-2 monoclonal antibodies, or with an anti-Fc antibody. B, Coomassie Blue staining of 6 μg/lane (except for AAB, 1 μg) of the indicated untagged single-chain BAFF-APRIL heteromers. WB, Western blot.
TABLE 3.
Sizes of single-chain constructs of BAFF and APRIL
| Constructsa | Theoretical no. of N-linkedb | Theoretical approximate mass (+N-linked)c | Observed approximate mass, WB + DTT | Observed approximate mass, S200 | Ratio, S200/WB |
|---|---|---|---|---|---|
| kDa | kDa | kDa | |||
| FLAG-AAA | 6 | 64.3 | 58 | 85 | 1.5 |
| FLAG-BAA | 5 | 62.5 | 60 | 90 | 1.5 |
| FLAG-ABB | 4 | 60.9 | 59 | 100 | 1.7 |
| FLAG-BBB | 3 | 59.3 | 58 | 95 | 1.6 |
| Fc-AAA | 7 | 92.7 | 98 | 270 | 2.8 |
| Fc-BAA | 6 | 91 | 98 | 330 | 3.4 |
| Fc-ABB | 5 | 89.4 | 93 | 500 | 5.4 |
| Fc-BBB | 4 | 88 | 100 | 500 | 5.0 |
| BAA, ABA, AAB | 3 | 56.1 | 51d | 40 | 0.8 |
| ABB, ABA, BBA | 3 | 56.8 | 54e | 45 | 0.8 |
a Tagged constructs were produced in CHO cells, and untagged constructs were produced in HEK 293 cells.
b Number of consensus N-glycosylation sites (NX(S/T)) is shown.
c We assume that all consensus sites are glycosylated and counted 2.5 kDa per N-linked glycan.
d 51- and 54-kDa bands were observed and are probably N-glycosylation species.
e 51-, 54-, and 57-kDa bands were observed and are probably N-glycosylation species.
FLAG-tagged ligands were eluted by size exclusion chromatography with apparent molecular masses 1.5–1.7-fold higher than the expected ones (Table 3). We find it unlikely that these molecules assemble as dimers and favor the hypothesis that the proteins have either nonglobular shapes and/or that the contribution of N-linked glycans to the hydrodynamic volume is higher than anticipated.
Fc-tagged single-chain ligands migrated by size exclusion chromatography with apparent sizes 2.8 to 5 times that of the monomeric single chain (Table 3), although in theory, given the dimeric nature of the Fc, it should have been only twice the size. Sizes of nonglobular proteins are overestimated by size exclusion chromatography, and we hypothesize that the presence of rod-like linkers (PQPQPKPQPKPEPEGS) between the Fc portion and the single-chain ligands might have contributed to the observed migration. Fc-tagged ABB and BBB were bigger by size exclusion chromatography than Fc-tagged BAA and AAA, and they might represent structures composed of four single chains (i.e. containing two dimeric Fc). When present, aggregates eluting in the void volume had been analyzed separately and share the same receptor-binding properties as nonaggregated ligands (data not shown).
Single-chain heteromers of BAFF and APRIL constructed in six possible combinations (AAB, ABA, BAA, ABB, BAB, and BBA) were also produced as untagged proteins in HEK 293 cells (Fig. 2B). In these constructs, one N-glycosylation site of APRIL was mutated (T126A). These proteins migrated by SDS-PAGE and size exclusion chromatography with sizes compatible with the theoretical ones (Fig. 2B and Table 3).
Taken together, these results indicate that single-chain homomers and heteromers of BAFF and APRIL can be produced but for single-chain APRIL only in low yield. All experiments were performed with size-fractionated ligands to exclude contributions of high molecular weight aggregates.
Crystal Structure of Single-chain APRIL-BAFF-BAFF Heteromer
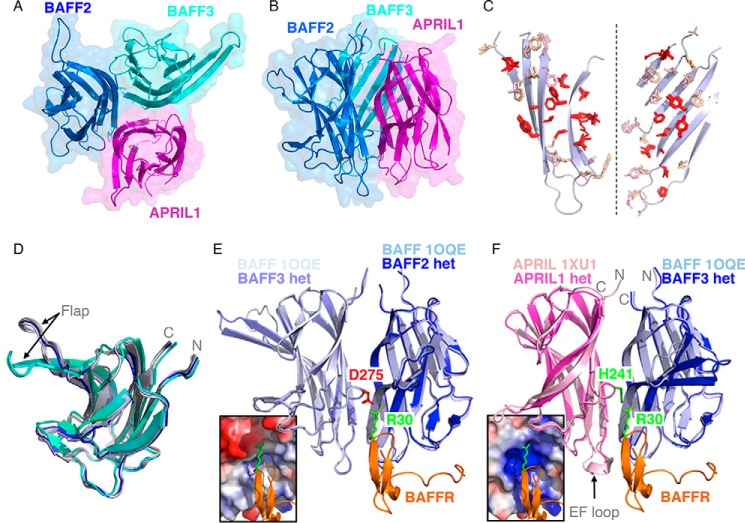
The structure of untagged single-chain ABB determined by crystallography at a resolution of 2.43 Å revealed the expected trimeric arrangement between the APRIL and the two BAFF subunits (Fig. 3, A and B). Interfaces between BAFF and APRIL involve residues that are mostly identical or conserved between both ligands, with conserved residues present at the core of the interaction surface, explaining why heteromerization can take place (Fig. 3C). Subunits of BAFF monomers adopt a folding very similar to that of BAFF previously crystallized as 3- or 60-mers (r.m.s.d. of 0.47 to 0.54 Å) (Fig. 3D) (13, 14, 36–38), and the same was true for the APRIL subunit that resembled previously crystallized mouse APRIL (r.m.s.d. of 1.56 or 0.76 Å when excluding loop EF that is different in both structures) (Fig. 3F) (32). In particular, the N and C termini of BAFF and APRIL in the heteromer superimposed with those of conventional BAFF and APRIL, indicating that GGGGS linkers between BAFF and APRIL subunits have no noticeable impact on the structure (Fig. 3, D–F). Except for a single glycine residue at the C terminus of the central BAFF subunit, linkers were flexible and unresolved in the structure. It is noteworthy that BAFF subunits at the C termini of both ABBs in the asymmetric unit of the crystal displayed a striking structural difference in the “flap” region, i.e. the loop between β-sheets D and E (r.m.s.d. of 2.25 Å between BAFF2 and BAFF3, but only 0.4 Å when excluding the DE loop). In these BAFF subunits, the flap adopts a different but well structured conformation in which the DE β-pleated sheet is longer, and the loop is reduced in size (Fig. 3D).
FIGURE 3.
Structural features of an APRIL-BAFF-BAFF heteromer. A, top view of a single-chain APRIL-BAFF-BAFF heteromer, with APRIL in magenta, and both BAFFs (BAFF2 and BAFF3) in blue and cyan. Ribbon drawing of the structure, in a semi-transparent space-filling representation. B, side view of the APRIL-BAFF-BAFF heteromer with the same color code as in A. C, residues that are involved in protomer interactions and that are conserved in the five interfaces examined (BAFF-BAFF in 1OQE, APRIL-APRIL in 1XU1, BAFF3-BAFF2, APRIL1-BAFF3, and BAFF2-APRIL1 in ABB) are shown in red. Other residues of BAFF (wheat) or APRIL (light pink) that participate in protomer-protomer interactions are also shown. Portions of the relevant β-sheets of BAFF are shown in pale blue (β-sheets of APRIL, not shown, are essentially superimposable). The two protomers were rotated by about 90° and −90°, respectively, so that both sides of the interaction surface face the reader. D, ribbon representation of superimposed monomers of BAFF taken from structures of BAFF 3-mers (shades of light blue, 1KD7 and 1KXG), BAFF 60-mers (shades of gray, 1ODQ, 1OQE, and 4V46), and ABB heteromer (second BAFF domain, dark blue; third BAFF domain, shades of cyan, as found in the two heteromers of the crystallization unit). The position of the DE loop (Flap) and of the N and C termini are indicated. E, two BAFF protomers (light shades of blue) and one BAFFR molecule (orange) taken from the crystal structure of BAFF 60-mer in complex with BAFFR (1OQE) were superimposed with the two adjacent BAFF domains (in dark shades of blue) of single-chain ABB. The side chains of Arg-30 of BAFFR and Asp-275 of BAFF, which are both determinants of BAFFR specificity for BAFF and not APRIL, are shown in green and red, respectively. The electrostatic potential of the relevant portion of the BAFF-BAFF interface in the heteromer is shown in the inset, from red (negative) to blue (positive). F, relevant ligand subunits of BAFF (1OQE), APRIL (1XU1), and single-chain ABB were superimposed. APRIL and BAFF subunits of the ABB heteromer are shown in dark shades of magenta and blue, respectively. One BAFF subunit (light blue) and one BAFFR (orange) of the BAFF-BAFFR complex (1OQE) are also shown, together with a relevant subunit of mouse APRIL (pink) of the APRIL-TACI complex (1XU1). N and C termini are indicated. Side chains of Arg-30 of BAFFR and His-241 of APRIL are shown in green. The electrostatic potential of the APRIL-BAFF interface is shown in the inset.
The receptor binding regions of the heteromer were examined for their predicted ability to accommodate BAFFR. Most TNF family receptors have elongated extracellular domains that contact ligands at the interface of two adjacent ligand protomers (39, 40). In contrast, BAFFR mainly contacts a single BAFF protomer (36, 37). Arg-30 of BAFFR, a known determinant of its specificity for BAFF and not APRIL (41), is also contacted by Asp-275 of the adjacent BAFF protomer (Fig. 3E). APRIL has a histidine at the corresponding position (His-241), and compared with the BAFF-BAFF interface, the APRIL-BAFF interface more generally lacks the negatively charged microenvironment that accommodates Arg-30 of BAFFR (Fig. 3F). Thus, ABB may present only one high affinity site for BAFFR instead of three in BBB, which would result in a decreased avidity for BAFFR.
In summary the crystal structure of single-chain ABB indicates the following: (i) linkers do not interfere with folding and assembly of BAFF and APRIL subunits; (ii) the structure of human and mouse APRIL monomers are very similar; (iii) the flap of the C-terminal BAFF subunit of ABB adopts a structure that has never been observed before; and (iv) ABB is predicted to bind BAFFR with a lower avidity than BAFF.
Receptor Binding Specificity of Single-chain BAFF, APRIL, and BAFF-APRIL Heteromers
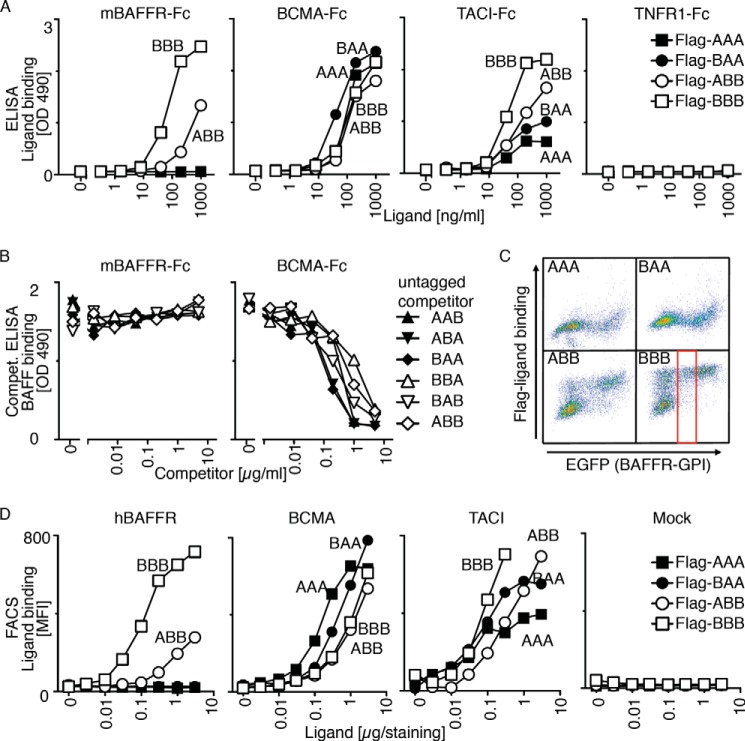
The binding of FLAG-tagged single-chain ligands to immobilized BAFFR-Fc, BCMA-Fc, and TACI-Fc was characterized in an ELISA-based assay. FLAG-BBB bound BAFFR, BCMA, and TACI but not the irrelevant TNFR1, and FLAG-AAA only bound BCMA and TACI, as observed previously for conventional ligands. For heteromers, FLAG-BAA behaved like AAA. FLAG-ABB bound all three receptors, but its binding to BAFFR, and to a lesser extent TACI, was clearly less efficient than that of BBB (Fig. 4A). In line with these results, untagged heteromers were unable to compete for FLAG-BAFF binding to BAFFR, indicating that the binding affinity of ABB (and BAB and BBA) for BAFFR is lower than that of BAFF (Fig. 4B). In contrast, all heteromers could compete with FLAG-BAFF binding to BCMA, indicating that BAFF and BAFF-APRIL heteromers have similar affinities for BCMA (Fig. 4B).
FIGURE 4.
Receptor binding specificity of BAFF-APRIL heteromers. A, binding of titrated amount of FLAG-tagged single-chain BAFF-APRIL heteromers to the indicated immobilized receptors-Fc was monitored in an ELISA-based assay. B, binding of a constant, nonsaturating amount of FLAG-BAFF to mBAFFR-Fc or BCMA-Fc was monitored in an ELISA-based assay. Before the addition of FLAG-BAFF, coated receptor Fcs were preincubated with titrated amounts of the indicated untagged single-chain BAFF-APRIL heteromers that served as competitors for FLAG-BAFF. C, 293T cells were co-transfected with plasmids coding for the following: (a) the extracellular domain of hBAFFR fused to the C-terminal GPI addition signal of TRAILR3, and (b) EGFP as a fluorescent tracer. These cells were stained with the indicated FLAG-tagged single-chain BAFF-APRIL heteromers (at 1 μg/staining = 40 μg/ml). FACS scattergrams show the binding of ligands (y axis) as a function of EGFP expression (which itself is proportional to BAFFR-GPI). Both axes show fluorescence intensities (from 100 to 104 arbitrary units) on a log scale. The gate shows the population of cells with intermediate EGFP expression that was selected to quantify mean fluorescence intensity in D. D, mean fluorescence intensity (MFI) of the binding of titrated amounts of the indicated single-chain FLAG-tagged heteromers to 293T cells expressing hBAFFR-GPI, BCMA-GPI, TACI-GPI or no transfected receptor (Mock). Experiment was performed as described in C. Experiments shown in A–D are representative of at least two with similar results.
The receptor-binding specificity of FLAG-tagged heteromers was also tested in a FACS-based assay, in which GPI-anchored receptors were expressed in 293T cells (Fig. 4C) (30). Binding results were virtually identical to those of the ELISA-based assay (Fig. 4D). Taken together, these data indicate that BAA is close to APRIL in terms of receptor binding and that ABB in addition displays some binding to BAFFR, but not to the same level as BAFF.
Activity of Single-chain BAFF, APRIL, and BAFF-APRIL Heteromers on Reporter Cells
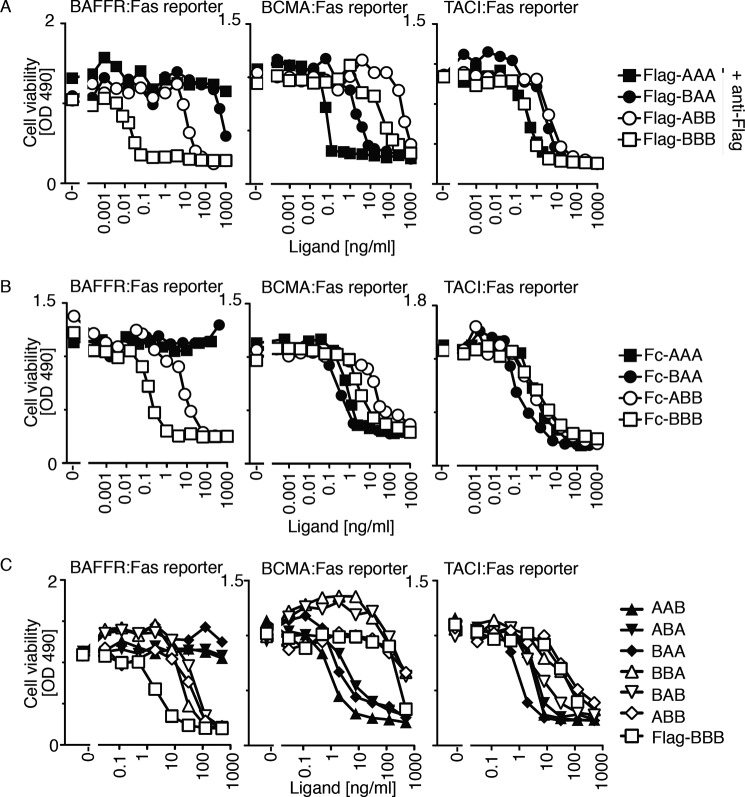
FLAG-tagged single-chain ligands were tested for their ability to induce death in apoptosis-sensitive Jurkat T cells expressing the extracellular domains of BAFFR, BCMA, or TACI fused to the transmembrane domain and intracellular tail of the death-inducing receptor Fas. Thus, successful binding and oligomerization of the receptor:Fas fusion proteins by BAFF or APRIL induces death of target cells through the surrogate Fas pathway. As triggering of the Fas pathway is intimately linked to the multimerization state of ligands (42), FLAG-tagged single-chain ligands were incubated in the presence of a cross-linking anti-FLAG antibody. All ligands killed TACI:Fas cells and BCMA:Fas cells, although AAA and to a lesser extent BAA were more active than ABB and BBB on BCMA:Fas cells. On BAFFR:Fas reporter cells, BBB was about 300-fold more efficient than ABB, in line with the poor binding of ABB to BAFFR (Figs. 4 and 5A). As expected from binding data, BAA and AAA have virtually no activity on BAFFR:Fas cells (Fig. 5A). Similar results were obtained for Fc-tagged single-chain ligands, except that differences between ligands were less marked than with FLAG-tagged ligands when assayed on TACI:Fas reporter cells (Fig. 5B). Whether this was due to an increased avidity of dimeric Fc ligands for TACI or to differences in protein preparation or assay conditions is not known. Finally, untagged single-chain ligands displayed activities mostly in line with those of the corresponding FLAG-tagged proteins, and there were no major differences between heterotrimers in which subunits of identical composition were present in a different order (Fig. 5C). Taken together, these results indicate that the activity of single-chain ligands on receptor:Fas cells reflects quite precisely their receptor binding ability and that the order in which BAFF and APRIL subunits are fused within a single-chain heteromer appears to be indifferent.
FIGURE 5.
Activity of BAFF-APRIL heteromers on BAFFR-, BCMA-, and TACI-specific reporter cell lines. Clones of Jurkat cells stably transduced with the chimeric receptors hBAFFR:Fas, hBCMA:Fas, or hTACI:Fas were exposed to titrated amounts of the indicated single-chain BAFF-APRIL heteromers which, upon binding, can activate the surrogate intracellular Fas apoptotic pathway that leads to cell death. A, effect of FLAG-tagged single-chain ligands cross-linked with a fixed amount of anti-FLAG antibody on the indicated reporter cell lines. B, same as A, but with Fc-tagged single-chain ligands in the absence of cross-linkers. C, same as A, but with untagged single-chain heteromers (and FLAG-BBB without anti-FLAG), all in the absence of cross-linker. Data shown in A–C are representative of at least two experiments with comparable results.
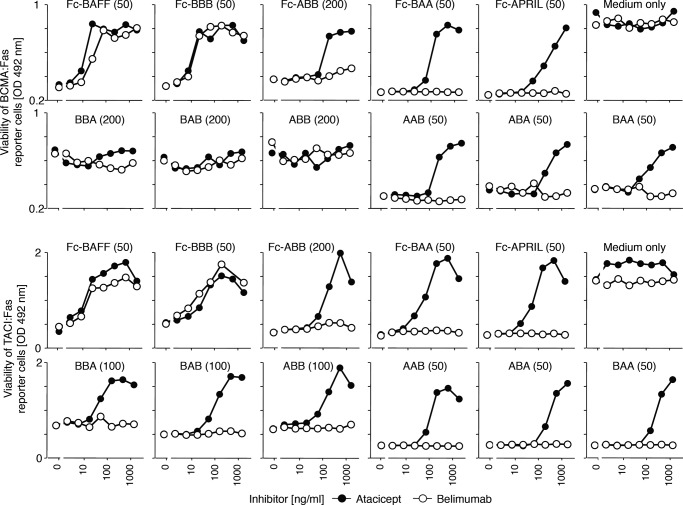
BAFF and APRIL Heteromers Are Differentially Inhibited by BAFF and APRIL Antagonist Drugs
Belimumab is an anti-BAFF antibody approved for the treatment of systemic lupus erythematosus, and atacicept is a TACI:Fc fusion protein currently in a phase II clinical trial for the same disease. The ability of these agents to inhibit BAFF-APRIL heteromers of defined stoichiometry was investigated. At a few-fold mass excess over the ligand, atacicept blocked not only BAFF and APRIL but also both types of heteromers (BAA and ABB) (Fig. 6). In contrast, belimumab efficiently inhibited Fc-BAFF and Fc-BBB but had very little to no activity on Fc-BBA, Fc-BAA, Fc-APRIL, and Fc-AAA (Fig. 6). Thus, the specificity of belimumab is limited to trimeric BAFF, whereas atacicept neutralizes BAFF and APRIL trimers and all heteromeric configurations thereof.
FIGURE 6.
Sensitivity of BAFF-APRIL heteromers of defined stoichiometries to BAFF and APRIL inhibitors. BCMA:Fas and TACI:Fas reporter cell lines were treated with lethal doses (except for BBA, BAB, and ABB on BCMA:Fas cells; dose in ng/ml indicated in parentheses) of the indicated single-chain or conventional BAFF and/or APRIL-containing ligands, in the presence or absence of titrated amounts of hTACI-Ig (atacicept) or anti-BAFF mAb (belimumab). Cell survival was monitored with a cell viability assay. One experiment out of two with similar results is shown.
Activity of Single-chain BAFF and BAFF-APRIL Heteromers on Primary B Cells in Vivo
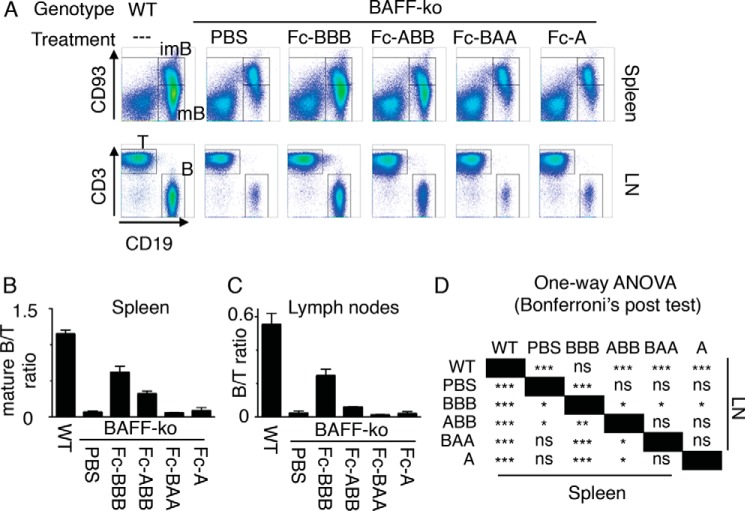
Although assays performed in the first part of this study are well defined from a molecular point of view and informative regarding receptor binding specificity of BAFF-APRIL heteromers, they do not address the capacity of heteromers to activate full-length receptors expressed at endogenous levels in a natural environment. To determine in particular whether the weak binding of ABB to BAFFR is physiologically relevant, Fc-tagged single-chain ligands were administered to BAFF-KO mice. Indeed recombinant BAFF can rescue a functionally mature B cell compartment in these mice, at least to some extent (20, 43). In BAFF-KO mice, CD93-positive immature B cells are normal, but there are only few CD93-negative mature B cells in the spleen and few mature B cells in lymph nodes (Fig. 7). As observed previously for Fc-BAFF, administration of Fc-BBB at 1.5 mg/kg for 2 weeks to BAFF-KO mice rescued both mature splenic and lymph node B cells (Fig. 7) (43). Fc-ABB was less efficient than Fc-BBB in this respect, but nevertheless it significantly increased the number of mature splenic B cells. There was also a trend for increased lymph node B cells in response to Fc-ABB, which did not reach statistical significance (Fig. 7). Finally, as anticipated, Fc-BAA and Fc-APRIL that do not bind to BAFFR had no effect on mature B cell populations (Fig. 7). Taken together, these results indicate that BAFF-rich heteromers, but not APRIL-rich ones, have the potential to functionally stimulate BAFFR in primary B cells in vivo and to allow B cell maturation.
FIGURE 7.
Effect of single-chain BAFF-APRIL heteromers in Baff-deficient mice. BAFF-KO mice were treated four times over 2 weeks with 1.5 mg/kg of the indicated Fc-tagged conventional or single-chain ligands or with buffer (PBS). Mice were sacrificed 4 days after the last injection. Spleens and lymph nodes (LN) were analyzed by FACS for the presence of T cells (T) (CD3+), B cells (B) (CD19+), immature splenic B cells (imB) (CD19+CD93+), and mature splenic B cells (mB) (CD19+CD93−). Untreated wild type (WT) mice were included as controls. A, representative scattergrams of splenic and lymph node cells from mice treated with the indicated Fc ligands. B, ratio of mature B cells to T cells in spleens of mice that received the indicated treatments. C, ratio of B to T cells in lymph nodes of mice that received the indicated treatments. D, results of statistical one-way analyses of variance (ANOVA) with Bonferroni's post test multiple comparisons for data shown in B and C. ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001. Experiment was performed once with three mice per condition (six mice for WT and six mice for PBS-treated BAFF-KO).
Discussion
The trimeric nature of both BAFF and APRIL makes the production and characterization of heteromers thereof a challenging issue, first to separate heteromers from homotrimers and then to resolve heteromers of different compositions. Although this is possible and has already been done for lymphotoxins (44) and for the APRIL2BAFF1 heteromer (18), it is difficult to exclude that exchanges of protomers at equilibrium might restore a proportion of homotrimers in a purified heteromer. In this regard, the strategy of expressing TNF family ligands as single-chain proteins, which was initially developed to specifically introduce mutations in one but not all subunits of TNF (45), has two undeniable advantages as follows: the generation of heteromers with defined stoichiometry, and a guarantee against exchanges of subunits. The size of FLAG-tagged and untagged heteromers and the structure of ABB undoubtedly confirmed that these constructs really formed heteromers. Interestingly, interfaces between adjacent BAFF-BAFF, APRIL-APRIL, BAFF-APRIL, or APRIL-BAFF subunits are virtually identical in the central region of the interaction, and they often involve similar residues in the peripheral part of the interaction (Fig. 3C). A potential concern with single-chain ligands is the C-terminal amino acid, which in most TNF family ligands is part of β-sheet H and intimately involved in the structure of the monomer (39), and it is appropriate to wonder whether the addition of a linker at the C terminus of BAFF or APRIL affects structure and activity of these ligands. Single-chain ligands of lymphotoxins α and β were not only competent to bind receptors (Fig. 1) (35) but also recapitulated known receptor-binding specificities for these ligands (Fig. 1) (30, 34). Similarly, single-chain heteromers of BAFF and APRIL retained receptor binding activity, and there were no noticeable differences in constructs when the single copies of BAFF or APRIL were placed in position 1 or 2 (i.e. with a linker at the C terminus) or in position 3 (i.e. with a natural C terminus). In addition, there was no evidence in the crystal structure of ABB that these short linkers would perturb the structure of BAFF or APRIL in any way. Taken together, these observations suggest that single-chain heteromers provide a valid representation of “classical” homo- or heterotrimers, at least for lymphotoxins and for BAFF and APRIL.
It is worth mentioning that although LTβ is usually reported to be active as heteromer with LTα (34), it was also shown to bind LTβR on its own (46). In line with this later observation, single-chain LTβββ bound LTβR with the same specificity as LTα1β2. The TNF ligand LIGHT binds to LTβR and HVEM, hence it will be interesting to test whether single-chain LTβββ interacts with receptors of the immediate TNF network (TNFR1, TNFR2, HVEM, DcR3, LTβR, and Fas).
A previously characterized APRIL2BAFF1 heteromer activated TACI as efficiently as APRIL in an NF-κB reporter assay, was less potent than either BAFF or APRIL taken alone in B cell proliferation assays, and could be inhibited by BCMA-Fc and TACI-Fc but not BAFFR-Fc (18). Our data are in line with these findings and extend them to APRIL1BAFF2. Briefly, BAA has a similar receptor-binding specificity as AAA, whereas ABB resembles BBB, except for a weaker binding to BAFFR. BAFF has a weaker affinity for BCMA than APRIL (reviewed in Ref. 47), and here we find that ABB is also a weaker binder and activator of BCMA than BAA. The difference is especially marked for uncross-linked heteromers, but it is attenuated in the multimerized Fc-fusion constructs. Whether BAFF-APRIL heteromers exist only as soluble trimeric molecules in vivo and/or whether they are also multimerized, for example if they exist in membrane-bound forms, remains to be investigated.
In contrast, the binding and activity of BAA and ABB on TACI were more comparable. ABB was severely impaired compared with BBB in all BAFFR binding and BAFFR activation assays, yet they always retained some binding and functional activity on BAFFR, including signaling in primary mouse B cells in vivo. The only test system in which ABB showed no activity was in a BAFF-BAFFR competition assay. This same ABB protein retained activity in a similar assay analyzing competition with the BAFF-BCMA interaction (Fig. 4B). This indicates that ABB has a lower affinity or avidity than BAFF for BAFFR, a conclusion re-enforced by the examination of the ABB crystal structure that suggests impaired binding to BAFFR. Therefore, a direct consequence of BAFF incorporation into heteromers is to strongly reduce (ABB) or even abrogate (BAA) its ability to activate BAFFR. Heteromers can account for a fair proportion of circulating BAFF in patient sera and may therefore control levels of BAFFR stimulating activity (one study found 0% heteromer in six controls, and 4–87% (average 32%) in 15 patients (19); a second study reported heteromers in 7/89 controls and 24/89 patients with systemic lupus erythematosus, with heteromer concentrations usually lower than those of BAFF, although some subjects had heteromers but undetectable BAFF (48)).
What about stimulation of BCMA and TACI by heteromers? It is predicted that the binding of BAFF, APRIL, or BAFF-APRIL heteromers to TACI will be similar. However, binding to and activation of TACI are distinct events in which ligand oligomerization (e.g. BAFF 60-mers or cross-linked APRIL) seems indispensable for TACI activation, at least in primary mouse B lymphocytes (20) and possible in human monocytes (49). Thus, beyond the ability of heteromers to bind to TACI, their ability to stimulate this receptor might well be determined by their capacity or incapacity to oligomerize. Whether endogenous heteromers can oligomerize via the Flap region that is present in BAFF only or via the proteoglycan-binding region that is present in APRIL only is presently an unresolved question. For BCMA, so little is currently known about its activation requirements that it is difficult to speculate whether heteromers will be worse, equal, or better ligands than BAFF or APRIL.
We therefore propose two main hypotheses for the physiological role of BAFF-APRIL heteromers as follows: (a) they act as partial functional antagonists of both BAFF and APRIL and down-modulate responses of these ligands on all cells known to respond to BAFF and/or APRIL; (b) conversely, they act as dominant negative inhibitors of BAFF signaling on BAFFR, which will affect naive mature B cells. However, they can signal via TACI and BCMA and will therefore support activated and differentiated B cells such as plasmablasts and plasma cells. In the later scenario, heteromers favor antigen-experienced B cells at the expense of naive ones.
Some hints to distinguish between one or the other hypothesis may come from clinical trials comparing single BAFF antagonists (belimumab/Benlysta) to dual BAFF- and APRIL-blocking reagents (i.e. atacicept, which is a TACI-Ig fusion protein) (10). Indeed, we show here that ABB and BAA heteromers are efficiently blocked by atacicept but not belimumab. If endogenous heteromers support survival of plasmablasts and plasma cells in vivo, then atacicept but not belimumab is expected to affect these cell types; the interpretation will, however, be complicated by the fact that atacicept also inhibits APRIL; the development of specific heteromer antagonists that inhibit neither BAFF nor APRIL will probably be necessary to definitively address the role of heteromers in health and disease. From a mechanistic point of view, the inability of belimumab to interfere with ABB is surprising, as one would expect that it should recognize at least the BB interface. A rational explanation for why belimumab fails to inhibit ABB will probably require crystallization of the belimumab-BAFF complex.
The Flap is a long loop between β-sheets D and E (specifically present in BAFF but no other TNF family ligand) that mediates contact between BAFF trimers and is responsible for the formation of BAFF 60-mers (14, 50). Interestingly, the arrangement of the Flap was not only well resolved in structures of BAFF 60-mers but also in crystals of BAFF 3-mers (Fig. 3D) (13, 14, 36–38, 41), suggesting that it may fulfill functions beyond the mere formation of 60-mers.4 In ABB, the Flap of only one BAFF subunit was similar to that found in BAFF 60-mers, and the second one was differently rearranged, suggesting that a single Flap-Flap interaction might take place between ABB heteromers, although this will be difficult to demonstrate experimentally in solution. Indeed, under conditions where BAFF oligomerizes into 60-mers, 3- and 60-mers are visible in the absence of any defined oligomers of intermediate sizes (14, 50, 51). The Flap region of the C-terminal BAFF subunit of ABB adopted a different, yet well defined conformation that was never observed before (Fig. 3D). In the structure, this Flap is also involved in the interaction with an adjacent ABB, but whether and how this affects ABB functionality will require further investigations.
In conclusion, the single-chain ligand technology was successful for studying the properties of BAFF-APRIL heteromers of defined stoichiometry and provided useful tools for the characterization of BAFF and/or APRIL inhibitors. In the future, these reagents will also help to determine whether reagents used to quantify BAFF or APRIL cross-react with the different forms of BAFF-APRIL heteromers.
Acknowledgments
We thank Peter Scheurich (University of Stuttgart) for advice in the initial state of this project. We are grateful to Susan Kalled (BiogenIdec, Boston) for providing BAFF−/− mice, Olivier Micheau (University of Dijon) for the gift of Fas-deficient JOM2 Jurkat cells, and Antonius Rolink (University of Basel) for the gift of mAb 493.
This work was supported by grants from the Swiss National Science Foundation (to P.S.), by the Institute of Arthritis Research, and by project funding from EMD Serono (to P. S.). P. S. is supported by a research grant from EMD Serono, a subsidiary of Merck, KGaA. X. J., H. H., and S. T. are employees of Merck, KGaA. T. Z. is an employee of BiogenIdec. A. L. and M. K. are employees of Proteros Biostructures GmbH. Other authors declare no competing financial interests.
The atomic coordinates and structure factors (code 4ZCH) have been deposited in the Protein Data Bank (http://wwpdb.org/).
M. Vigolo and P. Schneider, unpublished observations.
- BAFF
- B cell activating factor of the TNF family
- APRIL
- a proliferation-inducing ligand
- BAFFR
- BAFF receptor
- TACI
- transmembrane activator and CAML interactor
- BCMA
- B cell maturation antigen
- LTα
- lymphotoxin α
- LTβ
- lymphotoxin β
- LT-ββα
- single-chain LTβ-LTβ-LTα
- LTβR
- LTβ receptor
- AAA
- single-chain APRIL-APRIL-APRIL
- ABB
- single-chain APRIL-BAFF-BAFF
- BAA
- single-chain BAFF-APRIL-APRIL
- BBB
- single-chain BAFF-BAFF-BAFF
- h
- human
- EGFP
- enhanced GFP
- r.m.s.d.
- root mean square deviation
- GPI
- glycosylphosphatidylinositol.
References
- 1. Mackay F., Schneider P. (2009) Cracking the BAFF code. Nat. Rev. Immunol. 9, 491–502 [DOI] [PubMed] [Google Scholar]
- 2. Benson M. J., Dillon S. R., Castigli E., Geha R. S., Xu S., Lam K. P., Noelle R. J. (2008) Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 180, 3655–3659 [DOI] [PubMed] [Google Scholar]
- 3. Patke A., Mecklenbräuker I., Erdjument-Bromage H., Tempst P., Tarakhovsky A. (2006) BAFF controls B cell metabolic fitness through a PKCβ- and Akt-dependent mechanism. J. Exp. Med. 203, 2551–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shulga-Morskaya S., Dobles M., Walsh M. E., Ng L. G., MacKay F., Rao S. P., Kalled S. L., Scott M. L. (2004) B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J. Immunol. 173, 2331–2341 [DOI] [PubMed] [Google Scholar]
- 5. Treml L. S., Carlesso G., Hoek K. L., Stadanlick J. E., Kambayashi T., Bram R. J., Cancro M. P., Khan W. N. (2007) TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J. Immunol. 178, 7531–7539 [DOI] [PubMed] [Google Scholar]
- 6. von Bülow G. U., van Deursen J. M., Bram R. J. (2001) Regulation of the t-independent humoral response by taci. Immunity 14, 573–582 [DOI] [PubMed] [Google Scholar]
- 7. Yan M., Wang H., Chan B., Roose-Girma M., Erickson S., Baker T., Tumas D., Grewal I. S., Dixit V. M. (2001) Activation and accumulation of B cells in TACI-deficient mice. Nat. Immunol. 2, 638–643 [DOI] [PubMed] [Google Scholar]
- 8. Figgett W. A., Fairfax K., Vincent F. B., Le Page M. A., Katik I., Deliyanti D., Quah P. S., Verma P., Grumont R., Gerondakis S., Hertzog P., O'Reilly L. A., Strasser A., Mackay F. (2013) The TACI receptor regulates T-cell-independent marginal zone B cell responses through innate activation-induced cell death. Immunity 39, 573–583 [DOI] [PubMed] [Google Scholar]
- 9. O'Connor B. P., Raman V. S., Erickson L. D., Cook W. J., Weaver L. K., Ahonen C., Lin L. L., Mantchev G. T., Bram R. J., Noelle R. J. (2004) BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stohl W. (2014) Therapeutic targeting of the BAFF/APRIL axis in systemic lupus erythematosus. Expert Opin. Ther. Targets 18, 473–489 [DOI] [PubMed] [Google Scholar]
- 11. Moore P. A., Belvedere O., Orr A., Pieri K., LaFleur D. W., Feng P., Soppet D., Charters M., Gentz R., Parmelee D., Li Y., Galperina O., Giri J., Roschke V., Nardelli B., et al. (1999) BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 285, 260–263 [DOI] [PubMed] [Google Scholar]
- 12. Craxton A., Magaletti D., Ryan E. J., Clark E. A. (2003) Macrophage- and dendritic cell-dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood 101, 4464–4471 [DOI] [PubMed] [Google Scholar]
- 13. Karpusas M., Cachero T. G., Qian F., Boriack-Sjodin A., Mullen C., Strauch K., Hsu Y. M., Kalled S. L. (2002) Crystal structure of extracellular human BAFF, a TNF family member that stimulates B lymphocytes. J. Mol. Biol. 315, 1145–1154 [DOI] [PubMed] [Google Scholar]
- 14. Liu Y., Xu L., Opalka N., Kappler J., Shu H. B., Zhang G. (2002) Crystal structure of sTALL-1 reveals a virus-like assembly of TNF family ligands. Cell 108, 383–394 [DOI] [PubMed] [Google Scholar]
- 15. López-Fraga M., Fernández R., Albar J. P., Hahne M. (2001) Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2, 945–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huard B., McKee T., Bosshard C., Durual S., Matthes T., Myit S., Donze O., Frossard C., Chizzolini C., Favre C., Zubler R., Guyot J. P., Schneider P., Roosnek E. (2008) APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J. Clin. Invest. 118, 2887–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ingold K., Zumsteg A., Tardivel A., Huard B., Steiner Q. G., Cachero T. G., Qiang F., Gorelik L., Kalled S. L., Acha-Orbea H., Rennert P. D., Tschopp J., Schneider P. (2005) Identification of proteoglycans as the APRIL-specific binding partners. J. Exp. Med. 201, 1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dillon S. R., Harder B., Lewis K. B., Moore M. D., Liu H., Bukowski T. R., Hamacher N. B., Lantry M. M., Maurer M., Krejsa C. M., Ellsworth J. L., Pederson S., Elkon K. B., Wener M. H., Dall'Era M., Gross J. A. (2010) B-lymphocyte stimulator/a proliferation-inducing ligand heterotrimers are elevated in the sera of patients with autoimmune disease and are neutralized by atacicept and B-cell maturation antigen-immunoglobulin. Arthritis Res.Ther. 12, R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roschke V., Sosnovtseva S., Ward C. D., Hong J. S., Smith R., Albert V., Stohl W., Baker K. P., Ullrich S., Nardelli B., Hilbert D. M., Migone T. S. (2002) BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J. Immunol. 169, 4314–4321 [DOI] [PubMed] [Google Scholar]
- 20. Bossen C., Cachero T. G., Tardivel A., Ingold K., Willen L., Dobles M., Scott M. L., Maquelin A., Belnoue E., Siegrist C. A., Chevrier S., Acha-Orbea H., Leung H., Mackay F., Tschopp J., Schneider P. (2008) TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood 111, 1004–1012 [DOI] [PubMed] [Google Scholar]
- 21. Schneider P. (2000) Production of recombinant TRAIL and TRAIL receptor:Fc chimeric proteins. Methods Enzymol. 322, 322–345 [DOI] [PubMed] [Google Scholar]
- 22. Schneider P., Willen L., Smulski C. R. (2014) Tools and techniques to study ligand-receptor interactions and receptor activation by TNF superfamily members. Methods Enzymol. 545, 103–125 [DOI] [PubMed] [Google Scholar]
- 23. Kimberley F. C., van der Sloot A. M., Guadagnoli M., Cameron K., Schneider P., Marquart J. A., Versloot M., Serrano L., Medema J. P. (2012) The design and characterization of receptor-selective APRIL variants. J. Biol. Chem. 287, 37434–37446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kabsch W. (2010) XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bricogne G., Blanc E., Brandl M., Flensburg C., Keller P., Paciorek W., Roversi P., Sharff A., Smart O. S., Vonrhein C., Womack T. O. (2011) BUSTER, Version 2.11.5, Cambridge, UK [Google Scholar]
- 27. Chen V. B., Arendall W. B., 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 29. Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 30. Bossen C., Ingold K., Tardivel A., Bodmer J. L., Gaide O., Hertig S., Ambrose C., Tschopp J., Schneider P. (2006) Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J. Biol. Chem. 281, 13964–13971 [DOI] [PubMed] [Google Scholar]
- 31. Eck M. J., Sprang S. R. (1989) The structure of tumor necrosis factor-α at 2.6 A resolution. Implications for receptor binding. J. Biol. Chem. 264, 17595–17605 [DOI] [PubMed] [Google Scholar]
- 32. Hymowitz S. G., Patel D. R., Wallweber H. J., Runyon S., Yan M., Yin J., Shriver S. K., Gordon N. C., Pan B., Skelton N. J., Kelley R. F., Starovasnik M. A. (2005) Structures of APRIL-receptor complexes: like BCMA, TACI employs only a single cysteine-rich domain for high affinity ligand binding. J. Biol. Chem. 280, 7218–7227 [DOI] [PubMed] [Google Scholar]
- 33. Krippner-Heidenreich A., Grunwald I., Zimmermann G., Kühnle M., Gerspach J., Sterns T., Shnyder S. D., Gill J. H., Männel D. N., Pfizenmaier K., Scheurich P. (2008) Single-chain TNF, a TNF derivative with enhanced stability and antitumoral activity. J. Immunol. 180, 8176–8183 [DOI] [PubMed] [Google Scholar]
- 34. Browning J. L., Ngam-ek A., Lawton P., DeMarinis J., Tizard R., Chow E. P., Hession C., O'Brine-Greco B., Foley S. F., Ware C. F. (1993) Lymphotoxin β, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell 72, 847–856 [DOI] [PubMed] [Google Scholar]
- 35. Sudhamsu J., Yin J., Chiang E. Y., Starovasnik M. A., Grogan J. L., Hymowitz S. G. (2013) Dimerization of LTbetaR by LTα1β2 is necessary and sufficient for signal transduction. Proc. Natl. Acad. Sci. U.S.A. 110, 19896–19901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim H. M., Yu K. S., Lee M. E., Shin D. R., Kim Y. S., Paik S. G., Yoo O. J., Lee H., Lee J. O. (2003) Crystal structure of the BAFF-BAFF-R complex and its implications for receptor activation. Nat. Struct. Biol. 10, 342–348 [DOI] [PubMed] [Google Scholar]
- 37. Liu Y., Hong X., Kappler J., Jiang L., Zhang R., Xu L., Pan C. H., Martin W. E., Murphy R. C., Shu H. B., Dai S., Zhang G. (2003) Ligand-receptor binding revealed by the TNF family member TALL-1. Nature 423, 49–56 [DOI] [PubMed] [Google Scholar]
- 38. Oren D. A., Li Y., Volovik Y., Morris T. S., Dharia C., Das K., Galperina O., Gentz R., Arnold E. (2002) Structural basis of BLyS receptor recognition. Nat. Struct. Biol. 9, 288–292 [DOI] [PubMed] [Google Scholar]
- 39. Bodmer J. L., Schneider P., Tschopp J. (2002) The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 27, 19–26 [DOI] [PubMed] [Google Scholar]
- 40. Schneider P. (2009) in BLyS Ligands and Receptors (Cancro M., ed) pp. 1–18, Contemporary Immunology Series, Humana Press, New York [Google Scholar]
- 41. Gordon N. C., Pan B., Hymowitz S. G., Yin J., Kelley R. F., Cochran A. G., Yan M., Dixit V. M., Fairbrother W. J., Starovasnik M. A. (2003) BAFF/BLyS receptor 3 comprises a minimal TNF receptor-like module that encodes a highly focused ligand-binding site. Biochemistry 42, 5977–5983 [DOI] [PubMed] [Google Scholar]
- 42. Holler N., Tardivel A., Kovacsovics-Bankowski M., Hertig S., Gaide O., Martinon F., Tinel A., Deperthes D., Calderara S., Schulthess T., Engel J., Schneider P., Tschopp J. (2003) Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol. Cell. Biol. 23, 1428–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Swee L. K., Tardivel A., Schneider P., Rolink A. (2010) Rescue of the mature B cell compartment in BAFF-deficient mice by treatment with recombinant Fc-BAFF. Immunol. Lett. 131, 40–48 [DOI] [PubMed] [Google Scholar]
- 44. Browning J. L., Miatkowski K., Griffiths D. A., Bourdon P. R., Hession C., Ambrose C. M., Meier W. (1996) Preparation and characterization of soluble recombinant heterotrimeric complexes of human lymphotoxins α and β. J. Biol. Chem. 271, 8618–8626 [DOI] [PubMed] [Google Scholar]
- 45. Boschert V., Krippner-Heidenreich A., Branschädel M., Tepperink J., Aird A., Scheurich P. (2010) Single chain TNF derivatives with individually mutated receptor binding sites reveal differential stoichiometry of ligand receptor complex formation for TNFR1 and TNFR2. Cell. Signal. 22, 1088–1096 [DOI] [PubMed] [Google Scholar]
- 46. Crowe P. D., VanArsdale T. L., Walter B. N., Ware C. F., Hession C., Ehrenfels B., Browning J. L., Din W. S., Goodwin R. G., Smith C. A. (1994) A lymphotoxin-β-specific receptor. Science 264, 707–7108171323 [Google Scholar]
- 47. Bossen C., Schneider P. (2006) BAFF, APRIL and their receptors: structure, function and signaling. Semin. Immunol. 18, 263–275 [DOI] [PubMed] [Google Scholar]
- 48. Dillon S. R., Gross J. A., Ansell S. M., Novak A. J. (2006) An APRIL to remember: novel TNF ligands as therapeutic targets. Nat. Rev. Drug Discov. 5, 235–246 [DOI] [PubMed] [Google Scholar]
- 49. Chang S. K., Arendt B. K., Darce J. R., Wu X., Jelinek D. F. (2006) A role for BLyS in the activation of innate immune cells. Blood 108, 2687–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cachero T. G., Schwartz I. M., Qian F., Day E. S., Bossen C., Ingold K., Tardivel A., Krushinskie D., Eldredge J., Silvian L., Lugovskoy A., Farrington G. K., Strauch K., Schneider P., Whitty A. (2006) Formation of virus-like clusters is an intrinsic property of the tumor necrosis factor family member BAFF (B cell activating factor). Biochemistry 45, 2006–2013 [DOI] [PubMed] [Google Scholar]
- 51. Bossen C., Tardivel A., Willen L., Fletcher C. A., Perroud M., Beermann F., Rolink A. G., Scott M. L., Mackay F., Schneider P. (2011) Mutation of the BAFF furin cleavage site impairs B-cell homeostasis and antibody responses. Eur. J. Immunol. 41, 787–797 [DOI] [PubMed] [Google Scholar]