Background: Lysyl oxidase catalyzes collagen cross-link formation, which is essential for mechanically strong collagen fibrils.
Results: LOX inhibition stops early mechanical development of tendon constructs and leads to irregularly shaped collagen fibrils.
Conclusion: Collagen cross-linking is essential for successful fibrillogenesis and regulates fibril shape.
Significance: LOX activity is required in the control of collagen fibril architecture by a mechanism that remains to be explained.
Keywords: collagen, electron microscopy (EM), fibril, lysyl oxidase, tendon, Ehlers-Danlos Syndrome, collagen cross-linking, tendon construct, β-aminoproprionitrile (BAPN)
Abstract
Lysyl oxidases (LOXs) are a family of copper-dependent oxido-deaminases that can modify the side chain of lysyl residues in collagen and elastin, thereby leading to the spontaneous formation of non-reducible aldehyde-derived interpolypeptide chain cross-links. The consequences of LOX inhibition in producing lathyrism are well documented, but the consequences on collagen fibril formation are less clear. Here we used β-aminoproprionitrile (BAPN) to inhibit LOX in tendon-like constructs (prepared from human tenocytes), which are an experimental model of cell-mediated collagen fibril formation. The improvement in structure and strength seen with time in control constructs was absent in constructs treated with BAPN. As expected, BAPN inhibited the formation of aldimine-derived cross-links in collagen, and the constructs were mechanically weak. However, an unexpected finding was that BAPN treatment led to structurally abnormal collagen fibrils with irregular profiles and widely dispersed diameters. Of special interest, the abnormal fibril profiles resembled those seen in some Ehlers-Danlos Syndrome phenotypes. Importantly, the total collagen content developed normally, and there was no difference in COL1A1 gene expression. Collagen type V, decorin, fibromodulin, and tenascin-X proteins were unaffected by the cross-link inhibition, suggesting that LOX regulates fibrillogenesis independently of these molecules. Collectively, the data show the importance of LOX for the mechanical development of early collagenous tissues and that LOX is essential for correct collagen fibril shape formation.
Introduction
Collagen plays a major role in providing mechanical stability to tissues and structures such as skin, blood vessels, bones, and tendons (1–4). The mechanical integrity is based on a highly organized molecular structure in which three polypeptide chains are wound into a triple helix. Individual collagen molecules are organized in a head-to-tail quarter-staggered arrangement to make microfibrils and larger collagen fibrils (5). These fibrils are regularly arranged in increasingly complex hierarchical architectures such as parallel bundles in tendon (1), basket waves in skin (6), and orthogonal lattices in cornea (7). Collagen fibrils are strengthened by covalent cross-links formed enzymatically during assembly between the collagen molecules at specific locations. Lysyl oxidase (LOX)2 is the enzyme responsible for initiating covalent cross-link formation in collagen fibrils by oxidatively deaminating specific lysine and hydroxylysine residues to form allysines in the telopeptide domains of the collagen molecule (8). This enables the formation of cross-links between telopeptides and the helix of collagen molecules staggered by a 4D-period axially (8). During maturation, these cross-links can interact further, forming trivalent cross-links (9). Cross-link formation is an essential determinant of the material properties of developing and mature connective tissues.
The LOX family consists of LOX and the LOX homologues LOX-like 1 (LOXL1), LOXL2, LOXL3, and LOXL4 (10). Inactivation of the gene coding for LOX has earlier shown to be detrimental, leading to aortic aneurisms and thereby perinatal death in mice (11). Also, LOX inactivation led to disorganized elastin and collagen fibrils in the respiratory tract and skin in these animals, which hindered intact lung development (12). LOX was also recently discovered to play a major role in tumor growth and metastasis by increasing the stiffness of tumor substrate and thereby promoting cancer cell proliferation (13, 14). β-Aminopropionitrile (BAPN) is a competitive inhibitor of LOX (15–17) and has been shown to have detrimental effects upon several tissues. Early experiments have shown that injection of BAPN into fertilized eggs leads to extremely fragile chick embryos after 3 days of incubation. Furthermore, collagen became extractible from all matrix-rich tissues such as bone, skin, and tendons (18). Marturano et al. (19) have elegantly shown that BAPN specifically inhibits LOX without affecting total collagen content, macroscopic fibril organization, or cell fate in chick embryos. However, both the in ovo- and in vitro-treated chick tendons showed markedly reduced tensile moduli.
Apart from these considerable mechanical effects of LOX inhibition, very few studies have assessed the ultrastructure of these affected tissues. Mäki et al. (12) have shown disturbed collagen fibril bundles with slack packing density in lung and skin tissue from LOX−/− mouse embryos. Cell matrix constructs from chick embryonic corneal fibroblasts were further shown to develop impaired collagen fibril outlines when cultured with BAPN and transglutaminase inhibitor (20).
We recently introduced and characterized a tendon construct system from adult human tendon fibroblasts that is ideal to investigate collagen fibril formation in vitro (21–23). Primary fibroblasts from adult human tendon can produce new collagen fibrils in vitro that increase in diameter with time and strengthen the structure (22). In contrast to in vitro collagen self-assembly studies, the collagen fibrils are produced, aligned, and modified in a controlled fashion by adult human fibroblasts (21). This leaves the opportunity to manipulate the cell-driven collagen modification during development.
In this study we investigated the role of LOX by blocking its activity with BAPN in the controlled tendon construct system. We hypothesize that LOX inhibition will lead to reduced tensile mechanical properties. Furthermore, we will investigate the effect of LOX inhibition on collagen fibril formation and thereby elucidate the role of LOX in collagen fibrillogenesis.
Materials and Methods
Cell Culture
Tendon fibroblasts were isolated from human semitendinosus and gracilis tendon as previously described (21). Briefly, patients (18–32 years old) who underwent reconstructive surgery after anterior cruciate ligament (ACL) rupture gave their informed consent to donate excess tendon tissue to the present study. The tissue was transported to a cell culture laboratory immediately after harvest. Under aseptic conditions the tissue was minced into pieces of ∼2 mm3 and digested overnight in DMEM/F-12 (Gibco) supplemented with 0.1% collagenase type II (Worthington) and 20% fetal bovine serum (FBS) (Gibco). After repeated washes in culture medium (DMEM/F12, 10% FBS) the cells were seeded into flasks and cultured until the next passage. Cells from at least five different donors in the 2nd to 6th passage were used for experiments. The experiments were approved by the local ethics committee (Ref. H-3-2010-070).
Construct Formation
Tendon constructs from human tenocytes were assembled as described previously with minor modifications (21). Briefly, each well of a 6-well plate was coated with ∼1.5 ml of SYLGARD (Dow-Chemicals) and allowed to set at 55 °C for 48 h. Next, two short silk sutures (0.5 cm, Ethicon) were pinned onto the coated plates with insect pins (0.1 mm diameter) (Fine Science Tools GmbH) with a distance of 1 cm in between sutures. The plates were sterilized by immersion in 70% ethanol for 45 min, dried, and washed with sterile PBS. Human tendon fibroblasts were suspended in culture medium containing 4 mg of human fibrinogen, 10 μg/ml aprotinin, and 1 unit of human thrombin (all from Sigma) to a final concentration of 2 × 105 per 800 μl and rapidly spread over the complete surface of the coated wells. The cell-embedded fibrin gel was allowed to set for 30 min at 37 °C and cultured until the matrix was fully contracted between the anchor points. Every other day culture medium supplemented with 0.2 mm l-ascorbic acid 2-phosphate and 0.05 mm l-proline (Sigma Aldrich) was replaced, and adhesions to the side of the well were detached using a fine pipette tip to allow gel contraction. After 12–14 days the cells contracted the structure to a rod-like structure in between the anchor points (Fig. 1A).
FIGURE 1.

The tendon construct. A, tendon construct (central structure) grown for 14 days under control conditions. The black sutures at the ends anchor the structure to the base of the well as indicated in the image. B, when treated with 50 μm BAPN, the construct ruptures spontaneously around the time point of formation. On the right-hand side suture the retracted remnants of the construct are visible (arrow).
Initially, constructs were treated with the LOX inhibitor BAPN (50 μm; Sigma, A3134) at the time of cell seeding. However, this treatment led to spontaneous rupture of the construct around the time of formation (Fig. 1B, the arrow indicates retracted construct), thus excluding any usable phenotype for further studies. Therefore, we used a regime where constructs were allowed to form initially (14 days) and were subsequently supplemented with 50 μm BAPN or left as control until harvesting (Fig. 2A).
FIGURE 2.
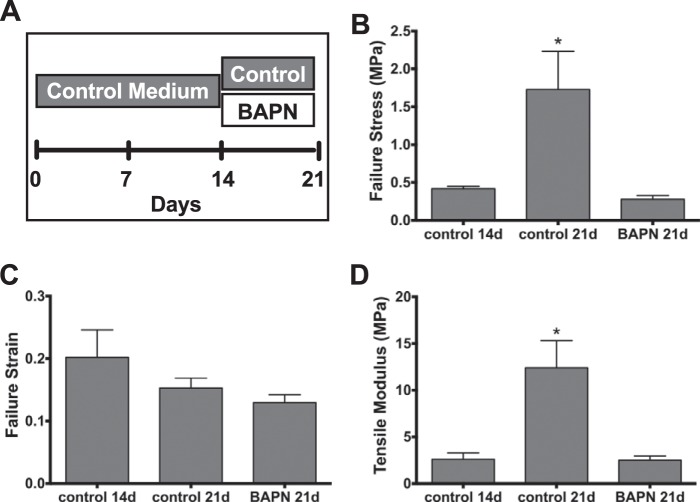
Construct mechanical data. A, due to the rupture as shown in Fig. 1, constructs were treated with control medium for 14 days and thereafter treated with 50 μm BAPN or left as controls, respectively, for 7 days. B, failure stress of control constructs develops from days 14 to 21 by ∼1.3 MPa. BAPN-treatment inhibits abolishes this development completely. C, despite a trend toward lower strains at 21 days, no statistical change in strain was found. D, the tensile modulus rises according to the increase in stress over similar strains by ∼10 MPa. Again, the BAPN treatment prohibits this increase. Data are presented as the mean ± S.E. *, p < 0.05 compared with other groups.
Mechanical Testing
Tensile testing of the tendon constructs at the different time points was performed in a PC-driven micromechanical rig with liquid chamber (20 Newton load-cell, sampling rate 10 Hz; Deben, Suffolk, UK). A stereoscopic microscope (SMZ1000, Nikon, Tokyo, Japan) with a C-mount lens (×8) equipped with a 15 Hz digital camera (DFWX700, Sony, Tokyo, Japan; 640 × 480 Pixel) was used for imaging during the test to verify clamping length and monitor the rupture site of the construct.
The tendon constructs were glued on specimen plates with a mounting distance of 10 mm. The glue was left to dry for 5 min, whereas the mid-portion was wrapped in PBS-soaked gauze. Subsequently, the specimen was transferred to a PBS bath, and after a short adaptation period, the test was started. The samples were stretched at 2 mm/min until the onset of force. After a 15-s relaxation period, the constructs were further stretched at 2 mm/min until failure.
The data analysis was based on the assumption that the construct had a circular cross-section. Construct diameter and mounting length were measured at the onset of force. For the calculation of ultimate tensile stress, the minimal diameter was used, and the strain was determined from the onset of force until failure. Tensile modulus was calculated based on stress and strain from the onset of force up to the point of failure. Three constructs per donor cell line were tested, and results of 6 (14-day control) and 8 cell lines (21-day control and BAPN), respectively, were compared.
Transmission Electron Microscopy (TEM)
After discarding culture medium and rinsing in PBS, constructs were fixed in 2% glutaraldehyde in 0.05 m phosphate buffer for 30 min at room temperature. The constructs were then cut in three equal pieces and fixed in fresh fixative for at least 2 h at 4 °C. After washing in 0.15 m phosphate buffer, the samples were postfixed with 1% OsO4 in 0.12 m sodium cacodylate buffer for 2 h at room temperature. After another wash in distilled H2O, the samples were stained en bloc with 1% aqueous uranyl for 16 h at 4 °C, dehydrated in a graded serious of ethanol, and embedded in Epon (Hexicon, Houston, TX) according to standard procedures.
Ultrathin cross-sections were cut with a Reichert-Jung Ultracut E microtome using a diamond knife and collected on one-hole copper grids with Formvar supporting membranes. Images were acquired in a Philips TM 100 transmission electron microscope, operated at an accelerating voltage of 80 kV, with a Megaview 2 camera and processed with the iTEM AnalySIS software package (ResAlta Research Technologies, Golden, CO). The NIH-based image-processing program, Image J, was used for measurement of collagen fibril diameters. On randomly selected micrographs, 300 fibrils were analyzed per specimen, cell line, treatment, and time point, respectively.
Serial Block Face-scanning Electron Microscopy
The samples were fixed as for TEM, but post-fixation was modified. Here, constructs were post-fixed in a solution of 1% (w/v) OsO4 and 1.5% (w/v) K4[Fe6] in 0.12 m sodium cacodylate buffer for 1 h. Subsequent to washing with water, samples were treated with 1% (w/v) tannic acid in 0.12 m cacodylate buffer, washed, and once more incubated with 1% (w/v) OsO4 in 0.12 m cacodylate buffer for an additional 30 min. Thereafter, samples were block-stained, dehydrated, and embedded as described above. Ultrathin sections were investigated by TEM to assure sample quality.
Samples were mounted on a Gatan 3view microtome within an FEI Quanta 250 scanning electron microscope as described previously (24). Section thickness was 100 nm over a Z range of 50 μm, resulting in 500 images. Image processing was conducted using the IMOD software package (University of Colorado, Department of Molecular, Cellular, and Developmental Biology, 347 UCB, Boulder, CO 80309 (61)), and videos were created.
Collagen Content Assay
Tendon constructs were snap-frozen in liquid nitrogen and stored at −80 °C until further use. Subsequent to thawing, the samples were freeze-dried and weighed at constant humidity using an ultra-microbalance scale (Mettler-Toledo GmBH, Gieβen, Germany). The samples were hydrolyzed in 6 m HCl at 110 °C for 18 h and dried at 95 °C followed by thorough washing in H2O. The remaining samples were diluted in 600 μl of acetate-citrate buffer (0.6% acetic acid, 130 mm citric acid, 440 mm sodium acetate, 425 mm sodium hydroxide) of which 150 μl were used for further analysis. 75 μl of chloramine-T solution (60 mm chloramine T in 50% 1-propanol) were added and incubated for 20 min at 20 °C. 75 μl of aldehyde-perchloric acid solution (1 m 4-dimethylaminobenzaldehyde, 60% 1-propanol, 22% perchloric acid (70–72%)) were added and further incubated at 60 °C for 25 min. The reaction was stopped by placing the samples on ice, and the absorbance was measured using a multi-plate reader at a wavelength of 570 nm. The samples were correlated with a standard curve from pure hydroxyproline (Sigma, H1637), and collagen content values were calculated using the sample weight and an estimated hydroxyproline/collagen conversion number of 11.4% (the multiplying factor was calculated from the measured ratio between pure bovine tendon collagen mass and hydroxyproline mass).
Gene Expression Analysis
The amount of mRNA for target genes was measured using quantitative real-time reverse transcriptase (RT) PCR. An overview over targets and primers sequences is provided in Table 1. First, tendon constructs were harvested and transferred to RNase free tubes containing 1 ml of TriReagent (Molecular Research Centre, Cincinnati, OH), 5 stainless steel beads of 2.3 mm in diameter, and 5 silicon-carbide sharp particles of 1 mm for mechanical disruption (BioSpec Products, Inc., Bartlesville, OK). For RNA isolation, samples were mechanically disrupted using a FastPrep®-24 instrument (MP Biomedicals, Inc., Illkirch, France) and subsequently bromo-chloropropane (Molecular Research Centre) was added to separate the samples into an aqueous and an organic phase. Glycogen was added to the tendon samples to improve RNA precipitation (120 μg/ml of TriReagent). After isolation of the aqueous phase, RNA was precipitated using isopropyl alcohol, washed in ethanol, and dissolved in RNase-free water. RNA concentrations were determined by spectroscopy at 260 nm, and RNA quality was confirmed by gel electrophoresis. Synthesis of complementary DNA (cDNA) was performed using the Omniscript reverse transcriptase (Qiagen, Hilden, Germany) on 500 ng of tendon cell RNA. For each target mRNA, 0.25 μl of 20× diluted cDNA (in 1× Tris/EDTA buffer with 1 ng/μl salmon DNA) was amplified in 25 μl of Quantitect SYBR Green Master Mix (Qiagen) with specific primers (100 nm each, Table 1) on a real-time PCR machine (MX3000P, Stratagene, La Jolla, CA). The thermal profile was 95 °C for 10 min → (95 °C for 15 s → 58 °C for 30 s → 63 °C for 90 s) × 50 → 95 °C for 60 s → 55 °C for 30 s → 95 °C for 60 s. Signal intensity was acquired at the 63 °C step, and the threshold cycle (Ct) values were related to a standard curve made with the cloned PCR product. Specificity was confirmed by melting curve analysis after amplification (the 55 °C to 95 °C step). The large ribosomal protein P0 (RPLP0) mRNA, which was stably expressed relative both to GAPDH mRNA and total RNA (data not shown), was chosen as the internal control. Values were normalized by RPLP0 expression and are presented as relative difference from 21-day control.
TABLE 1.
PCR primers
| Target | Sense | Antisense |
|---|---|---|
| RPLP0 | GGAAACTCTGCATTCTCGCTTCCT | GCTCCTTGCCGAGAAGCAGAAC |
| COL1A1 | GGCAACAGCCGCTTCACCTAC | GCGGGAGGACTTGGTGGTTTT |
| COL3A1 | CACGGAAACACTGGTGGACAGATT | ATGCCAGCTGCACATCAAGGAC |
| COL5A1 | AGCAGATGAAACGGCCCCTG | TCCTTGGTTAGGATCGACCCAGT |
| COL6A1 | CACACCGCTCAACGTGCTCTG | GCTGGTCTGAGCCTGGGATGAA |
| COL11A1 | ACCCTCGCATTGACCTTCCTCTT | ATCCCGTTGTTTTTGATATTCCCTCTG |
| COL12A1 | CCCAGGTCCTCCTGGATACTGTGA | GCAGCACTGGCGACTTAGAAAATGT |
| COL14A1 | AGCATGGGACCGCAAGGC | GACGCGCCACTGATCTCACC |
| Lysyl Oxidase | CGCTGTGACATTCGCTACACAGGAC | CATTGGGAGTTTTGCTTTGCCTTCT |
| LOXL1 | GGTGAGATGCAACATTCACTACACAGG | GCCTGCTTTGGAAGGGGAGAGA |
| LOXL2 | CCACCGCATCTGGATGTACAACTG | GAGCCCGCTGAAGTGCTCAAA |
| LOXL3 | CTGGGTGCACAACTGCCACAT | TCAAACCTCCTGTTGGCCTCTTC |
| LOXL4 | TATGATGGGCACCGGGTCTG | GGAGAGTTCTGCATTGGCTGGGTA |
| Transglutaminase 2 | CGGGAGGATATCACCCACACC | CTCCTTCTCGGCCAGTTTGTTCA |
| Decorin | GGTGGGCTGGCAGAGCATAAGT | TGTCCAGGTGGGCAGAAGTCA |
| Fibrilin-I | CGCTGCAATCATGGTTTCATCCTTT | ATTCCCATTTCCACTTGCACATTC |
| Elastin | GGCTTCGGATTGTCTCCCATTTT | CCAACGTTGATGAGGTCGTGAG |
| BMP-I | CAGACGGCACACAGCTCGTAAGT | TGGCAGCTGGGGGTAGAAGTGT |
| Fibromodulin | CAGTCAACACCAACCTGGAGAACC | TGCAGAAGCTGCTGATGGAGAA |
| Tenascin-C | CAACCATCACTGCCAAGTTCACAA | GGGGGTCGCCAGGTAAGGAG |
| Tenascin-X | GGAGGACTATGCCCATGGTTTTG | CGCATGGAGTAGTCACCTGCCTGT |
| Biglycan | AGGCCAAGCTGACTGGCATCC | TGGCCTGGATTTTGTTGTGGTC |
| Lumican | CCCTGGTTGAGCTGGATCTGTC | CCAGGATCTTGCAGAAGCTCTTTATGT |
Collagen Cross-link Analysis
Tendon constructs were washed twice in PBS, blotted dry, and subsequently heated in SDS-PAGE sample buffer (5 mg/ml) at 95 °C for 3 min. Samples were run on 5% SDS-PAGE according to standard procedures (25). For detection of type III collagen we used delayed reduction in which the electrophoresis was stopped after 20 min, and 10 μl of 0.5 m DTT (in water with 10% glycerol) were added and electrophoresis resumed. Gels were subsequently washed, stained with Coomassie Brilliant Blue, washed again, and analyzed. Collagen α1(I) chains were excised as individual bands (as marked in Fig. 3B) and digested in-gel with trypsin for mass spectral analysis to assess C-telopeptide lysine hydroxylation levels as described earlier (26).
FIGURE 3.
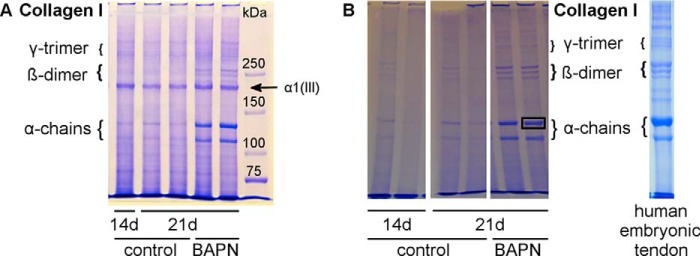
SDS-PAGE analysis of collagen chains. A, equal amounts of tendon constructs were analyzed by SDS-PAGE to assess the collagen cross-link abundance in 14- and 21-day controls versus BAPN treatment. The characteristic collagen type I pattern is shown with α-monomers, β-dimers, and γ-trimers. Overall, the BAPN samples show higher band intensity, particularly for monomers and dimers, suggesting higher collagen solubility due to fewer intermolecular covalent cross-links. Furthermore, the prominent collagen pN α1(III) chain is indicated. This is present in similar amounts per construct for all samples, even in the controls in which collagen type I chains are noticeably less soluble and abundant in the extracts due to lysyl oxidase cross-linking. B, SDS-PAGE without DTT sample reduction of tendon constructs and human embryonic tendon. Similar bands are seen as in A but without the pNα1(III) band. The human embryonic tendon extract shows a similar pattern to the tendon constructs. Single bands were excised for mass spectrometry analysis as indicated in the right 21d BAPN lane.
SDS-PAGE and Western Blotting
Tendon constructs for Western blotting were washed in PBS and blotted dry. Constructs were lysed by mechanical disruption with a pestle and sonication for 3 × 5 s in sample buffer (NuPAGE® LDS sample buffer; Invitrogen). Subsequently the samples were heated to 95 °C for 10 min and centrifuged at 10,000 × g for 10 min and stored at −80 °C until further use.
Samples were incubated at 70 °C for 10 min and separated on a NuPAGE® criterion XT 4–12% Bis-Tris gel using 1× NuPAGE® MES SDS running buffer (Invitrogen) at 700 V for 70 min, blotted with an iBlot™ device (Invitrogen) on PVFD membranes (GE Healthcare), and blocked with 5% milk powder in PBS plus 0.1% Tween 20. Primary antibodies against LOX (1:1000; NB100-2530), collagen V (1:500; NBP1-19633), decorin (1:500; NBP1–84970), fibromodulin (1:500; NBP2-16494), and tenascin-X (1:500; H00007-D01P) were incubated overnight at 4 °C (all acquired from Novus Biologicals, Littleton, CO). The complementary HRP-conjugated secondary antibody (anti-rabbit, 1:1000 dilution, swine, DAKO, #P0399) was incubated for 1 h. Membranes were analyzed using an Odyssey® FC system with complemented software (LI-COR Biotechnology, Lincoln, NE).
Statistics
Total collagen content, mean fibril diameter, and gene expression data were analyzed by one-way analysis of variance, and individual differences were determined by Tukey's multiple comparisons test. Construct mechanical data were acquired in triplicates per cell line and, therefore, analyzed by 2-way analysis of variance (cell line*treatment) with Tukey's multiple comparisons test using the statistical software package SPSS (version 22; IBM, Armonk, NY). Level of significance was set at p < 0.05.
Results
Mechanical Testing
Ultimate failure stress developed from 14 to 21 days from 0.41 MPa (±0.03; mean ± S.E.) to 1.73 MPa (±0.50) under control conditions, and this increase was completely abolished by BAPN (Fig. 2B). The tensile strain at failure did not change significantly with time and was unaffected by BAPN (Fig. 2C). The stress/strain curve resulted in a tensile modulus of 2.59 MPa (±0.68) after 14 days and was increased to 12.38 MPa (±2.90) after 21 days in controls, whereas BAPN-treated constructs did not show any development over 7 days and had a value of 2.48 MPa (±0.46) after 21 days (Fig. 2D).
To evaluate the quality of collagen cross-linking, equal amounts of homogenized tendon constructs were analyzed by SDS-PAGE staining the protein bands with Coomassie Brilliant Blue. The pattern given of collagen α chains, β dimers, and γ trimers is characteristic for different tissue types, and individual bands can be subjected to mass spectral analysis (26). Representative data for one 14-day control, two 21-day control constructs, and two 21-day BAPN-treated constructs are shown in Fig. 3A. The gel indicates that collagen from BAPN-treated constructs was more extractable than from controls as indicated by the higher band signal intensity of α chains and β dimers (Fig. 3A). A pNαI(III) chain of type III collagen is resolved by the interrupted PAGE technique. Notably, this band was in similar yield from the three groups (Fig. 3A). SDS-PAGE without sample DTT reduction (Fig. 3B) does not show any pNαI(III) band as it runs as a disulfide-bonded trimeric molecule close to the origin. Presumably, in the absence of BAPN some of the type III collagen monomers are cross-linked into the construct matrix (27). The difference between collagen type I band intensities remains the same between controls and BAPN-treated samples (Fig. 3B). Furthermore, human embryonic tendon was analyzed in comparison to tendon constructs. The visible collagen pattern is similar to the construct pattern for α1(I) and α2(I) bands and three β-dimers of about equal intensity (Fig. 3B). Single bands were excised as indicated by the black frame and further analyzed by mass spectrometry. The result showed that the α1(I) C-telopeptide lysine was fully hydroxylated so the cross-linking is predicted to be primarily of the hydroxylysine aldehyde variety, comparable to similar findings from human embryonic tendon α chains (data not shown).
Tendon Construct Ultrastructure
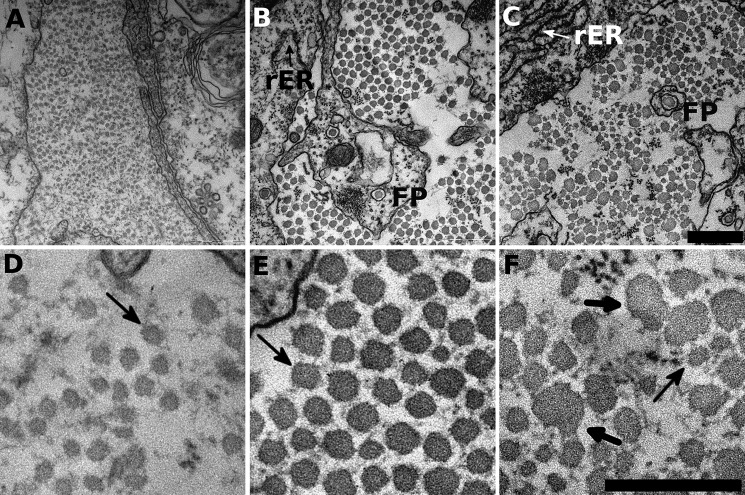
The TEM data of the tendon constructs showed regular collagen fibril distributions with circular outlines, a uniform distribution of fibrils in the extracellular space and healthy cells (as shown by intact membranes, intact nuclei, and the presence of rough ER). At 14 days, control constructs show small collagen fibrils with similar diameters (Fig. 4A), and after 21 days, the collagen fibrils were larger in diameter yet still with rather uniform diameters (Fig. 4B). At higher magnification the collagen fibrils of control constructs were not perfectly circular in profile but showed clear outlines with consistent fibril spacing (Fig. 4, D and E). Small dark circles in the extracellular matrix (ECM) are most probably microfibrils, which are characteristic for tendon tissue. Fibripositors containing collagen fibrils are also present, indicative of new collagen fibril development (indicated in image). In contrast, BAPN-treated constructs showed irregular collagen fibril shapes. The fibrils were of uneven size, and the spacing between fibrils was variable. At high magnification, some fibrils appeared to fuse (or split), whereas other fibrils were very small (Fig. 4, C and F). The cells in BAPN-treated samples looked nonetheless healthy, with rough ER indicating cell activity (Fig. 4) and intact nuclei (compare the supplemented videos). Fibrillin microfibrils and fibripositors were also visible in BAPN samples, suggesting that the ECM composition is normal but for the described collagen fibril impairment.
FIGURE 4.
BAPN precludes correct collagen fibril formation. Tendon construct cross-sections were investigated by TEM for their ultrastructure. A and D, 14-day control constructs show small diameter fibrils distributed in the extracellular space. At high resolution (D) the fibrils show almost circular outlines with large spacing in between individual fibrils (indicated by the arrow). B and E, at 21 days, the constructs show increased fibril diameters and tighter packing density. Moreover, the fibrils have similar fibril diameter with regular shapes. Rough endoplasmic reticulum (rER) indicate active cells, and fibripositors (FP) hint toward cell-collagen interactions and collagen production. A single collagen fibril is highlighted (arrow) that shows the typical close-to circular outline and is in regular distance to neighboring fibrils. C and F, BAPN-treated samples in contrast show heavily disrupted fibril shapes and irregular spacing in between individual fibrils. Fibril diameters vary considerably between very small (thin arrow) and very large fibrils (heavy arrows) of up to 100 nm. Moreover, the very large fibrils (heavy arrows) totally lost their circular shape. Compare the supplemental videos for a broader overview. Scale bar, 500 nm for A–C (in C); scale bar, 300 nm for (D–F) in F.
To quantify the effect of BAPN on collagen synthesis, total collagen content was measured and normalized to the dry weight of the construct before and after the BAPN treatment. 14-day constructs had a mean collagen content of 7.0% (±1.5; mean ± S.D.), whereas constructs at 21 days had significantly higher collagen contents at 11.6% (±1.9) (p < 0.05). However, BAPN-treated constructs had similar total collagen contents to controls with 11.8% (±3.2) (Fig. 5A). Similarly, the average fibril diameter developed significantly from 32.1 nm (±6.9; mean ± S.D.) at 14 days to 44.8 nm (±9.6) at 21 days under control conditions. The BAPN-treated samples showed a similar development to 44.7 nm (±13.8) (Fig. 5B). To understand the effect of BAPN on collagen fibrillogenesis further, the fibril diameter distributions for 14-day controls, 21-day controls, and 21-day BAPN-treated samples are shown in Fig. 5C. 14-day constructs show a unimodal Gaussian distribution around the mean fibril diameter. The distribution of 21-day control constructs is shifted to the right with few fibrils reaching high diameters larger than 60 nm. BAPN-treated constructs have a similar distribution to 21-day controls but have a flatter distribution; i.e. the BAPN-treated samples contain more very small diameter fibrils and a population of very large diameter fibrils as also visible in TEM images above. The three-dimensional ultrastructure was assessed using serial block face-scanning electron microscopy (supplemental Videos 1 and 2). The videos show sections through a 21-day control construct (Video 1) and a BAPN-treated construct (Video 2). Both samples show high cellular density, extensive cell connections, and open ECM spaces. This space is partly covered by collagen fibrils that run longitudinally. The collagen fibrils deviate slightly in x and y directions, and yet they do not change course in z direction. This shows that BAPN-treated fibrils have a similar three-dimensional organization compared with control tissue despite being disrupted in shape. Importantly, the large disrupted fibrils (recognizable by heavily stained larger structures in the ECM) appear to be irregular in shape over larger distances.
FIGURE 5.
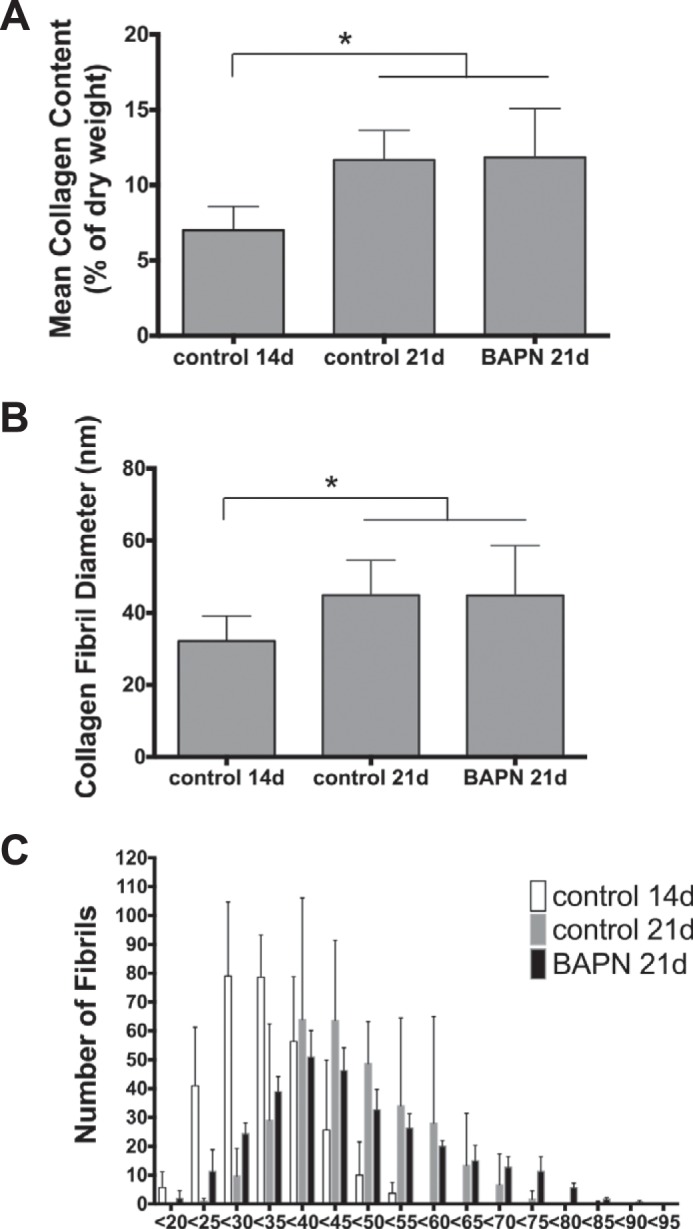
Collagen quantification. A, Total collagen content develops from 14 to 21 days by ∼4.5% of dry weight. BAPN does not affect the increase in collagen content. B, similarly, the mean collagen fibril diameter increases from day 14 to 21 by ∼12 nm without any effect of BAPN. C, the distributions for controls shift as expected from day 14 to 21 toward higher diameters. BAPN-treated constructs show a flattened distribution with more low and high diameter fibrils compared with 21-day controls. However, the irregular shapes of BAPN-treated fibrils are underestimated in size, as the minimal diameter was measured with the assumption of fibril circularity. Data are the mean ± S.E.; *, p < 0.05 toward other groups as indicated.
Gene Expression
To further address the response to LOX inhibition by the cells, gene expression of controls and BAPN-treated constructs was analyzed by RT-quantitative PCR. Fig. 6A shows that no significant changes in gene expression could be detected for the collagen cross-linker LOX, LOXL1–4, and transglutaminase 2. The group of the established collagen fibrillogenesis regulators decorin, collagen V, fibromodulin and tenascin-X, did not change due to BAPN treatment (Fig. 6B). Similarly, no changes could be detected in various important ECM molecules. This group included fibrillin I, the αI chain of the collagens I, III, VI, XI, XII, XIV, elastin, BMP-1, tenascin-C, biglycan, and lumican (primer sequences are provided in Table 1). Despite that the data indicate an overall reduction in gene expression of these molecules, no statistical differences could be detected (Fig. 6C). Moreover, as these data were only obtained after 7 days of LOX inhibition, the immediate response of the cells to BAPN treatment was monitored over 1, 6, 12, 24, and 48 h. Representative for these experiments, the expression profile for LOX over the time course is shown in Fig. 6D. As for the above-mentioned experiments, no changes were observed. Collagens I, III, V, decorin, fibromodulin, and tenascin-X did not change over time due to BAPN treatment neither (data not shown).
FIGURE 6.
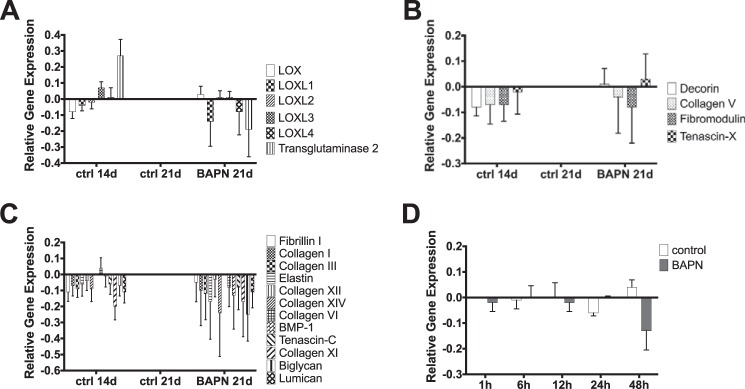
Gene expression analysis. Gene expression for 14- and 21-day controls as well as 21-day BAPN-treated samples was analyzed for collagen cross-linking enzymes (A), important fibrillogenesis regulators (B), and essential ECM components (C). Moreover, the immediate response toward BAPN-treatment was analyzed as presented here for LOX gene expression (D). A similar pattern to D was observed for all other molecules. Overall, no significant changes in gene expression were found. A–C, data are presented as -fold change from 21-day controls. D, data are presented as -fold change from 1 h control. Data are the mean ± S.E.
Western Blotting
Fig. 7A shows the results for LOX and for the fibrillogenesis regulators decorin, fibromodulin, and tenascin-X. LOX is equally present in control and BAPN-treated constructs at 80 kDa, which depicts the active form of LOX.3 Similarly, decorin, fibromodulin, and tenascin-X are present and not affected by the intervention.
FIGURE 7.
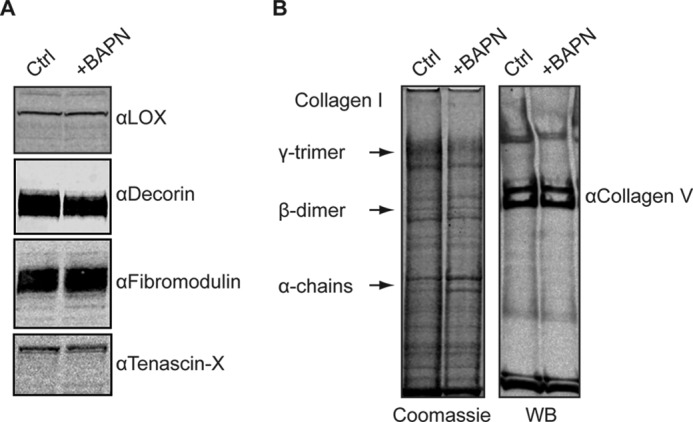
A, Western blotting results of 21-day controls and BAPN-treated samples were compared for LOX, decorin, fibromodulin, and tenascin-X. No change was found for these proteins due to BAPN treatment. B, direct comparison between a polyacrylamide gel stained with Coomassie Brilliant Blue (left side) and Western blot (WB) for collagen type V (right). The above-described pattern of collagen type I monomers, dimers, and trimers is visible. Splice or processing variants of the collagen pNα1(V) chain are indicated and are similarly positioned in both control and BAPN-treated construct, and only low levels of dimer bands are evident in both control and BAPN-treated lanes.
Construct extracts were analyzed by SDS/PAGE/Western blot for collagen type V and by Coomassie staining. A double band running above the β-dimer bands of the Coomassie gel is revealed in the Western blot where there are probably two pNα1(V) splice variants (Fig. 7B).
Discussion
LOX has previously been proven to be essential for the mechanical integrity of connective tissue (15, 18). The present findings show that this holds true for in vitro engineered tendon constructs from adult human tendon fibroblasts that present aligned collagen fibrils. Interestingly, blocking of LOX by BAPN leads to spontaneous rupture of constructs when introduced from early fibrillogenesis, and it stops mechanical development when added to already formed but still developing constructs (Figs. 1 and 2). This effect is most likely due to a loss of intra- and possibly interfibrillar collagen cross-links, as collagen solubility was increased with BAPN treatment (Fig. 3A). Furthermore, we were able to show that LOX inhibition leads to collagen fibrils with irregular profiles and broad non-physiological fibril diameter distributions despite no change in the total amount of collagen (Fig. 4). The average fibril diameter also remained unchanged as measured by the minimal width of the fibril. However, this method of measurement most probably underestimated the absolute size of the fibrils, particularly the irregular fibrils in BAPN-treated samples.
SDS-PAGE showed that the collagen profile of tendon constructs is strikingly similar to human embryonic tendon (Fig. 3B). Further analysis with mass spectrometry led to the result that most cross-links in embryonic tendon and tendon constructs are of the hydroxylysine aldehyde variety. Hence, the tendon construct can be used as a valid model for the collagen modification during human tendon development.
The effect of BAPN on tissues was originally shown by Levene and Gross (18) using chick embryos. Injections of BAPN into fertilized eggs led to exceptionally weak connective tissues and high collagen solubility (15, 18). BAPN causes osteolathyrism in humans and animals, a severe clinical condition caused by lacking collagen cross-linking (28). Furthermore, BAPN competitively inhibits LOX by inhibiting the active site of the enzyme (16, 17). We show here that constructs treated with BAPN from day 0 ruptured when the constructs were fully contracted, which coincides with maximal tensile force in the construct (Fig. 1). We have seen earlier that tendon constructs increase continuously in mechanical stiffness during 5 weeks post seeding (22). Here we show that mechanical development of tendon-like tissue can be stopped by BAPN between days 14 and 21. Irrespective of BAPN-treatment, the total collagen content increases similarly to controls during this phase (Fig. 5). Therefore, our data suggest that in early development continuing cross-link formation is essential for collagen fibrils that are constrained in diameter and regular in outline and maintain a mechanically cohesive tissue. In line with the unaltered total collagen content, gene expression of various ECM targets like collagens I, III, V, IX, and XII, biglycan, decorin, lumican, tenascin-C and elastin were equally expressed in BAPN-treated samples compared with controls at all times (Fig. 6). Gene expression of the cross-linking enzymes LOX, LOXL 1–4, and transglutaminase were also unaffected. This raises the question of whether the cells sense the alteration in collagen cross-linking. The results confirm that LOX is the rate-limiting step in collagen cross-link formation and that the cells do not possess an alternative mechanism for establishing the integrity of the collagen network. Moreover, BAPN does not affect the cells. This was also observed by Marturano et al. (19, 29), who found no change in collagen content or a toxic effect of BAPN treatment in chick embryos in vivo. Here, the fibroblasts applied a constant force that the impaired ECM could not withstand, leading to total rupture of the structure.
Collagen fibril diameters and shapes are distinct markers of tissue integrity (30). Throughout the body, the collagen network is arranged according to mechanical demands. This includes random networks of size-varying fibrils in skin (2, 31), fibrils internally mineralized with hydroxyapatite nanocrystals in bones (4), highly organized layers of small diameter fibrils in cornea (32, 33), linear arrays of intermediate-diameter fibrils in ligaments, and of large diameter fibrils in tendons (30, 34). Collagen fibrillogenesis starts with the production of procollagen molecules, which are converted to collagen molecules by cleavage of the terminal propeptides during assembly into fibrils (5). Early fibrils are uniform with about 20 nm in diameter, have circular outlines and tapered ends (5). These structural characteristics are notably impaired in BAPN-treated developing tendon constructs. The collagen fibrils are more heterogeneous in size, and the circular outline is disrupted. It is, therefore, plausible that these structural changes also relate to the mechanical weakness of the tendon construct. This phenotype is strikingly similar to the connective tissues of patients suffering from the Ehlers Danlos Syndrome (EDS). Various gene defects were described causing EDS, mostly involving mutations in collagen type V genes (35, 36). The irregular cauliflower-shaped collagen fibrils in EDS patients are consistently present in skin and tendon. These collagen fibrils can be of various sizes and shapes; often the regular circular outline is entirely lost, and the uniform size of fibrils is perturbed (36–38), but mechanical testing of these single fibrils has never been performed. Nevertheless, connective tissues from EDS patients have decreased mechanical strength and increased flexibility, which results in joint hypermobility, impaired skin wound healing, and an increased risk of bone fractures (36, 39). Also, tendon stiffness is markedly reduced in EDS patients in vivo (36). It has been suggested that collagen type V is polymerized as a template and internal copolymer for all collagen type I fibrils, which is disturbed during EDS (38, 40). Mice that are haploinsufficient for collagen type V develop irregular collagen fibrils in their tendons and skin that lead to reduced tissue stiffness similar to EDS patients. The irregular fibrils arise thereby from both fibril fusion and uncontrolled molecular accretion to the fibrils (38). Higher ratios of collagen types V/I are associated with thin fibrils as in cornea. Collagen types I, III, and V are cross-linked to each other in heterotypic fibrils (41–44). Apart from collagen type V, decorin, fibromodulin, and tenascin-X regulate collagen fibril diameters (40, 45–47). A lack of the small leucine-rich proteoglycan decorin has been shown to have a similar effect on collagen fibril formation as the above-described mutations in collagen V (45). Decorin binds to collagen fibrils (48, 49), and it was proposed that it tightly controls collagen fibril structure by binding four single collagen molecules (50). Fibromodulin also binds to collagen fibrils (51) and fibromodulin knock-out mice show a similar phenotype to decorin knock-out mice with irregular fibrils and hyper-flexible tissues (46). These mice have, despite significantly weaker tendons, higher amounts of lysyl oxidase-derived cross-links in tendon collagen (26). It was proposed, therefore, that fibromodulin has a site-specific LOX-inhibiting role that regulates the temporal sequence of cross-linking as fibrils grow, which is disrupted in the null mice (26). In another recessive form of EDS, mutations in tenascin-X (47) cause elastic skin and irregular fibrils phenocopied in tenascin X-null mice (52, 53). Taken together, these various studies indicate a very sensitive regulation system for collagen fibril formation involving a close interplay of collagen V, decorin, fibromodulin, tenascin-X, and probably other matrix proteins including type III collagen. Importantly, none of the described regulators is redundant, as no regulator can fulfill the others' function, and lack of each of the regulators leads to similar phenotypes. The present study showed that LOX inhibition leads to similarly disrupted collagen fibrils, adding LOX to the list of essential fibrillogenesis regulators.
To explore potential changes in molecular networks that could account for the LOX inhibition effect, gene expression profiles were analyzed. However, no change in gene expression was found for the collagen cross-link regulating proteins (Fig. 6B). Thus, the effect seems to be primarily extracellular and beyond cellular control. Western blotting confirmed the gene expression data with no effect on these proteins (Fig. 7). Collagen type V expression was also examined by Western blotting compared with the SDS-PAGE profile of total construct collagen. No difference in expression was evident, but this analysis may not detect the likely effects of BAPN treatment on collagen V/I coassembly and cross-linking. LOX does not act on procollagen monomers or soluble forms of collagen but is active when associated with native fibrils (54). The enzyme directly binds to the triple helical collagen molecules within fibrils, yet cannot penetrate the fibril core (55). This supports the theory that collagen cross-linking is initiated at the surface of growing collagen fibrils. Collagen type V was proposed as the key fibrillogenesis initiator or template. Yet despite a theoretical model (38), there is no experimental evidence as yet for a regulating mechanism. One possibility is that cross-link formation within and to type V oligomers is needed for ordered growth of the I/V copolymer.
Despite the proposed mechanism here of BAPN-induced LOX inhibition that impairs integer structural assembly of collagen fibrils and thereby the constructs' mechanical development, other possible causes need to be addressed. LOX is currently the subject of many scientific studies largely because of its essential role in collagen cross-linking, but other possible functions of the enzyme remain unknown. To what extend LOX might play a role in collagen fibrillogenesis remains to be discovered. A detailed mechanism of involvement in fibril shape regulation, as described in this study, might be among the yet unknown functions of LOX in connective tissues. Because we were unable to establish a direct mechanism linking the morphological to the mechanical impairments, LOX might be involved in two independent mechanisms: the well established collagen cross-linking that provides mechanical integrity and the regulation of fibril morphology. The latter mechanism can be modulated in two ways: by molecular accretion of collagen to existing fibrils or by fusion of existing fibrils. Wenstrup et al. (38) have proposed that irregular fibril shapes arise from a combination of both mechanisms, although experimental evidence is still lacking. A possible interplay between collagen type V and LOX would in this context be a reasonable hypothesis to explain the development of irregular fibrils.
BAPN is widely accepted to specifically inhibit the active site of the LOX family members. The current study could not show a toxic or inhibiting effect on cells, which is in line with other studies (19, 56), but it cannot be excluded that BAPN might directly interfere with collagen fibrillogenesis or other regulating proteins. However, it has been shown in vitro that BAPN does not bind directly to collagen fibrils (57, 58) and does not interfere with procollagen processing (59), which argues against a direct effect of BAPN on fibrillogenesis. To date, no study has shown that BAPN affects regulatory molecules other than the LOX family. If mechanical and morphological irregularity are not connected by a single mechanism as indicated above, the possibility that BAPN also interferes with an alternative fibrillogenesis-regulating molecule needs to be considered. However, we could not demonstrate an effect of BAPN on gene expression or protein modification of the so far established fibrillogenesis regulators collagen type V, decorin, fibromodulin, and tenascin-X. Yet it cannot entirely be excluded that BAPN interferes with one of these molecules or other as-yet-unknown regulators. Nevertheless, the well described LOX-inhibition mechanism (16, 17), the increased collagen solubility, and reduced mechanical strength of tendon constructs (19, 29) in combination with unaltered gene expression and protein content of established key regulators coincide with the so-far-established function of BAPN.
A recent study focusing on corneal development investigated the effects of LOX and transglutaminase induced cross-linking on cornea collagen fibrils (20). Three-dimensional tissue constructs from chick corneal fibroblasts were manipulated with BAPN and transglutaminase inhibitor. Transglutaminase inhibition again had a similar effect as the above described collagen V absence: collagen fibrils showed broadened diameter spectra and random shapes (20). Interestingly, BAPN treatment had no effect on fibril shape in contrast to the present study. This might be due either to the fact that fibril formation is different in tendon versus cornea cells or that the corneal constructs were not cultured under tensile tension as described here. Moreover, earlier studies of decorin-deficient mice showed highly impaired collagen fibrils in tendons and skin but no change in corneal tissue, which supports the notion that collagen fibrillogenesis in cornea is regulated differently (60).
Serial block face-scanning electron microscopy provided valuable insight into the three-dimensional tissue architecture before and after BAPN treatment. The data show that the collagen fibrils in BAPN-treated samples were similarly arranged to control tissue (supplemental videos). Fibril populations were in close proximity to the cells and were longitudinally aligned with minor variations in BAPN-treated samples and controls, respectively. Some large (probably irregularly shaped) collagen fibrils evident in the BAPN-treated samples appeared to be fusing with thinner fibrils. This observation and the overall structural differences were, however, difficult to quantify due to technical limitations.
In summary, we demonstrate that LOX is essential for the assembly of uniformly shaped collagen fibrils in a tendon-like construct from human tenocytes grown under tensile load in vitro. Cross-link formation by LOX was also required to gain mechanical stiffness and load resistance in the tendon-like constructs.
Supplementary Material
Acknowledgments
We gratefully acknowledge the help with tissue acquisition of the Department of Orthopaedic Surgery at Bispebjerg Hospital, Copenhagen. The Core Facility for Integrated Microscopy at the University of Copenhagen is thankfully acknowledged for support and access to electron microscopes.
This work was supported, in whole or in part, by National Institutes of Health Grant AR037318 (NIAMS; to D. E.). This work was also supported by the Wellcome Trust (to K. E. K) and, in whole or in part, by the Nordea Foundation (a Healthy Aging grant) and the University of Copenhagen, Department of Health Sciences, Academy of Muscle Biology, Exercise, and Health Research. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Videos 1 and 2.
J. Erler, BRIC Copenhagen, personal communication.
- LOX
- lysyl oxidase
- BAPN
- β-aminopropionitrile
- TEM
- transmission electron microscopy
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- EDS
- Ehlers Danlos Syndrome
- ECM
- extracellular matrix.
References
- 1. Kastelic J., Galeski A., Baer E. (1978) The multicomposite structure of tendon. Connect. Tissue Res. 6, 11–23 [DOI] [PubMed] [Google Scholar]
- 2. Silver F. H., Freeman J. W., DeVore D. (2001) Viscoelastic properties of human skin and processed dermis. Skin Res. Technol. 7, 18–23 [DOI] [PubMed] [Google Scholar]
- 3. Wang R., Brewster L. P., Gleason R. L., Jr. (2013) In-situ characterization of the uncrimping process of arterial collagen fibers using two-photon confocal microscopy and digital image correlation. J. Biomech. 46, 2726–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiner S., Traub W. (1992) Bone structure: from angstroms to microns. FASEB J. 6, 879–885 [PubMed] [Google Scholar]
- 5. Kadler K. E., Holmes D. F., Trotter J. A., Chapman J. A. (1996) Collagen fibril formation. Biochem. J. 316, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown I. A. (1972) Scanning electron microscopy of human dermal fibrous tissue. J. Anat. 113, 159–168 [PMC free article] [PubMed] [Google Scholar]
- 7. Young R. D., Knupp C., Pinali C., Png K. M., Ralphs J. R., Bushby A. J., Starborg T., Kadler K. E., Quantock A. J. (2014) Three-dimensional aspects of matrix assembly by cells in the developing cornea. Proc. Natl. Acad. Sci. U.S.A. 111, 687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kagan H. M., Li W. (2003) Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem. 88, 660–672 [DOI] [PubMed] [Google Scholar]
- 9. Eyre D. R., Paz M. A., Gallop P. M. (1984) Cross-linking in collagen and elastin. Annu. Rev. Biochem. 53, 717–748 [DOI] [PubMed] [Google Scholar]
- 10. Molnar J., Fong K. S., He Q. P., Hayashi K., Kim Y., Fong S. F., Fogelgren B., Szauter K. M., Mink M., Csiszar K. (2003) Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim. Biophys. Acta 1647, 220–224 [DOI] [PubMed] [Google Scholar]
- 11. Mäki J. M., Räsänen J., Tikkanen H., Sormunen R., Mäkikallio K., Kivirikko K. I., Soininen R. (2002) Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106, 2503–2509 [DOI] [PubMed] [Google Scholar]
- 12. Mäki J. M., Sormunen R., Lippo S., Kaarteenaho-Wiik R., Soininen R., Myllyharju J. (2005) Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am. J. Pathol. 167, 927–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baker A. M., Bird D., Lang G., Cox T. R., Erler J. T. (2013) Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene 32, 1863–1868 [DOI] [PubMed] [Google Scholar]
- 14. Cox T. R., Bird D., Baker A. M., Barker H. E., Ho M. W., Lang G., Erler J. T. (2013) LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 73, 1721–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pinnell S. R., Martin G. R. (1968) The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to α-aminoadipic-δ-semialdehyde (allysine) by an extract from bone. Proc. Natl. Acad. Sci. U.S.A. 61, 708–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang S. S., Trackman P. C., Kagan H. M. (1983) Reaction of aortic lysyl oxidase with β-aminopropionitrile. J. Biol. Chem. 258, 4331–4338 [PubMed] [Google Scholar]
- 17. Wilmarth K. R., Froines J. R. (1992) In vitro and in vivo inhibition of lysyl oxidase by aminopropionitriles. J. Toxicol. Environ. Health 37, 411–423 [DOI] [PubMed] [Google Scholar]
- 18. Levene C. I., Gross J. (1959) Alterations in state of molecular aggregation of collagen induced in chick embryos by β-aminopropionitrile (lathyrus factor). J. Exp. Med. 110, 771–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marturano J. E., Arena J. D., Schiller Z. A., Georgakoudi I., Kuo C. K. (2013) Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc. Natl. Acad. Sci. U.S.A. 110, 6370–6375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang L., Uhlig P. C., Eikenberry E. F., Robenek H., Bruckner P., Hansen U. (2014) Lateral growth limitation of corneal fibrils and their lamellar stacking depend on covalent collagen cross-linking by transglutaminase-2 and lysyl oxidases, respectively. J. Biol. Chem. 289, 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bayer M. L., Yeung C.-Y., Kadler K. E., Qvortrup K., Baar K., Svensson R. B., Peter Magnusson S. P., Krogsgaard M., Koch M., Kjaer M. (2010) The initiation of embryonic-like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension. Biomaterials 31, 4889–4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herchenhan A., Bayer M. L., Svensson R. B., Magnusson S. P., Kjaer M. (2013) In vitro tendon tissue development from human fibroblasts demonstrates collagen fibril diameter growth associated with a rise in mechanical strength. Dev. Dyn. 242, 2–8 [DOI] [PubMed] [Google Scholar]
- 23. Kapacee Z., Richardson S. H., Lu Y., Starborg T., Holmes D. F., Baar K., Kadler K. E. (2008) Tension is required for fibripositor formation. Matrix Biol. 27, 371–375 [DOI] [PubMed] [Google Scholar]
- 24. Starborg T., Kalson N. S., Lu Y., Mironov A., Cootes T. F., Holmes D. F., Kadler K. E. (2013) Using transmission electron microscopy and 3View to determine collagen fibril size and three-dimensional organization. Nat Protoc 8, 1433–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 26. Kalamajski S., Liu C., Tillgren V., Rubin K., Oldberg Å., Rai J., Weis M., Eyre D. R. (2014) Increased C-telopeptide cross-linking of tendon type I collagen in fibromodulin-deficient mice. J. Biol. Chem. 289, 18873–18879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pingel J., Lu Y., Starborg T., Fredberg U., Langberg H., Nedergaard A., Weis M., Eyre D., Kjaer M., Kadler K. E. (2014) 3-D ultrastructure and collagen composition of healthy and overloaded human tendon: evidence of tenocyte and matrix buckling. J. Anat. 224, 548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deyl Z., Adam M., Macek K. (1981) 3-Hydroxypyridinium cross-links in lathyritic tissues. Biochem. Biophys. Res. Commun. 101, 1026–1030 [DOI] [PubMed] [Google Scholar]
- 29. Marturano J. E., Xylas J. F., Sridharan G. V., Georgakoudi I., Kuo C. K. (2014) Lysyl oxidase-mediated collagen crosslinks may be assessed as markers of functional properties of tendon tissue formation. Acta Biomater. 10, 1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parry D. A., Barnes G. R., Craig A. S. (1978) A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc. R. Soc. Lond. B Biol. Sci. 203, 305–321 [DOI] [PubMed] [Google Scholar]
- 31. Smith L. T., Holbrook K. A., Byers P. H. (1982) Structure of the dermal matrix during development and in the adult. J. Invest. Dermatol. 79, 93s–104s [DOI] [PubMed] [Google Scholar]
- 32. Birk D. E., Trelstad R. L. (1984) Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J. Cell Biol. 99, 2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Komai Y., Ushiki T. (1991) The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest. Ophthalmol. Vis. Sci. 32, 2244–2258 [PubMed] [Google Scholar]
- 34. Zhu J., Zhang X., Ma Y., Zhou C., Ao Y. (2012) Ultrastructural and morphological characteristics of human anterior cruciate ligament and hamstring tendons. Anat. Rec. (Hoboken) 295, 1430–1436 [DOI] [PubMed] [Google Scholar]
- 35. Symoens S., Syx D., Malfait F., Callewaert B., De Backer J., Vanakker O., Coucke P., De Paepe A. (2012) Comprehensive molecular analysis demonstrates type V collagen mutations in over 90% of patients with classic EDS and allows to refine diagnostic criteria. Hum. Mutat. 33, 1485–1493 [DOI] [PubMed] [Google Scholar]
- 36. Nielsen R. H., Couppé C., Jensen J. K., Olsen M. R., Heinemeier K. M., Malfait F., Symoens S., De Paepe A., Schjerling P., Magnusson S. P., Remvig L., Kjaer M. (2014) Low tendon stiffness and abnormal ultrastructure distinguish classic Ehlers-Danlos syndrome from benign joint hypermobility syndrome in patients. FASEB J. 28, 4668–4676 [DOI] [PubMed] [Google Scholar]
- 37. Piérard G. E., Lê T., Piérard-Franchimont C., Lapière C. M. (1988) Morphometric study of cauliflower collagen fibrils in Ehlers-Danlos syndrome type I. Coll. Relat. Res. 8, 453–457 [DOI] [PubMed] [Google Scholar]
- 38. Wenstrup R. J., Florer J. B., Davidson J. M., Phillips C. L., Pfeiffer B. J., Menezes D. W., Chervoneva I., Birk D. E. (2006) Murine model of the Ehlers-Danlos syndrome: col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J. Biol. Chem. 281, 12888–12895 [DOI] [PubMed] [Google Scholar]
- 39. Beighton P., De Paepe A., Steinmann B., Tsipouras P., Wenstrup R. J. (1998) Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am. J. Med. Genet. 77, 31–37 [DOI] [PubMed] [Google Scholar]
- 40. Wenstrup R. J., Florer J. B., Brunskill E. W., Bell S. M., Chervoneva I., Birk D. E. (2004) Type V collagen controls the initiation of collagen fibril assembly. J. Biol. Chem. 279, 53331–53337 [DOI] [PubMed] [Google Scholar]
- 41. Birk D. E. (2001) Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 32, 223–237 [DOI] [PubMed] [Google Scholar]
- 42. Birk D. E., Fitch J. M., Babiarz J. P., Linsenmayer T. F. (1988) Collagen type I and type V are present in the same fibril in the avian corneal stroma. J. Cell Biol. 106, 999–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niyibizi C., Eyre D. R. (1994) Structural characteristics of cross-linking sites in type V collagen of bone. Chain specificities and heterotypic links to type I collagen. Eur. J. Biochem. 224, 943–950 [DOI] [PubMed] [Google Scholar]
- 44. Wu J. J., Weis M. A., Kim L. S., Eyre D. R. (2010) Type III collagen, a fibril network modifier in articular cartilage. J. Biol. Chem. 285, 18537–18544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang G., Ezura Y., Chervoneva I., Robinson P. S., Beason D. P., Carine E. T., Soslowsky L. J., Iozzo R. V., Birk D. E. (2006) Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell. Biochem. 98, 1436–1449 [DOI] [PubMed] [Google Scholar]
- 46. Svensson L., Aszódi A., Reinholt F. P., Fässler R., Heinegård D., Oldberg A. (1999) Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J. Biol. Chem. 274, 9636–9647 [DOI] [PubMed] [Google Scholar]
- 47. Schalkwijk J., Zweers M. C., Steijlen P. M., Dean W. B., Taylor G., van Vlijmen I. M., van Haren B., Miller W. L., Bristow J. (2001) A recessive form of the Ehlers-Danlos syndrome caused by tenascin-X deficiency. N. Engl. J. Med. 345, 1167–1175 [DOI] [PubMed] [Google Scholar]
- 48. Fleischmajer R., Fisher L. W., MacDonald E. D., Jacobs L., Jr., Perlish J. S., Termine J. D. (1991) Decorin interacts with fibrillar collagen of embryonic and adult human skin. J. Struct. Biol. 106, 82–90 [DOI] [PubMed] [Google Scholar]
- 49. Pringle G. A., Dodd C. M. (1990) Immunoelectron microscopic localization of the core protein of decorin near the d and e bands of tendon collagen fibrils by use of monoclonal antibodies. J. Histochem. Cytochem. 38, 1405–1411 [DOI] [PubMed] [Google Scholar]
- 50. Orgel J. P., Eid A., Antipova O., Bella J., Scott J. E. (2009) Decorin core protein (decoron) shape complements collagen fibril surface structure and mediates its binding. PLoS ONE 4, e7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hedbom E., Heinegård D. (1993) Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J. Biol. Chem. 268, 27307–27312 [PubMed] [Google Scholar]
- 52. Bristow J., Carey W., Egging D., Schalkwijk J. (2005) Tenascin-X, collagen, elastin, and the Ehlers-Danlos syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 139C, 24–30 [DOI] [PubMed] [Google Scholar]
- 53. Mao J. R., Taylor G., Dean W. B., Wagner D. R., Afzal V., Lotz J. C., Rubin E. M., Bristow J. (2002) Tenascin-X deficiency mimics Ehlers-Danlos syndrome in mice through alteration of collagen deposition. Nat. Genet. 30, 421–425 [DOI] [PubMed] [Google Scholar]
- 54. Siegel R. C. (1974) Biosynthesis of collagen crosslinks: increased activity of purified lysyl oxidase with reconstituted collagen fibrils. Proc. Natl. Acad. Sci. U.S.A. 71, 4826–4830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cronlund A. L., Smith B. D., Kagan H. M. (1985) Binding of lysyl oxidase to fibrils of type I collagen. Connect. Tissue Res. 14, 109–119 [DOI] [PubMed] [Google Scholar]
- 56. Chvapil M., Misiorowski R., Eskelson C. (1981) On the mechanisms of β-aminopropionitrile toxicity. J. Surg. Res. 31, 151–155 [DOI] [PubMed] [Google Scholar]
- 57. Orloff S. D., Gross J. (1963) Experimental lathyrism in the chick embryo. The distribution of β-aminopropionitrile. J. Exp. Med. 117, 1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tanzer M. L., Hunt R. D. (1964) Experimental lathyrism: an autoradiographic study. J. Cell Biol. 22, 623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Canty E. G. (2004) Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J. Cell Biol. 165, 553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Danielson K. G., Baribault H., Holmes D. F., Graham H., Kadler K. E., Iozzo R. V. (1997) Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 136, 729–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kremer J. R., Mastronarde D. L., McIntosh J. R. (1996) Computer visualization of three-dimensional image data using IMOD. J Struct. Biol. 116, 71–76 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.