Introduction
Osteoarthritis (OA) is a chronic, progressive disease resulting in loss of function and pain due to articular cartilage loss and represents the most common disease among musculoskeletal disorders [7, 8, 15].
Various strategies for the treatment for medial OA of the knee including conservative and operative treatment options are currently used. Surgical treatment includes, among others, high tibial osteotomy (HTO), unicompartmental knee arthroplasty (UKA), and total knee arthroplasty (TKA). Conservative treatment comprises a variety of different options including analgesic and/or anti-inflammatory medication, bracing, physiotherapy, and intra-articular injections of corticosteroids or viscosupplements. The general problem with conservative treatment is that despite amelioration of symptoms, the progressions of OA are usually unhalted [10].
London et al. [12] illustrated an additional problem: There are patients unwilling or unsuitable to undergo extensive surgery by means of arthroplasty or HTO. These patients are often young and show mild to moderate but symptomatic OA on radiographs that limits their inactivities of daily life, especially participation in recreational sports. These patients are reported to be within a “treatment gap” with no perfectly suitable treatment concept available [12]. A recently published survey illustrated that the majority of orthopedic surgeons are aware of this treatment gap and feel the need for a suitable treatment option for the young patient with symptomatic early osteoarthritis [11].
Recently, a new surgical implant was introduced for the treatment of medial knee osteoarthritis trying to address this treatment gap. The device consists of titanium alloy base plates rigidly attached to the medial aspect of tibia and femur connected to a cobalt/cobalt chrome alloy spring crossing the joint line. This is meant to reduce the load of the medial knee joint compartment while preserving the joint and not significantly affecting the lateral joint contact mechanics [6]. It was designed to reduce pain and stop progression of OA of the medial compartment [5].
Our purpose is to describe the clinical course of a 52-year-old patient implanted with this type of device. In this case, the device failed necessitating complete implant removal 18 months following implantation due to pain, metallosis, and penetration of the joint capsule.
Case Report
A 52-year-old patient was referred to our hospital for ongoing pain localized over the medial aspect of the right knee joint following implantation of a medial knee joint load absorber device (KineSpring, Moximed®, Hayward, CA, USA) at an outside institution 18 months prior to admission. The patient reported no history of trauma or injury to the operated leg after or before surgery. The patient was mostly satisfied with the outcome of the procedure for 1 year despite recurrent tenderness of the tissue around the implant and recurrent swelling of the joint. Effusions and pain over the medial aspect of the joint increased 18 months after implantation and limited pain-free walking distance to approximately 500 m. Additionally, the patient reported that the pain over the device excelled that of the former osteoarthritis by far.
Clinical examination revealed tenderness and pain localized at the medial joint line as well as directly over the implant combined with a mild joint effusion. Range of motion was measured as zero to 120°. No signs of joint instability were present on examination. No clinical signs of infection could be perceived. Serum analysis revealed a normal blood cell count, a normal C-reactive protein, and a normal amount of cobalt ions, while there was a sevenfold elevated chromium ion level (2.9 μg/l).
Radiographic evaluation by means of radiographs in two plains of the knee joint (Fig. 1) as well as a long leg anterior-posterior radiograph (Fig. 2) showed an intact device and no signs of fracture or other bone lesions.
Fig. 1.
The lateral and anterior-posterior radiographs of the right knee with the intact device prior to surgery.
Fig. 2.
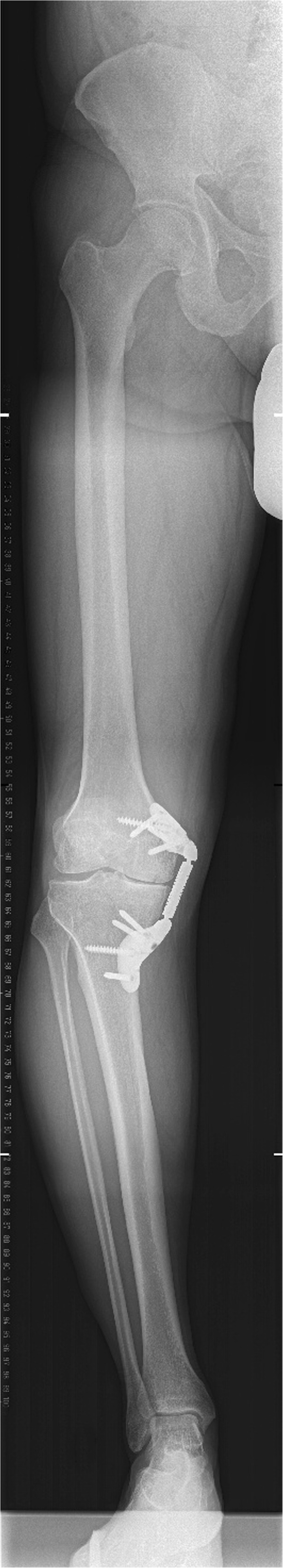
The long leg anterior-posterior radiograph of the right leg with the implanted device in place prior to revision surgery.
Surgery
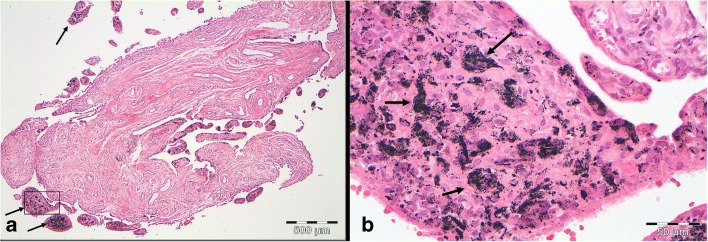
Arthroscopy of the knee joint was performed via standard antero-medial and antero-lateral portals. Extensive synovitis and metallosis were noted (Fig. 3). There was full thickness cartilage erosion on the medial femoral condyle and the tibia. The medial joint capsule showed disruption with free visualization of the implant spring underneath the pars intermedia of the medial meniscus due to penetration of the device into the joint (Fig. 4). Arthroscopic partial synovectomy was performed, and biopsies were obtained for analysis followed by open hardware removal of the implant. Figure 5 shows the intra-operative site with the implant still in place (Fig. 5a) and after removal (Fig. 5b). After removal of the implant, the full extent of metallosis could be perceived. Histopathological examination of a synovial biopsy showed synovial hyperplasia combined with an extensive burden of metal wear particles (Fig. 6).
Fig. 3.

An arthroscopic view of the superior articular recessus of the patients affected the right knee with extensive synovitis and metallosis.
Fig. 4.
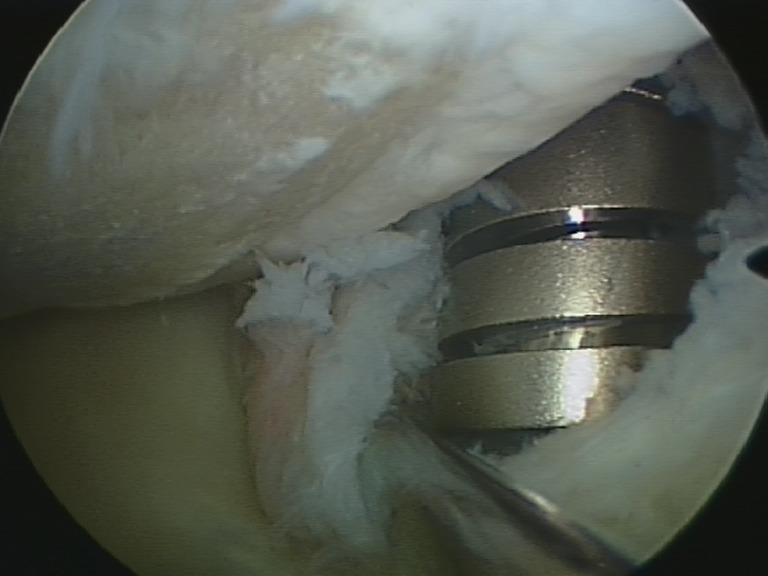
An arthroscopic view of the medial joint compartment with disruption of the joint capsule and penetration of the implant into the joint space.
Fig. 5.
a This intra-operative image documents the exposure of the tibial component of the KineSpring device. b This intra-operative photograph documents the extensive metallosis remaining after removal of the implant.
Fig. 6.
a This histology specimen prepared with hematoxylin and eosin staining of a synovial biopsy shows extensive amounts of metal wear particles indicated by the black arrows (×40 magnification). b This part of the figure shows a higher power photograph of the area indicated by the black box of a. Black metal wear particles are indicated by the black arrows (×400 magnification).
After removal of the device, the knee joint was found to be instable medially. The knee was treated with an orthotic for 3 months. All incisions healed primarily without complications. At the 6-month follow-up visit, medial knee instability was persistent but well tolerated by the patient. Total knee arthroplasty was discussed with the patient in the case of worsening complaints of his medial osteoarthritis.
Discussion
Altered biomechanics and excessive joint load are arguably the primary reasons for the development and progression of medial OA [3]. Consequently, an approach for treatment of initial medial OA could be conceived that focuses on reducing excessive joint load [14]. The KineSpring device was designed to achieve medial joint load reduction without affecting lateral contact mechanics [6]. The device is currently still investigational and not FDA-approved.
Current literature regarding this new device shows promising results. After its introduction and description of its biomechanical characterization by Clifford et al. [6], Allen et al. [1] reported safe usage of the device in sheep over a period of 52 weeks. No conclusion on the functionality of the device could be drawn from this animal study, as joint loading patterns between sheep and men differ enormously [1]. Additionally, a case report of successful patient treatment with the device in two patients has been published [9].
Recently, London et al. [13] presented midterm results of 99 patients treated with the novel device comprising the collective data from three clinical trials reporting favorable clinical results. Within the study collective, the Western Ontario and McMaster Universities Osteoarthritis index (WOMAC) improved regarding the pain score from 45 ± 17 at pretreatment to 20 ± 18 and regarding the function score from 44 ± 18 at pretreatment to 22 ± 18, respectively [13]. Bowditch et al. also addressed an infection following implantation and performed a two-step revision surgery due to infection of the absorber by coagulase-negative staphylococci. After removal of the absorber and antibiotic therapy, a new absorber unit was implanted 3 months later [2].
In spite of these favorable reports, there are several others reporting on failure of the device due to breakage and metallosis. Citak et al. reported of a broken absorber unit without trauma and extensive extra-articular metallosis 7 months after implantation in a 75-year-old woman. Knee arthroscopy was not performed. The authors removed the KineSpring® device and recommended total knee arthroplasty [4]. On the other hand, Clifford et al. [5] reported no signs of wear or cracking of the components of the new device after 15 million loaded motion cycles in an in vitro model. Although Allen et al. [1] reported no metallosis or adverse soft tissue reaction 52 weeks after implantation of the device in a sheep model, they did document the formation of mature fibrous tissue around the implant [1].
In the present case report, the use of the new device leads to ongoing pain and extensive extra- and intra-articular metallosis. In the light of the current case and the case reported by Citak et al. [4], metallosis and wear of the new device seem to represent a possible problem necessitating revision surgery. Whether these two case reports indicate a device inherit problem or are results of incorrect implant positioning cannot be assessed by this article. Further research especially regarding the biomechanics of the device and possible soft-tissue reaction after implantation is needed. This case highlights several risks of the unloader device that we believe have not been described and merit caution in recommending this implant. These include (1) development of local intra- and extra-articular metallosis potentially leading to elevated chromium serum levels, (2) soft tissue damage especially to the medial joint capsule and the medial collateral ligament, (3) intra-articular metallosis possibly contributing to synovitis and joint effusion, and (4) medial joint instability after removal of the device.
In conclusion, despite the initial promising results and the theoretically appealing idea behind the KineSpring® device, further research regarding its safety and biomechanical properties is needed. During informed consent, patients should be counseled on the risks of local metallosis and elevated ion levels in the serum, soft tissue damage to medial joint structures possibly leading to instability after implant removal as well as synovitis and joint effusion.
Electronic supplementary material
(PDF 510 kb)
(PDF 510 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Disclosures
Conflict of Interest
Karl Friedrich Schüttler, MD, Marion Roessler, MD and Susanne Fuchs-Winkelmann, MD, have declared that they have no conflict of interest. Turgay Efe, MD is a paid consultant for Smith & Nephew and Arthrokinetics, receives grants and payments from Smith & Nephew and Arthrokinetics, outside the work. Thomas J. Heyse, MD is a paid consultant for Smith & Nephew, receives grants from Smith & Nephew and Biomet and receives payments for other from Smith & Nephew and Biomet, outside the work.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 [5].
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Work performed at the University Hospital Marburg.
References
- 1.Allen MJ, Townsend KL, Bauer TW, Gabriel SM, O'Connell M, Clifford A. Evaluation of the safety of a novel knee load-bypassing device in a sheep model. J Bone Joint Surg Am. 2012;94(1):77–84. doi: 10.2106/JBJS.J.00918. [DOI] [PubMed] [Google Scholar]
- 2.Bowditch M, Miller LE, Block JE. Successful two-stage revision of a KineSpring(R) joint unloading implant: a case study. Int Med Case Rep J. 2012;5:91–5. doi: 10.2147/IMCRJ.S38486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93(1):1–24. doi: 10.1016/j.mcna.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Citak M, Kendoff D, O Loughlin PF, Klatte TO, Gebauer M, Gehrke T, et al. Failed joint unloading implant system in the treatment of medial knee osteoarthritis. Arch Orthop Trauma Surg. 2013;133(11):1575–8. doi: 10.1007/s00402-013-1830-6. [DOI] [PubMed] [Google Scholar]
- 5.Clifford AG, Gabriel SM, O'Connell M, Lowe D, Miller LE, Block JE. The KineSpring((R)) Knee Implant System: an implantable joint-unloading prosthesis for treatment of medial knee osteoarthritis. Med Devices (Auckl) 2013;6:69–76. doi: 10.2147/MDER.S44385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifford A, O'Connell M, Gabriel S, Miller LE, Block JE. The KineSpring load absorber implant: rationale, design and biomechanical characterization. J Med Eng Technol. 2011;35(1):65–71. doi: 10.3109/03091902.2010.535592. [DOI] [PubMed] [Google Scholar]
- 7.Dominick KL, Ahern FM, Gold CH, Heller DA. Health-related quality of life among older adults with arthritis. Health Qual Life Outcomes. 2004;2:5. doi: 10.1186/1477-7525-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30(8):914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 9.Hayes DA, Miller LE, Block JE. Knee Osteoarthritis Treatment with the KineSpring Knee Implant System: A Report of Two Cases. Case Rep Orthop. 2012;2012:297326. doi: 10.1155/2012/297326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henriksen M, Simonsen EB, Alkjaer T, Lund H, Graven-Nielsen T, Danneskiold-Samsoe B, et al. Increased joint loads during walking–a consequence of pain relief in knee osteoarthritis. Knee. 2006;13(6):445–50. doi: 10.1016/j.knee.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Li CS, Karlsson J, Winemaker M, Sancheti P, Bhandari M. Orthopedic surgeons feel that there is a treatment gap in management of early OA: international survey. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):363–78. doi: 10.1007/s00167-013-2529-5. [DOI] [PubMed] [Google Scholar]
- 12.London NJ, Miller LE, Block JE. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses. 2011;76(6):887–92. doi: 10.1016/j.mehy.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 13.London NJ, Smith J, Miller LE, Block JE. Midterm Outcomes and Predictors of Clinical Success With the KineSpring Knee Implant System. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:19–28. doi: 10.4137/CMAMD.S11768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waller C, Hayes D, Block JE, London NJ. Unload it: the key to the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1823–9. doi: 10.1007/s00167-011-1403-6. [DOI] [PubMed] [Google Scholar]
- 15.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)
(PDF 510 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)