Abstract
Background
Traditionally, blood transfusions in the perioperative setting are used to maintain adequate delivery of nutrients and oxygen to organs. However, the effect of blood administration on tissue oxygenation in the perioperative setting remains poorly understood.
Questions/Purposes
The aim of this study was to determine changes in muscle tissue oxygenation saturation (SmO2) in response to perioperative blood transfusions.
Patients and Methods
Patients undergoing total knee arthroplasty were enrolled. SmO2, continuous hemoglobin (SpHb), stroke volume (SV), cardiac index, and standard hemodynamic parameters including heart rate (HR), mean arterial blood pressure (MAP), and arterial oxygen saturation (SO2) were recorded. To assess fluid responsiveness, a passive leg raise (PLR) test was performed before the transfusions were started.
Results
Twenty-eight patients were included in the analysis. Mean (±SD) SmO2 before transfusion was 63.18 ± 10.04%, SpHb was 9.27 ± 1.16 g/dl, and cardiac index was 2.62 ± 0.75 L/min/m2. A significant increase during the course of blood transfusion was found for SmO2 (+3.44 ± 5.81% [95% confidence interval (CI) 1.04 to 5.84], p = 0.007), SpHb (0.74 ± 0.92 g/dl [95% CI 0.35 to 1.12], p < 0.001), and cardiac index (0.38 ± 0.51 L/min/m2 [95% CI 0.15 to 0.60], p = 0.002), respectively. However, the correlation between SmO2 and SpHb over the course of the transfusion was negligible (ρ = 0.25 [95% CI −0.03 to 0.48]). A similar lack of correlation was found when analyzing data of those patients who showed a positive leg raise test before the start of the transfusion (ρ = 0.37 [95% CI −0.11 to 0.84]).
Conclusion
We detected a statistically significant increase in SmO2 during the course of a single unit blood transfusion compared to baseline. However, there was no evidence of a correlation between longitudinal SmO2 and SpHb measurements.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-015-9434-z) contains supplementary material, which is available to authorized users.
Keywords: blood transfusion, muscle tissue oxygenation, total knee arthroplasty, near-infrared spectroscopy
Introduction
Total knee arthroplasty (TKA) often leads to blood loss resulting in the need for blood transfusions which are intended to maintain end organ perfusion. Each year, approximately 13 million units of red blood cells are transfused throughout the USA [21]. Many effects on organ systems and specifically those on muscle tissue oxygenation remain largely unstudied [14, 15, 20]. Tissue oxygenation and delivery of O2 has been reported to be optimal at hemoglobin and hematocrit values of 10 g dl−1 and 30%, respectively [1, 19]. More recent studies, suggesting that various other hemodynamic and/or clinical parameters should be taken into account when transfusing patients, failed to show a positive impact [12]. According to the ASA guidelines on perioperative blood transfusion, the literature to date is insufficient to evaluate the efficacy of specific intraoperative or postoperative monitoring techniques to detect the presence of inadequate perfusion of vital organs or as indicators for the transfusion of red blood cells [3].
The concept of near-infrared spectroscopy (NIRS) has long been used to measure arterial oxygen saturation (SaO2) [22]. Technological advances have allowed for the non-invasive determination of muscle tissue oxygenation (SmO2), muscle oxygen tension (PmO2), and muscle pH [17]. As PmO2 and SmO2 have proven to be very early indicators of central hypovolemia [18], they could serve as ideal measurements to assess critical changes in volume status and replace more subjective and likely unreliable measures of organ perfusion used in clinical practice such as urine output, blood pressure, and mental status changes. Studies suggest that NIRS-obtained values may predict the need for blood transfusion after severe trauma [4]. It is unclear if muscle hypoperfusion due to surgical anemia detected through SmO2 readings may be used as a real-time indicator for blood transfusion.
We hypothesized that blood transfusions in the perioperative setting would increase SmO2 in the postoperative period irrespective of starting hematocrit. In conducting this pilot study using NIRS in patients undergoing TKA, we sought to investigate (1) if real-time change of SmO2 and various other hemodynamic parameters before, during, and after administration of red blood cells in the postoperative period would occur and (2) if we could find a correlation between the SmO2, hemoglobin levels, and other hemodynamic parameters.
Patients and Methods
After obtaining approval from the Institutional Review Board (Hospital for Special Surgery, New York, NY), potential participants in this prospective, observational, pilot study were identified and consented by a research assistant on the day of surgery at the Hospital for Special Surgery (HSS) from March 2012 to February 2013. All adult patients, age 18–99, undergoing primary total knee arthroplasty with a starting hematocrit of 38% or less on preoperative testing, were eligible for inclusion. As the obtainment of consent was only approved before surgery, this cutoff was used to minimize enrollment failures, as historically patients in this group were more likely to require blood transfusions postoperatively. Exclusion criteria were patients with a hematocrit of >38% on preoperative testing, patients taking vasopressors and/or inotropic agents postoperatively, minors, mentally disabled patients, pregnant women, and employees. Patients enrolled in the study were only followed up in the post-anesthesia care unit (PACU).
Data on demographics (age, gender, ethnicity, comorbidities) and perioperative fluid administration were collected. Transfusions were ordered as per the treating clinician’s judgment, without a mandate for the use of specific transfusion triggers in order to obtain data in an actual patient care setting. These clinicians were not actively involved in the study and were blinded to the measurements. As soon as the clinical care team deemed that a blood transfusion was necessary, patients were connected to the monitors as follows: SmO2 was measured by continuous sampling of near-infrared spectroscopy spectra at the deltoid muscle using the CareGuide™ NIRS device (Reflectance Medical, Westborough, MA). Continuous hemoglobin (SpHb) was measured using non-invasive Rainbow® ReSposable™ Pulse CO-Oximeter™ Sensor System (Masimo®; Irvine, CA). Stroke volume (SV) and cardiac index were measured continuously using a non-invasive bioreactance monitor (NICOM™; Cheetah Medical, Vancouver, WA). In addition, standard hemodynamic parameters including heart rate (HR), invasive mean arterial blood pressure (MAP), and arterial oxygen saturation (SO2) were obtained from the standard monitoring equipment according to institutional practice. All hemodynamic parameters were recorded continuously throughout the blood transfusion and up to 10 min thereafter. In addition, relevant intraoperative data (fluid balance, tourniquet times, length of surgery, type of anesthesia, and postoperative events (complications)) were collected.
A passive leg raise test was performed before blood transfusion to determine fluid responsiveness [13]. This test has been used widely in clinical practice to determine a patient’s potential response to the administration of volume. The leg raise test was considered positive when a change in cardiac index of >10% was found. Blood transfusions were administered in a standardized fashion. We recorded the type and volume of blood transfused.
Study data were collected and managed using REDCap (Research Electronic Data Capture) (Vanderbilt University, Nashville, TN) electronic data capture tools hosted at the Hospital for Special Surgery [10]. REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
This study was exploratory, so a formal power analysis was not performed. The primary outcome of interest was a change in SmO2 measured continuously during the transfusion of blood in the recovery room. Baseline values and absolute and percentage change in SmO2, SpHb, MAP, HR, and cardiac index from baseline to middle, end, 5, and 10 min post-infusion are presented as means and standard deviations. Mean absolute changes in SmO2, SpHb, MAP, HR, and cardiac index are presented along with 95% confidence intervals and evaluated using paired t tests. Data from the beginning, middle, end, 5, and 10 min post-infusion were analyzed via the generalized estimating equations method (GEE) [11, 23] using an autoregressive [AR(1)] correlation structure and adjusting for age, sex, and BMI. The correlation between SmO2 and SpHb, MAP, HR, and cardiac index over time was assessed via bivariate linear mixed modeling [9]. Correlation coefficients are presented as point estimates along with 95% bootstrap confidence intervals. Exploratory subgroup correlation analyses were carried out based on PLR test result. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). All statistical tests were two-sided with a p value < 0.05 considered statistically significant.
Results
A total of 57 patients were consented for this study. Twenty-seven of those patients were ineligible because the PACU clinician determined that no blood transfusions would be administered during their PACU stay. Of the 30 remaining patients, 2 had to be excluded from analysis because of equipment failure. Patient-related demographics as well as procedure-related data are shown in Table 1.
Table 1.
Patient and procedure-related information
| Variable | Value |
|---|---|
| Age, mean (SD), years | 66.5 (9.21) |
| BMI, mean (SD), kg/m2 | 28.82 (6.03) |
| Female sex, n (%) | 25 (89.3) |
| Race, n (%) | |
| White | 24 (85.7) |
| Black | 1 (3.6) |
| Asian | 2 (7.14) |
| Other | 1 (3.6) |
| Type of anesthesia, n (%) | |
| CSE/peripheral nerve block | 24 (85.7) |
| Epidural/peripheral nerve block | 1 (3.6) |
| Spinal/peripheral nerve block | 3 (10.7) |
| Length of surgery, mean (SD), min | 100.56 (43.43) |
| PACU LOS, mean (SD), min | 1011.04 (554.78) |
| Hospital LOS, mean (SD), days | 3.89 (1.03) |
| Lactated ringers, mean (SD), ml | 1932.14 (726.77) |
SD standard deviation, BMI body mass index, CSE combined spinal-epidural, LOS length of stay
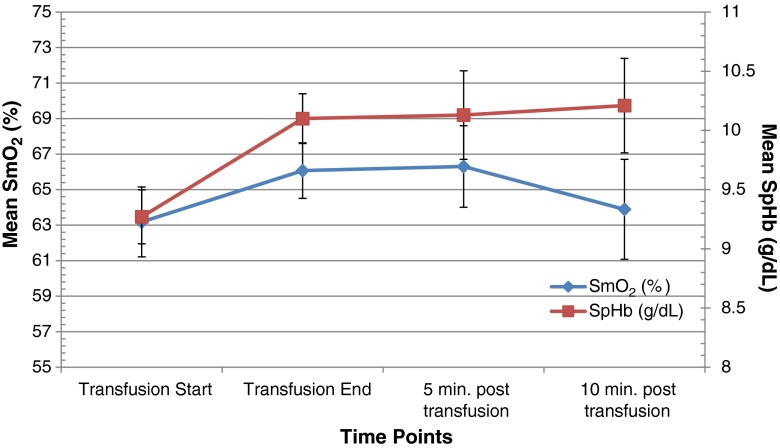
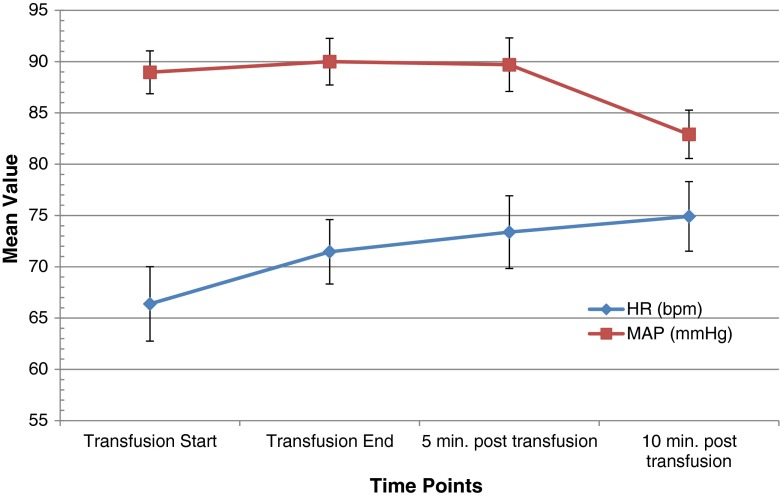
There was a statistically significant increase over the time frame of the transfusion in SmO2 (3.44% [95% confidence interval (CI) 1.04–5.84], p = 0.007), SpHb (0.74 g/dl [95% CI 0.35–1.12], p = 0.001), and cardiac index (0.38 L/min/m2 [95% CI 0.15–0.60], p = 0.002, Table 2). However, SmO2 reached pre-transfusion levels again 10 min after transfusion was completed (Fig. 1). Mean SmO2 at the beginning of transfusion was 63.18 + 10.04, mean SpHb was 9.27 + 1.16 g/dl, and mean cardiac index was 2.62 + 0.75 L/min/m2 s. No change was observed from the beginning to the end of transfusions in HR (2.37 bpm [95% CI −0.51–5.24], p = 0.102) or MAP (0.35 mmHg [95% CI −3.16–2.85], p = 0.839). However, there was a significant increase in HR 10 min after the end of transfusion (4.10 bpm [95% CI 0.46–7.74], p = 0.031, Fig. 2). After adjustment for longitudinal measurements, age, sex, and BMI, there was evidence of an increase in all measures besides MAP between the beginning and end of transfusion (Table 3).
Table 2.
Mean change (95% confidence interval) of hemodynamic parameters during the course of the blood transfusion
| Mean change (95% confidence interval) during the course of transfusion | ||||
|---|---|---|---|---|
| Absolute change | p value | Percent change | p value | |
| SmO2 (%) | 3.44 (1.04–5.84) | 0.007 | 6.52% (1.78–11.26) | 0.009 |
| SpHb (g/dL) | 0.74 (0.35–1.12) | 0.001 | 8.61% (4.34–12.88) | <0.001 |
| MAP (mm Hg) | 0.35 (−3.16–3.85) | 0.839 | 0.63% (−3.46–4.73) | 0.751 |
| CI (l/min/m2) | 0.38 (0.15–0.6) | 0.002 | 13.45% (5.48–21.42) | 0.002 |
| HR (bpm) | 2.37 (−0.51–5.24) | 0.102 | 5.39% (0.28–10.51) | 0.040 |
| SV (ml) | 6.69 (1.8–11.59) | 0.010 | 10.8% (2.74–18.86) | 0.011 |
SmO 2 muscle tissue oxygenation, SpHb hemoglobin, MAP mean arterial pressure, CI cardiac index, HR heart rate, SV stroke volume
Fig. 1.
Muscle tissue oxygenation (SmO2) and total hemoglobin (SpHb) over time. There were significant increases in the absolute mean values of SmO2 (p = 0.007) and SpHb (p = 0.001) during the course of the transfusion. Vertical bars represent standard errors.
Fig. 2.
Heart rate (HR) and mean arterial pressure (MAP) over time. There were no significant changes in the absolute mean values of HR (p = 0.102) or MAP (p = 0.839) during the blood transfusion. Vertical bars represent standard errors.
Table 3.
Mean change from baseline of hemodynamic parameters: unadjusted and adjusted for demographic variables
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Mean change from pre-transfusion (95% confidence interval) | p value | Mean change from pre-transfusion (95% confidence interval) | p value | |
| SmO2 (%) | ||||
| Middle | 1.53 (−0.57–3.64) | 0.146 | 1.54 (−0.43–3.50) | 0.125 |
| End | 3.44 (1.04–5.84) | 0.007 | 3.67 (1.39–5.94) | 0.002 |
| 5 min post | 3.88 (0.55–7.21) | 0.026 | 4.17 (2.12–6.22) | <0.001 |
| 10 min post | 5.66 (2.34–8.98) | 0.004 | 4.11 (2.03–6.20) | 0.000 |
| SpHB (g/dL) | ||||
| Middle | 0.38 (0.08–0.69) | 0.017 | 0.39 (0.10–0.67) | 0.007 |
| End | 0.74 (0.35–1.12) | 0.001 | 0.74 (0.39–1.08) | <0.001 |
| 5 min post | 1.15 (0.2–2.1) | 0.023 | 0.81 (0.29–1.32) | 0.002 |
| 10 min post | 0.93 (0.06–1.79) | 0.040 | 0.63 (0.25–1.02) | 0.001 |
| MAP (mm Hg) | ||||
| Middle | 1.35 (−2.06–4.77) | 0.421 | 1.51 (−1.66–4.68) | 0.349 |
| End | 0.35 (−3.16–3.85) | 0.839 | 0.15 (−2.99–3.29) | 0.925 |
| 5 min post | 0.73 (−3.03–4.5) | 0.681 | 0.99 (−2.40–4.39) | 0.567 |
| 10 min post | −0.54 (−5.14–4.06) | 0.789 | −0.82 (−4.65–3.01) | 0.675 |
| CI (l/min/m2) | ||||
| Middle | 0.06 (−0.07–0.19) | 0.360 | 0.06 (−0.06–0.18) | 0.314 |
| End | 0.38 (0.15–0.6) | 0.002 | 0.38 (0.19–0.58) | <0.001 |
| 5 min post | 0.47 (0.15–0.79) | 0.008 | 0.49 (0.26–0.72) | <0.001 |
| 10 min post | 0.51 (0.1–0.92) | 0.021 | 0.54 (0.24–0.85) | 0.001 |
| HR (bpm) | ||||
| Middle | 2.54 (−1.75–6.83) | 0.233 | 2.86 (−1.16–6.87) | 0.163 |
| End | 2.37 (−0.51–5.24) | 0.102 | 3.05 (0.44–5.65) | 0.022 |
| 5 min post | 6.43 (−2.48–15.34) | 0.143 | 6.17 (−0.93–13.27) | 0.088 |
| 10 min post | 9.88 (4.36–15.39) | 0.004 | 10.43 (4.13–16.73) | 0.001 |
| SV (mL) | ||||
| Middle | 0.3 (−1.88–2.48) | 0.778 | 0.29 (−1.73–2.31) | 0.777 |
| End | 6.69 (1.8–11.59) | 0.010 | 6.38 (2.03–10.73) | 0.004 |
| 5 min post | 8.76 (2–15.52) | 0.015 | 8.75 (3.90–13.60) | 0.000 |
| 10 min post | 8.81 (−0.05–17.66) | 0.051 | 8.94 (3.05–14.83) | 0.003 |
SmO 2 muscle tissue oxygenation, SpHb hemoglobin, MAP mean arterial pressure, CI cardiac index, HR heart rate, SV stroke volume
There was no evidence of a correlation between SmO2 and SpHb (ρ = 0.25 [95% CI −0.03–0.48]). There was similarly no evidence of a correlation between SmO2 and MAP (ρ = 0.22 [95% CI 0.05–0.40]), HR (ρ = 0.20 [95% CI 0–0.42]), or CI (ρ = 0.08 [95% CI −0.22–0.34]). A subgroup analysis revealed no evidence of a correlation between SmO2 and SpHb in patients who had a positive PLR test (n = 5) before transfusion start (ρ = 0.37 [95% CI −0.11–0.84]), or for those who had a negative PLR test (n = 23) (ρ = 0.21 [95% CI −0.11–0.50]).
Discussion
When measuring the effect of blood transfusions on SmO2, as determined by NIRS, in patients after TKA, we were able to find a statistically significant increase in SmO2 during the course of a single unit blood transfusion compared to baseline. A similar increase was found for continuously measured SpHb and CI, respectively. Interestingly, the increase in SmO2 was only transient and a decline to baseline levels was found 10 min after the end of transfusion, while SpHb and cardiac index remained elevated. However, no correlation could be established between continuously measured SmO2 and SpHb during the course of the blood transfusion.
This study has several limitations. Firstly, this trial was designed as a pilot study with a small number of participants and a limited data collection period. The patient population chosen may be of limited clinical relevance due to the relative lack of major comorbidity burden and absence of those with extreme blood loss. However, our findings provide a good hypothesis base for future studies including sample size calculations and point towards the need for longer observation periods of SmO2 and other parameters. The transfusion threshold for patients in our study was not standardized and might be considered as high. The average hemoglobin before transfusion was greater than 9 g/dl. Recent studies have suggested a more restrictive transfusion strategy due to safety concerns [6]. However, one has to consider the fact that in this patient population, blood loss is ongoing and thus clinical decisions to transfuse may partially be based on the patient’s trajectory. Considering the lack of correlation between SmO2 and hemoglobin concentration, this practice may be questioned. However, it remains debated if the prevention of low, although transient, hemoglobin levels during ongoing blood loss could be beneficial.
Within our SpHb measurements, we encountered a wide standard deviation of our readings. However, the continuous collection of SpHb is one of the advantages of this trial as it provides real-time information on changes in SpHb related to blood transfusion. In addition, it is not clear if a statistically significant increase of median 3.16% in SmO2 during transfusion has any clinical impact. Of note however is the fact that SpHb increased significantly over the same time interval in the expected and clinically as significant accepted range.
Despite accumulating evidence in favor of more restrictive transfusion protocols, safe transfusion triggers on an individual level remain elusive, particularly in the setting of complex patient comorbidities and ongoing blood loss. Recent data has shown a lack of benefit associated with liberal transfusion regimens both in critically ill patients, as well as those undergoing elective orthopedic surgery, even in higher-risk patient populations [5, 6]. Reliable monitors able to measure microvascular blood flow may be useful adjuncts to guide transfusion therapy in a more accurate manner [16]. However, this technology is not readily available in clinical medicine to date, and no conclusive evidence exists about the effect of blood transfusions on SmO2 in the perioperative setting. We observed a significant increase in SmO2 in response to a transfusion of blood, which was short-lived as SmO2 returned to baseline values within 10 min of termination of the transfusion. Sadaka et al. reported that SmO2 was not affected globally by transfused blood in critically ill patients. Nevertheless, blood transfusions resulted in a significant increase in hemoglobin [15]. Similar results in critically ill patients were reported by Creteur et al. in 2009 [7]. Donati et al. observed an increase in SmO2 with non-leukodepleted blood compared to leukodepleted blood. Conversely, they did observe increases in microvascular flow index and blood flow velocity with leukodepleted blood [8]. In all three of these studies, SmO2 was measured 1 h before and 1 h after the transfusion. Since the increase in SmO2 in our study was transient and lasted only for 10 min, it is possible that some of these studies missed this effect. Our population was also different as the three other studies examined patients with sepsis. The microvascular circulation of postoperative patients may respond differently to transfusions than septic patients.
SmO2 has been shown to correlate with central hypovolemia in volunteers [18]. It is perplexing why we did not find a correlation between SmO2 and blood transfusions in both patients with and without signs of fluid responsiveness. One explanation may be that central hypovolemia (generation of negative pressure in the lower extremities via a neoprene skirt to decrease central blood volume) is an artificial physiological construct and does not pertain to postoperative patients who demonstrate they may be fluid responsive with the straight leg test. Alternatively, perfusion to capillary beds may still be adequate even in patients with a positive straight leg test.
It remains speculative why the increase of SmO2 is a short-term effect only. One possible explanation might be a transient increase in the delivery of oxygen to the tissues. Once the transfused blood has become adequately mixed with the rest of the blood, any overall increase may be negligible. Alternatively, because of altered deformability, a decrease in mean corpuscular hemoglobin concentration and/or lower ATP concentrations in stored red blood cells may prevent oxygen from being released to the tissues at the capillary level [2]. In this scenario, oxygen-rich blood may travel from the arteriolar to the venous side, where increased oxygen hemoglobin saturation is detected by the NIRS sensor for a limited period of time. To elucidate this question further, analysis of the saturation pattern after transfusion accounting for the age of transfused blood would have been required.
While SmO2 has been shown to be a sensitive parameter to detect occult hypoperfusion in a human model of non-resuscitated hemorrhagic shock [18], we could not establish a significant correlation between SmO2 and hemodynamic parameters including MAP, HR, cardiac index, and SmO2 over time. This most likely is due to the fact that (1) patients undergoing TKA rarely have sudden and large volume loss and (2) patients in the perioperative period undergo continuous volume resuscitation and are less likely to develop a state of acute hypovolemia. Furthermore, no association with SmO2 and continuously measured hemoglobin (SpHb) was found in this pilot study.
In conclusion, in this pilot study, we found a statistically significant increase in SmO2 during the course of a blood transfusion in orthopedic surgical patients. However, this increase was short-lived and not associated with a significant correlation between SmO2 and SpHb or any hemodynamic parameter analyzed. Further research, including larger scaled trials, is needed to determine whether the short-term increase in SmO2 following transfusion is of clinical relevance.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1226 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Acknowledgments
We would like to thank the Anna Maria and Stephen Kellen Career Development Award (Memtsoudis) and the Department of Anesthesia at Hospital for Special Surgery for funding this study. The study was also supported by the Clinical and Translational Science Center at Weill Medical College—Cornell University through grant number UL1 TR000457-06 for the use of REDCap.
Disclosures
ᅟ
Conflict of Interest:
Stavros G. Memtsoudis, MD, PhD, FCCP, Thomas Danninger, MD, Ottokar Stundner, MD, Daniel Yoo, MB, Federico P. Girardi, MD, Isabelle Kao, BS, Kara G. Fields, MS, and Michael K. Urban, MD, PhD have declared that they have no conflict of interest. Friedrich Boettner, MD reports grants and personal fees from Smith and Nephew and personal fees and other from Orthodevelopment, outside the work. Stephen O. Heard, MD reports non-financial support and other from Reflectance Medical, during the study. J. Matthias Walz, MD, FCCP reports other from Reflectance Medical, during the study.
Human/Animal Rights:
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent:
Informed consent was obtained from all patients for being included in the study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Level IV: Prognostic Study
Work performed at Hospital for Special Surgery
References
- 1.Adams RCLJ. Anesthesia in cases of poor surgical risk: Some suggestions for decreasing the risk. Surg Gynecol Obstet. 1942;74:1011–1015. [Google Scholar]
- 2.Almizraq R, Tchir JD, Holovati JL, et al. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013 doi: 10.1111/trf.12080. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the American society of anesthesiologists task force on perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Beekley AC, Martin MJ, Nelson T, et al. Continuous noninvasive tissue oximetry in the early evaluation of the combat casualty: A prospective study. J Trauma. 2010;69(Suppl 1):S14–S25. doi: 10.1097/TA.0b013e3181e42326. [DOI] [PubMed] [Google Scholar]
- 5.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4 doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creteur J, Neves AP, Vincent JL. Near-infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation. Crit Care. 2009;13(Suppl 5):S11. doi: 10.1186/cc8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donati A, Damiani E, Luchetti MM, et al. Microcirculatory effects of the transfusion of leukodepleted or non-leukodepleted red blood cells in septic patients: A pilot study. Crit Care. 2014;18:R33. doi: 10.1186/cc13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamlett A, Ryan L, Serrano-Trespalacios P, et al. Mixed models for assessing correlation in the presence of replication. J Air Waste Manag Assoc. 2003;53:442–450. doi: 10.1080/10473289.2003.10466174. [DOI] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Mazumdar M, Memtsoudis SG. Beyond repeated-measures analysis of variance: Advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg Anesth Pain Med. 2012;37:99–105. doi: 10.1097/AAP.0b013e31823ebc74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland JG. Perioperative blood transfusions: Indications and options. Chest. 1999;115:113S–121S. doi: 10.1378/chest.115.suppl_2.113S. [DOI] [PubMed] [Google Scholar]
- 13.Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402–1407. doi: 10.1097/01.CCM.0000215453.11735.06. [DOI] [PubMed] [Google Scholar]
- 14.Roberson RS, Lockhart E, Shapiro NI, et al. Impact of transfusion of autologous 7- versus 42-day-old AS-3 red blood cells on tissue oxygenation and the microcirculation in healthy volunteers. Transfusion. 2012;52:2459–2464. doi: 10.1111/j.1537-2995.2012.03615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadaka F, Aggu-Sher R, Krause K, O'Brien J, Armbrecht ES, Taylor RW. The effect of red blood cell transfusion on tissue oxygenation and microcirculation in severe septic patients. Ann Intensive Care. 2011;1:46-5820-1-46. doi:10.1186/2110-5820-1-46. [DOI] [PMC free article] [PubMed]
- 16.Sakr Y, Chierego M, Piagnerelli M, et al. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med. 2007;35:1639–1644. doi: 10.1097/01.CCM.0000269936.73788.32. [DOI] [PubMed] [Google Scholar]
- 17.Soller BR, Idwasi PO, Balaguer J, et al. Noninvasive, near infrared spectroscopic-measured muscle pH and PO2 indicate tissue perfusion for cardiac surgical patients undergoing cardiopulmonary bypass. Crit Care Med. 2003;31:2324–2331. doi: 10.1097/01.CCM.0000086999.21673.6A. [DOI] [PubMed] [Google Scholar]
- 18.Soller BR, Yang Y, Soyemi OO, et al. Noninvasively determined muscle oxygen saturation is an early indicator of central hypovolemia in humans. J Appl Physiol. 2008;104:475–481. doi: 10.1152/japplphysiol.00600.2007. [DOI] [PubMed] [Google Scholar]
- 19.Stehling L, Zauder HL. Acute normovolemic hemodilution. Transfusion. 1991;31:857–868. doi: 10.1046/j.1537-2995.1991.31992094675.x. [DOI] [PubMed] [Google Scholar]
- 20.Stundner O, Chiu YL, Sun X, et al. Comparative perioperative outcomes associated with neuraxial versus general anesthesia for simultaneous bilateral total knee arthroplasty. Reg Anesth Pain Med. 2012;37:638–644. doi: 10.1097/AAP.0b013e31826e1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services. The 2011 national blood collection and utilization survey report; 2011.
- 22.Wahr JA, Tremper KK, Diab M. Pulse oximetry. Respir Care Clin N Am. 1995;1:77–105. [PubMed] [Google Scholar]
- 23.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. doi: 10.2307/2531734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1226 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)