Abstract
Background
While the gold standard of treatment of nonunion is open autologous bone grafting, studies have shown that injecting bone marrow aspirate concentrates (BMAC) is effective in treating tibial nonunions with fracture gaps less than 5 mm.
Questions/Purposes
We aim to demonstrate that combining BMAC with osteoinductive agents can effectively treat delayed or nonunion regardless of fracture gap size, nonunion site, or osteoinductive agent used.
Methods
In this non-randomized retrospective-prospective cohort study, 49 patients with tibial nonunion met the inclusion criteria and underwent BMAC injection with demineralized bone matrix (DBM) and/or recombinant human bone morphogenic protein-2 (rhBMP-2). Radiologic healing of the fracture was the primary outcome. Patients were followed until radiographic union was achieved or another procedure was performed. Radiographic healing was defined as bridging of three out of four cortices on anteroposterior and lateral films.
Results
There was no difference in the healing rate (p = 0.81) between patients with fracture gaps less than and greater than 5 mm. On multivariate analysis, the use of rhBMP-2 was associated with a lower healing rate compared to DBM (p = 0.036). Patients who underwent early intervention (within 6 months of fixation) had higher union rates (p = 0.04).
Conclusion
This study shows that percutaneous BMAC injection combined with either DBM and/or rhBMP-2 is a safe and effective treatment for delayed or nonunion regardless of the fracture gap size or fracture site. DBM may be superior to rhBMP-2 in this procedure.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-015-9432-1) contains supplementary material, which is available to authorized users.
Keywords: modified Hernigou technique, nonunion, delayed union, BMAC (concentrated autogenous iliac crest bone marrow aspirate), demineralized bone matrix, bone morphogenic protein
Introduction
The treatment of nonunion continues to be a major challenge in orthopedic surgery. An estimated 5% to 10% of fractures result in delayed or nonunion causing patients to endure impaired function as well as repeated hospitalizations and surgeries [15, 19]. The total estimated cost of these complications is between US$23,246 and US$58,525 per patient [6]. The current gold standard of treatment for atrophic nonunion includes surgical stabilization and open autologous bone grafting with success rates as high as 97%–99% [24]. This method provides a limited source of material for grafting and is associated with a number of complications, including persistent donor site pain in up to 49% of patients [6]. Increasingly, alternative methods such as injections of bone marrow aspirate concentrate (BMAC) are being investigated to facilitate bone healing [5].
Goujon et al. first demonstrated in rabbits that bone marrow has osteogenic properties [14]. This led to the further interest in studies regarding the osteogenic potential of the bone marrow [8]. A number of studies after that have shown mixing of autograft with allograft, xenograft, and composite grafts can be useful [3, 18, 21]. At the end of the growth, the adult human skeleton consists of red and hematopoietic active bone marrow and yellow bone marrow containing fat which is hematopoietically inactive. Study has shown that primitive osteogenic cells or hematopoietic cells present in the red bone marrow are responsible for the physiologic effect of the bone graft [3]. A number of studies in both animals and human beings have shown that these osteogenic precursor cells can contribute to the healing of the bone [1, 3, 5, 11, 17, 18, 21, 22].
BMAC injection is a minimally invasive procedure shown to successfully treat nonunion and is not associated with any local or systemic complications [13]. Although this approach has demonstrated success for nonunions with small fracture gaps, there is currently a lack of evidence to support the use of this technique in nonunions with fracture gaps greater than 5 mm or long bones other than tibia.
Commercial osteoinductive biomaterials such as demineralized bone matrix (DBM) and recombinant human bone morphogenic protein-2 (rhBMP-2) have become popular adjuncts to stimulate bone formation in a variety of settings [1, 17, 22]. The combination of BMAC and DBM has been shown to be comparable to standard cancellous bone grafting in the treatment of nonunion in both animals and humans [17, 22]. The study of human subjects included nonunions of the tibia with fracture gap less than 5 mm. Animal studies have also shown that a composite graft of rhBMP-2 and bone marrow results in higher rates of union than autologous bone grafting alone [16]. Although both rhBMP-2 and DBM are widely used, no prior studies have directly compared their efficacy. There is also a report of safety issue regarding use of rhBMP-2 [4].
The purpose of this study was to prospectively evaluate our results using either DBM or rhBMP-2 in combination with percutaneous BMAC injection for a variety of long bone nonunions. We refer to this surgical procedure as the modified Hernigou technique. Our specific aims were to (1) document and compare the radiographic rate of healing of nonunions of the tibia and those of other long bones treated with BMAC, (2) to compare the radiographic healing rate between nonunions with fracture gaps greater than 5 mm and those less than 5 mm, and (3) to compare the radiographic healing of nonunions treated with BMAC combined with rhBMP-2 to BMAC combined with DBM. Finally, we assessed whether any clinical characteristics of the patients could be identified which impacted the rate of healing.
Patients and Methods
Following approval from the institutional review board, we retrospectively enrolled patients for modified Hernigou procedure by a single surgeon at two institutions for nonunion or delayed union between October 2006 and December 2012. The patients were followed prospectively. All the patients were followed until union was achieved, for a minimum of 2 years without evidence of union, or until any additional procedure was performed to treat the fracture.
Eighty-two patients were enrolled for the study. All the patients treated with modified Hernigou technique for atrophic nonunion without modification of hardware were included in the study. Patients with any surgical fixation with our procedure, open bone grafting, stress fractures where fracture gap could not be measured, and patients with genetic bone disorders were excluded from the study. Forty-nine patients met the inclusion criteria while 33 patients were excluded for not meeting the inclusion criteria. Of those excluded, ten had open bone grafting, seven had stress fractures, four had genetic bone disorders, four had osteotomy nonunion, four had concurrent operative intervention, and four had some modification of implant along with our procedure. One of the patients with concurrent operative intervention was diagnosed with infection based on positive cultures from the fracture site.
Patient demographics and laboratory values are shown in Table 1. The age range of patients was between 19 and 93 years and all were in good health with stable internal fixation of long bone involved. Out of 49 patients, 20 were on bisphosphonate treatment for at least 1 year prior to the fracture.
Table 1.
Clinical characteristics of patient population
| Variables | All (N = 49) |
|---|---|
| Age (years) | 53.9 (19–93) |
| Sex (% female) | 33 (67.3%) |
| Race (% Caucasian) | 38 (77.6%) |
| Charlson comorbidity index | 0.67 (0–6) |
| Months between fracture fixation and modified Hernigou procedure | 6.9 (4–30) |
| Early intervention (% treated within 6 months after fixation) | 29 (60.4%) |
| Previous surgical fixation (%) | 49 (100%) |
| Fracture gap (mm) | 5.2 (1.0–20) |
| Gap ≥ 5 mm | 23 (46.9%) |
| Open fracture | 11 (22.4%) |
| Comminuted fracture | 13 (26.5%) |
| Fracture site | p = 0.4 |
| Femur | 19 (38.8%) |
| Tibia-fibula | 23 (46.9%) |
| Humerus | 7 (14.3%) |
Modified Hernigou Procedure
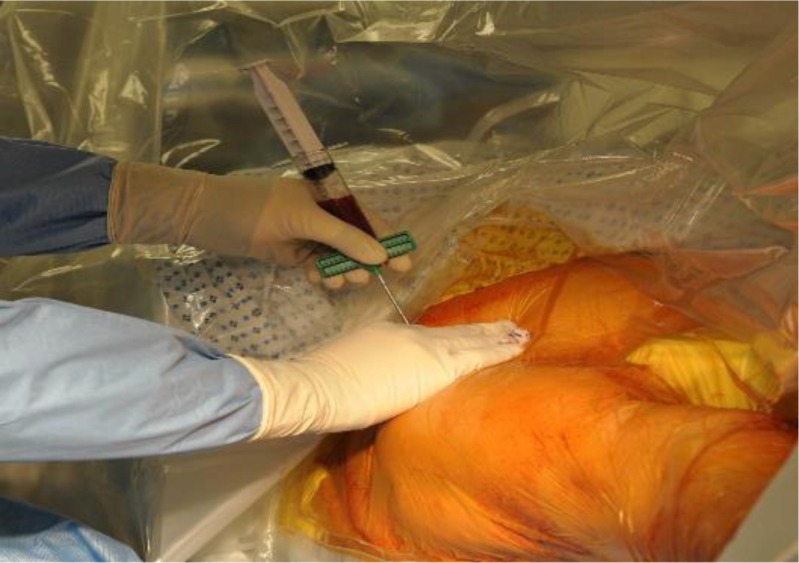
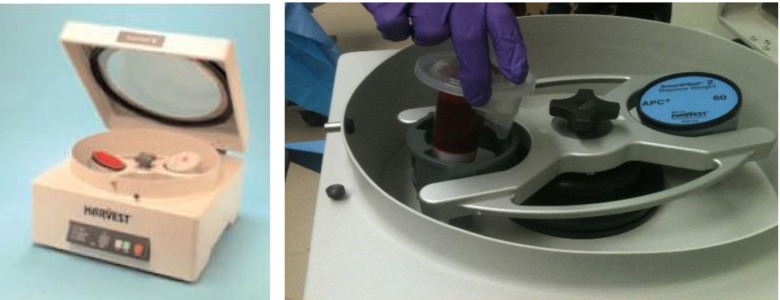
Spinal anesthesia was preferred for lower extremity nonunion/delayed union and general anesthesia for upper extremity nonunion/delayed union. The patient under anesthesia was placed in a supine position on the operating table. The affected long bone and the iliac crest of the same side were usually chosen for ease of execution of the procedure. After prepping and draping, the anterior iliac crest was identified. A horizontal incision of about 0.5 cm is made about 2-cm posterior to the anterior superior iliac spine. A 16-gauge Jamshidi needle for bone marrow aspiration is then placed at the incision site between the inner and outer tables of the iliac bone and hammered approximately 6 cm into the cancellous bone. Incision helped in the exact positioning of the needle as well as prevention of multiple entry sites within 1 cm of the bone if the needle was accidentally pulled out during the procedure and to prevent any iatrogenic injury to iliac bone. The marrow was aspirated using a 30-cm3 syringe (Fig. 1). If no marrow can be aspirated after entry, the needle was reoriented. The trocar was turned 45 after every 10 cm3 aspirated to permit aspiration from the largest possible space. The marrow aspirated was not more than 10 cm3 every time to reduce dilution by peripheral blood. After aspirating 30 cm3 of bone marrow, the trocar was withdrawn 1 cm, and the process was repeated. Multiple perforations of the iliac crest can be made but should be spaced at least 2 cm apart to avoid aspirating in the same area. The aspirates were then pooled and transferred to the Harvest system to concentrate the bone marrow (Fig. 2). The 60 cm3 of bone marrow is typically concentrated to 10 cm3 for injection. In our first 20 patients, we sent 1 cm3 of aspirate and 1 cm3 of concentrate to the laboratory for the analysis of nucleated cells and colony forming unit (CFU) count. The count of nucleated cells in aspirate was 18.62 ± 12.16 and in concentrate was 101.48 ± 64.13 while CFU count per milliliter in aspirate was 205 ± 152 and in concentrate was 1014 ± 958. The CFU count per milliliter and concentration of nucleated cells in concentrate was consistently higher in our first 20 patients, which was required for union as described by Hernigou for healing of nonunion [13]. So, the count was not repeated for further patients in our study.
Fig. 1.
A Jamshidi needle is inserted at the incision site between the inner and outer tables of the iliac bone and hammered approximately 6 cm into the cancellous bone. The bone marrow is aspirated using a 30-cm3 syringe.
Fig. 2.
The bone marrow aspirates are pooled and transferred to the Harvest system for concentration.
While the marrow was being concentrated, percutaneous incision was made over the fracture site. Biopsies and cultures were taken from the fracture site to rule out infection or any other bone pathology that may affect healing. Using curettage and rongeur, space was created over the fracture site and around the bone.
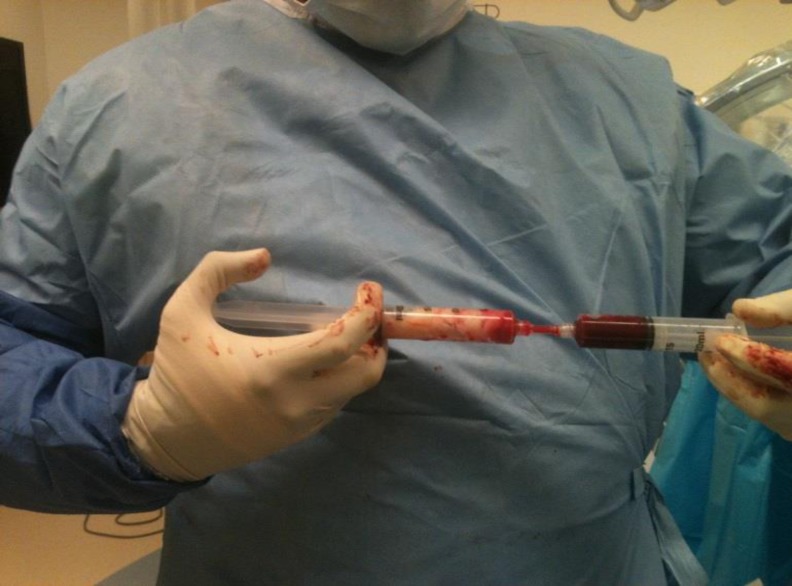
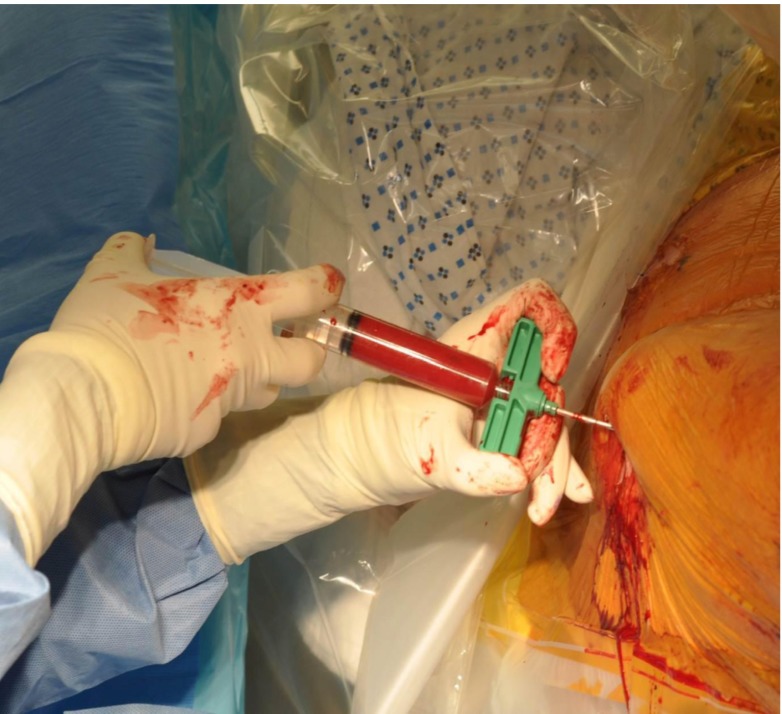
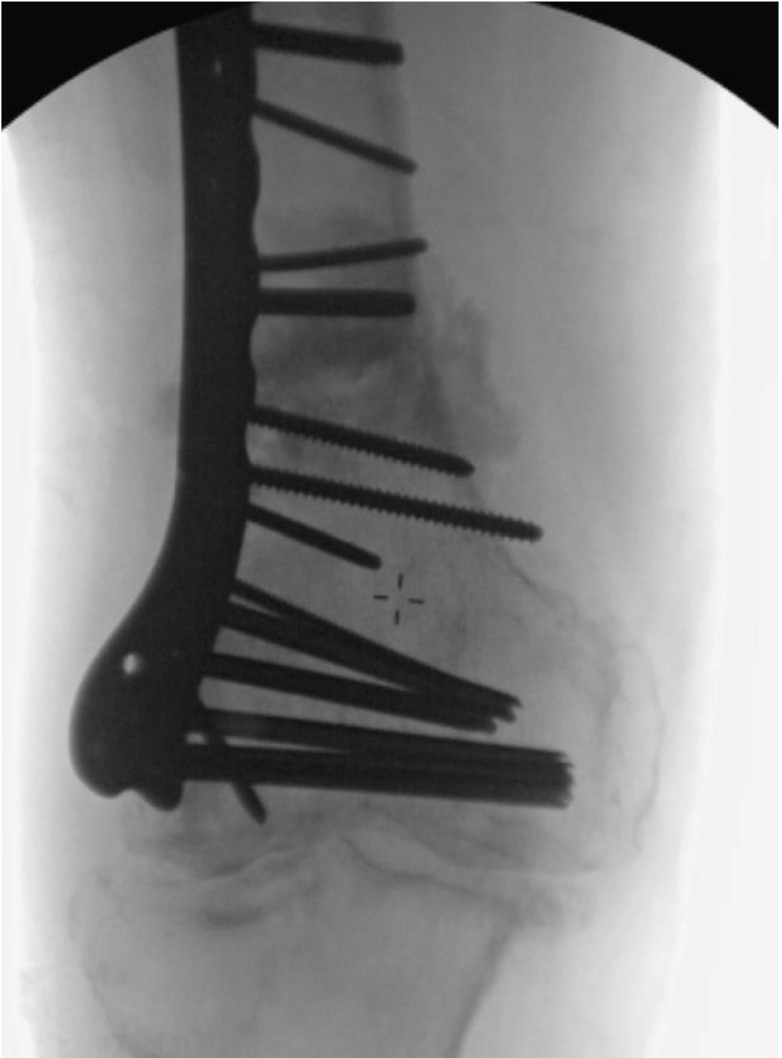
The BMAC was mixed with adjuvants such as DBM and/or rhBMP-2 (Fig. 3). The total volume of the marrow was then slowly injected into the space created previously (Fig. 4). Prior to injection, iohexol was also added to allow fluoroscopic visualization (Fig. 5). After injection, the trocar is slowly withdrawn and the skin is then sutured closed to avoid the leakage of marrow. The skin over donor site was also sutured.
Fig. 3.
The concentrated bone marrow aspirate (BMAC) is mixed with osteoinductive biomaterials (DBM and/or rhBMP-2) and iohexol for visualization under fluoroscopy.
Fig. 4.
The BMAC and bioadjuvant mixture is injected into the fracture site.
Fig. 5.
Intraoperative fluoroscopy illustrating injection site with iohexol dye.
In this study, patients had similar demographic characteristics in terms of age and sex for BMAC with DBM and BMAC with rhBMP-2 and all other relevant clinical information was collected. In all patients, calcium and vitamin D levels were checked after initial procedure and treatment was started if those levels were low. All patients had vitamin D level 30 μg/dl or more prior and during follow-up. At the initial consultation, both anteroposterior (AP) and lateral radiographs were obtained for patients referred to our clinic with diagnosis of nonunion or delayed union. As described in prior literature, nonunion was defined as “A fracture that has no potential to heal without further intervention.” And delayed union was defined as “A fracture that takes more than usual time to heal” [25].
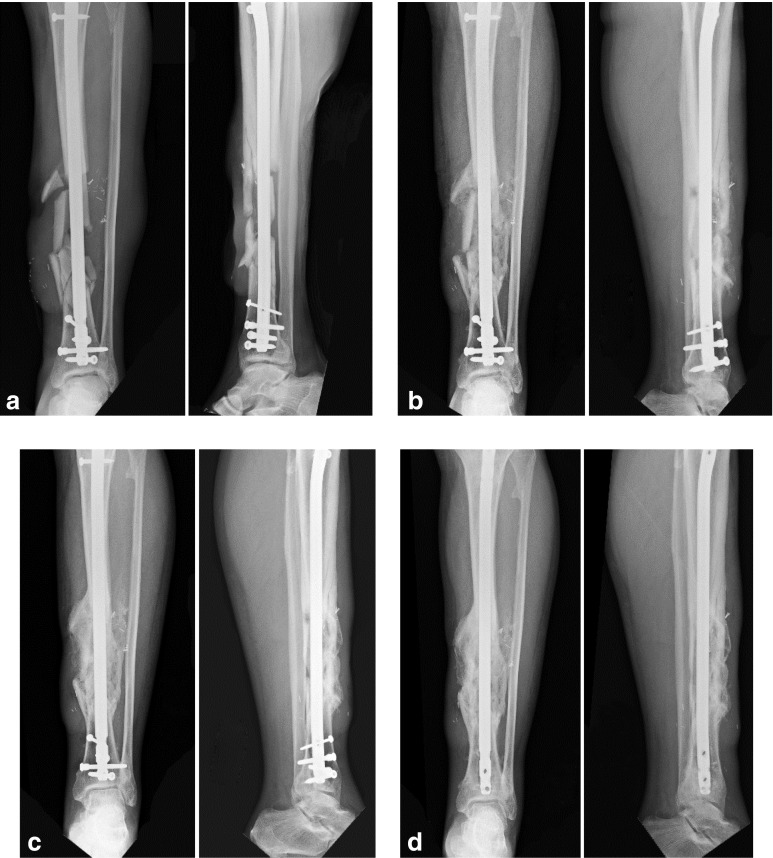
Radiographs were reviewed by three independent orthopedic surgeons. The first one (JML) was a Professor of orthopedics at both institutions, and the other two were fellowship-trained orthopedic trauma surgeons. The fracture gap was measured as the largest distance between two cortical fragments at the fracture site. Inter-observer variability was resolved by re-reviewing the radiographs until all three orthopedic surgeons reached a consensus (Fig. 6).
Fig. 6.
Anteroposterior (AP) and lateral radiographs of a 59-year-old man with a comminuted tibial fracture with a gap of 7.7 mm taken a pre-operatively; then post-operatively at b 3 weeks, c 6 weeks, and d 12 weeks. Note that the patient had removal of distal screws over tibia due to pain over site of screw insertion 1 week prior to our follow-up at 12 weeks. Fracture had healed and so both static and dynamic locking screws over distal tibia were removed.
Patients remained non-weight bearing on the operated extremity for the first 3 weeks. Radiographs were taken after 3 weeks and weight bearing was permitted if callus formation was present. Radiographs were then repeated every 4 weeks until the fracture healed or nonunion was confirmed. Our primary outcome was radiographic healing of the fracture. Bridging of three out of four cortices on AP and lateral radiographs was considered as radiographic healing.
Descriptive statistics were calculated using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Following the descriptive analysis, differences in clinical characteristics between patients that healed and those that did not heal were evaluated using independent samples t tests for continuous variables and chi square or Fisher’s exact test for categorical variables. Crude odds ratios (cOR) and their respective 95% confidence intervals (95% CI) were calculated to assess the magnitude and precision of their associations. A multivariable binary logistic regression model was then created to evaluate the adjusted associations of each potential explanatory variable to predict the likelihood of healing. Variables with a univariate significance level of 0.25 or less or those deemed to be clinically relevant were eligible for inclusion in the analysis. Using a forward stepwise procedure, variables that failed to achieve a p value of 0.15 or below were removed from the final model. Because of the explanatory nature of the analyses, 0.15 was chosen as the threshold for retention in the final model; however, statistical significance was still set at p ≤ 0.05.
Results
In our study, the concentration of nucleated cells was 18.62 ± 12.16 in the aspirate from bone marrow and it increased to 101.48 ± 64.3 after concentration which is higher than other commercially available concentrate systems. “Absolute CFU” count in our study was 205 ± 152/ml in aspirate and 1014 ± 958/ml in concentrate. The level of concentration of nucleated cells and absolute CFU was steadily elevated in our first 20 patients higher than other commercially available concentrate systems, and so, we did not repeat them for other patients in our study.
The overall healing rate following the modified Hernigou procedure was 79.6% with an average healing time of 4.7 months. Univariate analysis showed that healing rate was not affected by nonunion location (tibia, femur, or humerus) (p = 0.481), fracture gap size (> or <5 mm) (p = 0.828), (Table 2) or presence of an atypical bisphosphonate fracture (p = 0.2).
Table 2.
Healed vs nonhealed groups
| Healed | % or SD | Non Healed | % or SD | p value | |
|---|---|---|---|---|---|
| Fracture location | 0.481 | ||||
| Femur | 14 | 73.7% | 5 | 26.3% | |
| Tibia | 20 | 87.0% | 3 | 13.0% | |
| Humerus | 5 | 71.4% | 2 | 28.6% | |
| Open vs closed fracture | 0.673 | ||||
| Open fracture | 8 | 72.7% | 3 | 27.3% | |
| Closed fracture | 31 | 81.6% | 7 | 18.4% | |
| Communited fracture | 10 | 76.9% | 3 | 23.1% | |
| Early vs late intervention | 0.004 | ||||
| Early intervention (≤6 months) | 27 | 93.1% | 2 | 6.9% | |
| Late intervention | 11 | 57.9% | 8 | 42.1% | |
| Gap | 0.711 | ||||
| <5 mm | 21 | 80.8 | 5 | 19.2% | |
| ≥5 mm | 18 | 78.3% | 5 | 21.7% |
The DBM and rhBMP-2 groups did not differ in any clinical characteristics (Table 3). The rate of healing was 86.4% with DBM vs 70.8% with rhBMP-2 and on multivariate logistic regression model showed decreased likelihood of healing in patients with rhBMP-2 treatment vs DBM (p = 0.036) and late intervention (≥6 months after the initial fixation) (p = 0.04). All three patients who received both rhBMP-2 and DBM with BMAC healed. There was no significant association of poor healing with age, sex, fracture gap size (≥ or <5 mm), fracture location, or the presence of atypical bisphosphonate fracture. The multivariate linear regression model (Table 4) showed slower healing in patients with increased fracture gap size. Healing time was prolonged by 2 days (p = 0.026) for each year of age and by 11.25 days (p = 0.011) for each 1 mm of fracture gap. The remaining factors evaluated showed no association with healing time.
Table 3.
BMP vs DBM cohorts
| Variables | Total patients N = 49 |
BMP group N = 24 |
DBM group N = 22 |
p value |
|---|---|---|---|---|
| Age in years | 53.9 (19–93) | 53.8(19–84) | 54.9(27–3) | 0.72 |
| Sex (% females) | 67.3% | 70.8% | 68.2% | 0.62 |
| Early treatment (less than 6 months) | 60.4% | 52.2% | 68.2% | 0.211 |
| Late treatment (after 6 months) | 39.6% | 47.8% | 31.8% | |
| Fracture characteristics | ||||
| Femur/tibia/humerus | 19/23/7 | 12/11/1 | 6/11/5 | 0.21 |
| Fracture gap (mm) | 5.2(1–20) | 5(1–16) | 5.7(1–20) | 0.51 |
| Fracture gap ≥5 mm | 46.9% | 41.7% | 54.5% | 0.623 |
| Fracture healing | ||||
| Rate | 79.6% | 70.8% | 86.4% | 0.033 |
| Time (months) | 4.7(2–12) | 4.8(2–12) | 4.5(2–12) | 0.5 |
p = 0.033 for healing rate between DBM and BMP group as per multivariate analysis
p > 0.05 for all others
Table 4.
Results of multivariate linear regression model for time of healing
| Unstandardized beta coefficient | p value | 95% confidence interval | ||
|---|---|---|---|---|
| Age | 0.067 | 0.026 | 0.009 | 0.126 |
| Gap (mm) | 0.375 | 0.011 | 0.093 | 0.658 |
The beta coefficient indicates the factor by which a one-unit increase in the variable increases the outcome. For example, for every 1 year increase in age, the amount of time expected for healing increases by 0.067 months. As a result, healing time was prolonged by approximately 2 days (p = 0.026) for each year of increased age and by 11.25 days (p = 0.011) for each 1 mm increase in fracture
The healing rate of fracture was same in bisphosphonate user vs nonuser group (Table 5). However, time to healing of fracture in bisphosphonate user was 6.1 month vs nonuser group which was 4 months (p = 0.03).
Table 5.
Bisphosphonate user vs nonuser
| Bisphosphonate user | Bisphosphonate nonuser | p value | |
|---|---|---|---|
| Average age in years | 67.7 | 54.9 | 0.08 |
| Time to intervention after original procedure | 0.4 | ||
| Within 6 months | 12/20 (60%) | 18/29 (62%) | |
| After 6 months | 8/20 (40%) | 11/29 (38%) | |
| Fracture gap | 0.5 | ||
| ≤5 mm | 14/20 (70%) | 12/29 (41.3%) | |
| >5 mm | 6/20 (30%) | 17/29 (59.7%) | |
| Healing | 15/20 (75%) | 24/29 (82.7%) | 0.5 |
| Time to healing (in months) | 6.1 | 4.0 | 0.03 |
Of the ten patients who failed to achieve radiographic healing, all were offered surgical intervention in form of open autologous bone grafting combined with osteoinductive materials. Six underwent further surgical treatment. One patient achieved clinical healing with a residual fracture site defect and refused for surgery. Three other patients opted for pain control during our last follow-up. There were no local or systemic complications associated with this procedure.
Discussion
In this study, the rate of healing was the same in femur, tibia, and humerus. There was no difference in healing rate in open vs closed fractures and simple vs comminuted fractures [23]. The rate of healing of the fracture was not affected by fracture gap. The other characteristics that increased time to healing were increase in age of patient and increase in fracture gap. There was increase in time to healing of fracture in patients with history of bisphosphonate use.
This study has several limitations. Firstly, our sample size is small. The group treated with BMAC and DBM as well as the group treated with BMAC and rhBMP-2 were similar in most clinical characteristics. However, there was no randomization of the groups like in prospective cohort study. Furthermore, there was no control group receiving BMAC without osteoinductive biomaterials. However, superior results of combination of BMAC with DBM over BMAC with rhBMP-2 make us conclude better result of BMAC with DBM over BMAC alone.
Hernigou demonstrated that growth factors are released from the bone marrow and they are useful in healing of the bone [12]. In a separate study, he looked at the concentration and number of “progenitor cells” needed for the healing of atrophic nonunion in tibia [13]. He measured the concentration of progenitor cells aspirated from bone marrow in the form of “fibroblast colony forming units” (CFU-F). The amount of healing was directly related to the concentration of CFU-F and time to healing was indirectly related to CFU-F. The number of progenitor cells was less than normal in the aspirate obtained from the bone marrow and required concentration for the healing of the bone. In the same study, he showed that absolute CFU less than 634 ± 187/ml led to the failure of the procedure. In our study, absolute CFU count was 1270 ± 1009/ml, which is much higher. The bone marrow aspirate contains only 0.1% of progenitor or nucleated cells and requires concentration. Spinning of the concentrate for 5 min at 400-g force leads to collection of cells which are heavier due to the presence of nucleus to the periphery. These cells are collected and separated from the remainder and one can get concentrated progenitor cells [5]. In our study, the concentration of nucleated cells was 18.62 ± 12.16 in the aspirate from the bone marrow and it increased to 66.52 ± 19.47 after concentration which is higher than other commercially available concentrate systems.
In this study, 79.6% of patients attained radiographic healing at an average of 4.7 months. The only other large study of the treatment of nonunion with BMAC injection showed a higher healing rate (88%) and a faster healing time (12 weeks) [13]. In that study, all patients underwent external fixation or conservative therapy for the initial fracture for tibia, whereas all of our patients had nonunion after some form of internal fixation and possibility of nonunion without any further intervention was confirmed. Thus, our data may reflect the treatment of more refractory nonunions. The longer healing times in our study may be due to the inclusion of patients with larger fracture gaps, patients with bisphosphonate use, and older age (54 vs 40 years), all of which are associated with prolonged healing time in our analysis (Tables 4 and 5). We have successfully demonstrated that our technique could be used for the treatment of nonunion in other long bones in addition to tibia.
There are no studies which include method of percutaneous BMAC injection for fracture gap greater than 5 mm. By adding osteoinductive biomaterials, we have augmented the previously described technique of BMAC injection to treat patients with nonunions of various long bones and with fracture gaps ≥5 mm [13]. Time to union increases by 11.25 days for every 1-mm increase in fracture gap.
There is a lack of studies comparing the efficacy of bone morphogenic proteins (BMPs) and DBM in bone healing. While BMP is proven to effectively treat nonunion, DBM is not currently recommended as monotherapy due to a broad range of healing rates (52% to 85.1%) and a lack of randomized controlled trials comparing DBM to autologous bone grafting [9, 24, 26]. However, our study demonstrates the efficacy of DBM combined with BMAC for nonunion therapy, with a healing rate of 86.4%. These results are consistent with the “diamond concept” of fracture healing—the regeneration of skeletal defects requires osteogenic cells, osteoinductive stimulants, an osteoconductive matrix, and mechanical stability [10]. Unlike rhBMP-2, DBM consists of an osteoconductive matrix that recruits osteoprogenitor cells. Furthermore, it contains a number of osteoinductive growth factors, including BMPs, growth differentiation factors, and TGF-β [1]s. In contrast, rhBMP-2 is a solitary growth factor without a physiologic scaffold, which may explain its inferiority to DBM in our study. Notably, all three patients that we treated with both DBM and rhBMP-2 achieved radiographic healing. While this subgroup is too small to include in our statistical analyses, further studies can elucidate the efficacy of this combined therapy.
The only previously identified risk factors for poor healing following BMAC injection are a lower number and concentration of progenitor cells in the bone marrow aspirate [13]. Our analysis identified several risk factors for failure to heal, including late treatment of nonunion and treatment with BMAC and rhBMP-2. The relationship between late treatment for nonunion and poor prognosis is less clear. We do accept the possibility that patients treated earlier may not have a true nonunion and may be able to heal intrinsically; biological changes may occur over time at the site of nonunion, thereby perpetuating the inability to heal. However, resorption of bone on x-ray and increase in fracture gap with good internal fixation led the authors to consider a high possibility of nonunion as early as 4 months after initial procedure. Increased age is associated with decreased bone marrow cellularity and connective tissue progenitors, which may explain our findings of increased healing time with increase in age [7, 20]. A long-term use of bisphosphonate, the drug, causes alterations to normal pattern of collagen cross-linking in bone, increased mineralization, reduced heterogeneity of mineralization, reduced vascularity, and eventually reduced bone turnover. A reduction in bone turnover causes ineffective curing of cracks in the bone. This results in delayed fracture healing in patients with bisphosphonate use [2].
In conclusion, this study introduces a technique involving the injection of concentrated bone marrow aspirates combined with osteoinductive biomaterials for the treatment of nonunion. Our data show that this treatment strategy is effective for nonunions of the femur, tibia, and humerus; with fracture gaps greater than 5 mm; and in the presence of previous internal fixation. Furthermore, DBM appears to be more effective than rhBMP-2 in this procedure when used as adjuvant with BMAC. We also identified that late treatment after nonunion is associated with poor prognosis. We plan to enroll more patients to assess the efficacy of using both DBM and rhBMP-2 with BMAC as well as BMAC without any biological additives. Based on the findings of the current study, we make following recommendations:
The modified Hernigou technique is a minimally invasive option for the treatment of nonunions of long bone.
This procedure is effective when performed within 6 months of the initial fracture fixation.
DBM is superior to the more costly rhBMP-2 in healing nonunions when combined with BMAC.
Healing rate is not affected by previous bisphosphonate use, long bone involved, or fracture characteristics.
Electronic supplementary material
(PDF 1225 kb)
Acknowledgments
We wish to thank Joseph Nguyen, MPH, Hospital for Special Surgery, for his assistance with the statistical analysis.
Disclosures
ᅟ
Conflict of Interest
Pingal Desai, MD; Saad Mumtaz Hasan, MD; Lester Zambrana, BA; Vishal Hegde, MD; Anas Saleh, MD; and Matthew R. Cohn, BS have declared that they have no conflict of interest. Joseph M. Lane, MD reports personal fees from DFIN E, Bone Therapeutics SA, Graftys, Amgen, BioMimetics, Zimmer, CollPlant Ltd., Royal Consulting and Marketing, Eli Lilly, Novartis, and Warner Chilcott, outside the work.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
Informed consent was waived from all patients for being included in the study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Level III, Therapeutic study
References
- 1.Agarwal R, Williams K, Umscheid CA, Welch WC. Osteoinductive bone graft substitutes for lumbar fusion: A systematic review. J Neurosurg Spine. 2009;11(6):729–740. doi: 10.3171/2009.6.SPINE08669. [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis–for whom and for how long? N Engl J Med. 2012;366(22):2051–2053. doi: 10.1056/NEJMp1202623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burwell RG. The function of bone marrow in the incorporation of a bone graft. Clin Orthop Relat Res. 1985;(200)(200):125-141. [PubMed]
- 4.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Connolly JF, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991;(266)(266):259–270. [PubMed]
- 6.Dahabreh Z, Calori GM, Kanakaris NK, Nikolaou VS, Giannoudis PV. A cost analysis of treatment of tibial fracture nonunion by bone grafting or bone morphogenetic protein-7. Int Orthop. 2009;33(5):1407–1414. doi: 10.1007/s00264-008-0709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14(7):1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 8.dos Reis FB, Faloppa F, Fernandes HJ, Albertoni WM, Stahel PF. Outcome of diaphyseal forearm fracture-nonunions treated by autologous bone grafting and compression plating. Ann Surg Innov Res. 2009;3:5-1164-3-5. [DOI] [PMC free article] [PubMed]
- 9.Friedlaender GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A(Suppl 1(Pt 2)):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 10.Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: The diamond concept. Injury. 2007;38(Suppl 4):S3–S6. doi: 10.1016/S0020-1383(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 11.Healey JH, Zimmerman PA, McDonnell JM, Lane JM. Percutaneous bone marrow grafting of delayed union and nonunion in cancer patients. Clin Orthop Relat Res. 1990;(256)(256):280–285. [PubMed]
- 12.Hernigou P. Growth factors released from bone marrow are promising tools in orthopedic surgery. Rev Rhum Engl Ed. 1998;65(2):79–84. [PubMed] [Google Scholar]
- 13.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 14.Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg (Br) 2005;87(7):896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 15.Hierholzer C, Sama D, Toro JB, Peterson M, Helfet DL. Plate fixation of ununited humeral shaft fractures: Effect of type of bone graft on healing. J Bone Joint Surg Am. 2006;88(7):1442–1447. doi: 10.2106/JBJS.E.00332. [DOI] [PubMed] [Google Scholar]
- 16.Lane JM. Bone morphogenic protein science and studies. J Orthop Trauma. 2005;19(10 Suppl):S17–S22. doi: 10.1097/00005131-200511101-00006. [DOI] [PubMed] [Google Scholar]
- 17.Liebergall M, Schroeder J, Mosheiff R, et al. Stem cell-based therapy for prevention of delayed fracture union: A randomized and prospective preliminary study. Mol Ther. 2013;21(8):1631–1638. doi: 10.1038/mt.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindholm TS, Urist MR. A quantitative analysis of new bone formation by induction in compositive grafts of bone marrow and bone matrix. Clin Orthop Relat Res. 1980;(150)(150):288–300. [PubMed]
- 19.Mollon B, da Silva V, Busse JW, Einhorn TA, Bhandari M. Electrical stimulation for long-bone fracture-healing: A meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2008;90(11):2322–2330. doi: 10.2106/JBJS.H.00111. [DOI] [PubMed] [Google Scholar]
- 20.Quarto R, Thomas D, Liang CT. Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif Tissue Int. 1995;56(2):123–129. doi: 10.1007/BF00296343. [DOI] [PubMed] [Google Scholar]
- 21.Salama R, Weissman SL. The clinical use of combined xenografts of bone and autologous red marrow. A preliminary report. J Bone Joint Surg (Br) 1978;60(1):111–115. doi: 10.1302/0301-620X.60B1.342531. [DOI] [PubMed] [Google Scholar]
- 22.Tiedeman JJ, Connolly JF, Strates BS, Lippiello L. Treatment of nonunion by percutaneous injection of bone marrow and demineralized bone matrix. an experimental study in dogs. Clin Orthop Relat Res. 1991;(268)(268):294–302. [PubMed]
- 23.Veillette CJ, McKee MD. Growth factors–BMPs, DBMs, and buffy coat products: Are there any proven differences amongst them? Injury. 2007;38(Suppl 1):S38–S48. doi: 10.1016/j.injury.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Wilkins RM, Kelly CM. The effect of allomatrix injectable putty on the outcome of long bone applications. Orthopedics. 2003;26(5 Suppl):s567–s570. doi: 10.3928/0147-7447-20030502-08. [DOI] [PubMed] [Google Scholar]
- 25.Wiss DA, Stetson WB. Tibial nonunion: Treatment alternatives. J Am Acad Orthop Surg. 1996;4(5):249–257. doi: 10.5435/00124635-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Ziran B, Cheung S, Smith W, Westerheide K. Comparative efficacy of 2 different demineralized bone matrix allografts in treating long-bone nonunions in heavy tobacco smokers. Am J Orthop (Belle Mead NJ) 2005;34(7):329–332. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)