Abstract
Background
Dual antiplatelet therapy is recommended after coronary stenting to prevent thrombotic complications, yet the benefits and risks of treatment beyond 1 year are uncertain.
Methods
Subjects were enrolled after a drug-eluting coronary stent procedure. After 12 months of thienopyridine (clopidogrel bisulfate [Plavix] or prasugrel [Effient/Efient]) with aspirin, subjects were randomized to continued thienopyridine or placebo for another 18 months; all continued aspirin. The co-primary effectiveness end points were stent thrombosis and major adverse cardiovascular and cerebrovascular events (a composite of death, myocardial infarction, or stroke) at 12 to 30 months. The primary safety end point was moderate or severe bleeding.
Results
Subjects (N=9,961) were randomized to continued thienopyridine or placebo. Continued thienopyridine reduced stent thrombosis (0.4% vs. 1.4%, hazard ratio 0.29, 95% confidence interval [CI] 0.17-0.48, P<0.001) and major adverse cardiovascular and cerebrovascular events (4.3% vs. 5.9%, hazard ratio 0.71, 95% CI 0.59-0.85, P<0.001). Myocardial infarction was reduced (2.1% vs. 4.1%, hazard ratio 0.47, P<0.001). Rates of all-cause mortality in the continued thienopyridine and placebo groups were 2.0 and 1.5%, respectively (hazard ratio 1.36, 95% CI 1.00-1.85, P=0.052). Moderate or severe bleeding was increased with continued thienopyridine (2.5% vs. 1.6%, P=0.001). An elevated hazard for stent thrombosis and myocardial infarction was observed in both groups during the 3 months following thienopyridine discontinuation.
Conclusion
Dual antiplatelet therapy beyond one year after drug-eluting stent placement significantly reduced the risks of stent thrombosis and major adverse cardiovascular and cerebrovascular events compared with aspirin alone, but was associated with increased bleeding.
Introduction
Millions of patients worldwide receive coronary stents each year for the treatment of ischemic heart disease.1,2 Although drug-eluting stents reduce restenosis compared with bare metal stents, there is concern that drug-eluting stents may be associated with risks of stent thrombosis occurring beyond one year after treatment.3 Stent thrombosis, while rare, is frequently associated with myocardial infarction, and may be fatal.3 Furthermore, ischemic events such as myocardial infarction, stroke, or cardiovascular death, unrelated to the treated coronary lesion, also occur beyond one year.4,5
The use of dual antiplatelet therapy combining a P2Y12 receptor inhibitor with aspirin is critically important to prevent coronary stent thrombosis, and is currently recommended for 6 to 12 months after implantation of a drug-eluting stent.6,7 While some observational studies suggest that extending dual antiplatelet therapy beyond one year is associated with a lower risk of myocardial infarction following drug-eluting stent treatment8, several trials have also demonstrated increased risk of bleeding without lowering myocardial infarction incidence with longer therapy.9-12 Whether treatment with dual antiplatelet therapy beyond one year reduces either coronary stent thrombosis or ischemic events remote to the stent has not been determined by an adequately powered randomized trial.
The Dual Antiplatelet Therapy (DAPT) Study was an international, multicenter, randomized placebo-controlled trial to determine the benefits and risks of continuing dual antiplatelet therapy beyond one year after treatment with coronary stents.
Methods
Study Design
The DAPT Study design has been described previously.13 The trial was designed in response to a request from the United States Food and Drug Administration (FDA) to manufacturers of coronary stents, and was conducted under an investigational-device exemption through a public-private collaboration involving the FDA, eight funding stent and pharmaceutical manufacturers (see Supplementary Appendix), and Harvard Clinical Research Institute (HCRI). The stent manufacturers who contributed to the funding of the trial had contributing roles in trial design and in data collection as detailed in the Supplementary Appendix. HCRI was responsible for the scientific conduct and independent analysis of the trial.
A single uniform randomized trial was designed incorporating five individual component studies to facilitate enrollment (Supplementary Appendix). Subjects were enrolled into the trial either by HCRI or from one of four post-marketing surveillance studies designed to collect similar clinical data in similar patient populations. Each contributing study followed uniform randomization criteria and follow-up as specified by the overall DAPT Study protocol. A single clinical events committee blinded to the randomized treatment assignment adjudicated events, and an unblinded independent central data monitoring committee oversaw the safety of all subjects. All participating institutions received institutional review board approval.
The first three authors and the last author, who wrote the manuscript under the coordination of HCRI, had full access to the data; they vouch for the integrity of the analyses presented and for the fidelity of this report to the trial protocol, which is available with the full text of this article at NEJM.org. The manuscript was provided to the funding manufacturers for review in advance of publication; however, they did not have the right of refusal except with regard to individual manufacturer confidential information.
Study Population
Adults who were candidates for dual antiplatelet therapy following treatment with FDA-approved drug-eluting or bare metal stents were enrolled. Detailed inclusion and exclusion criteria are listed in the Supplementary Appendix. Each subject provided written informed consent at enrollment.
The primary analytic population and focus of this report is subjects treated with drug-eluting stents only (results in subjects treated with bare metal stents will be reported in a separate publication; see Figure 1). Drug-eluting stents included sirolimus-eluting stents (Cypher, Cordis), zotarolimus-eluting stents (Endeavor, Medtronic), paclitaxel-eluting stents (TAXUS, Boston Scientific), and everolimus-eluting stents (Xience, Abbott Vascular and PROMUS, Boston Scientific). It was recommended that all subjects receive either clopidogrel at a maintenance dose of 75 mg daily or prasugrel at a maintenance dose of 10 mg daily (a dose of 5 mg daily was recommended in subjects weighing less than 60 kg).13 The recommended maintenance aspirin dosage was 75 to 162 mg daily, continued indefinitely.
Figure 1. Subject Randomization and Follow-Up.
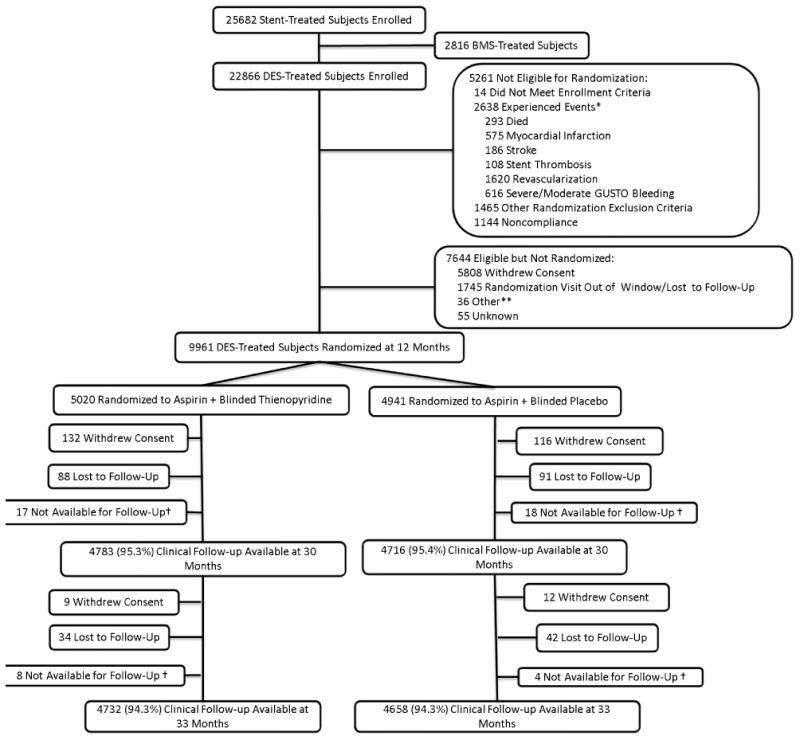
Subjects were enrolled within 72 hours of stent placement, followed for 12 months on open-label thienopyridine plus aspirin, then randomized to 18 months of thienopyridine or placebo (each in addition to aspirin). Randomized treatment ended at 30 months and subjects remained on aspirin only and were followed for another 3 months. While the number of subjects with clinical follow-up available is reported in each arm, the co-primary efficacy endpoints were analyzed according to the principle of intention to treat, including all randomized subjects and last available follow-up information.
*Subjects may have >1 event.
**Site terminated participation, randomization target met prior to subject follow-up, or subject not recognized to be eligible by site
† Subjects moved, were incarcerated, or were prematurely exited from the study.
Study Procedures
Subjects were enrolled within 72 hours of stent placement and were given open-label aspirin and thienopyridine for 12 months. At 12 months, subjects who were both event-free (from major adverse cardiovascular and cerebrovascular events, repeat revascularization, or moderate or severe bleeding) and compliant with thienopyridine (defined as having taken 80% to 120% of the drug without an interruption of longer than 14 days) were eligible for randomization (see Figure 1).
Eligible subjects continued aspirin and were randomized in a 1:1 ratio to continued thienopyridine or placebo for an additional 18 months (months 12 to 30 after enrollment). A computer-generated randomization schedule stratified subjects by stent type (drug-eluting versus bare metal stent), hospital site, thienopyridine type, and presence or absence of at least one prospectively-defined clinical or lesion-related risk factor for stent thrombosis (risk factors listed in Table S1 of the Supplementary Appendix).13 After completing the randomized treatment period, subjects were followed for a 3-month observational period on aspirin alone (months 30 to 33 after enrollment) to assess the effect of thienopyridine discontinuation on end point event rates.
End Points
The co-primary effectiveness end points were the cumulative incidence of definite or probable stent thrombosis14 and major adverse cardiovascular and cerebrovascular events (defined as the composite of death, myocardial infarction or stroke) during the randomized treatment period (months 12 to 30). The primary safety end point was the incidence of moderate or severe bleeding during this same period (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries [GUSTO] classification).15 Bleeding was also evaluated according to the Bleeding Academic Research Consortium [BARC] definitions.16 End point definitions are provided in the Supplementary Appendix. After the primary analysis had been completed, a second blinded clinical events committee was convened to adjudicate non-cardiovascular causes of death.
Statistical Analysis
The primary efficacy analysis was a superiority analysis performed using the log-rank test, stratified by geographic region (North America, Europe, or Australia and New Zealand), thienopyridine type at randomization, and presence or absence of stent thrombosis risk factors, controlling the two-sided family-wise error rate of 0.05 across the two co-primary end points using the Benjamini-Hochberg method.17 Using this method, the null hypothesis of randomized treatment equivalence is rejected if significance is achieved on both end points at a two-sided alpha = 0.05, or on one end point at a two-sided alpha = 0.025. We assumed annual placebo event rates of 0.5% for stent thrombosis and 2.9% for major adverse cardiovascular and cerebrovascular events; hazard ratios of 0.45 for stent thrombosis and 0.75 for major adverse cardiovascular and cerebrovascular events for continued thienopyridine vs. placebo; and up to 3% annual loss to follow-up. Given these assumptions, a sample size of 9,800 randomized subjects receiving drug-eluting stents yielded at least 85% power. This sample size was reduced from the 12,196 specified in the original protocol based on changes made in statistical parameters before enrollment was completed and without inspection of the study data (as described in the Supplementary Appendix).
The primary safety analysis was a non-inferiority analysis performed using the Farrington-Manning risk difference approach18. Assuming an annualized moderate or severe bleeding rate of 1.9%, and an absolute non-inferiority margin (δ) of 0.8%, at an alpha = 0.025 (1-sided) significance, a sample size of 9,960 subjects provided 80% power to detect non-inferiority.
For major adverse cardiovascular and cerebrovascular events and stent thrombosis, the primary analyses were performed on all randomized subjects treated with drug-eluting stents (the intention-to-treat principle). Kaplan-Meier estimates of the cumulative incidence of each end point are presented by treatment group, with 2-sided 95% confidence intervals (CI) of stratified hazard ratios. Subjects not experiencing an end point were censored for the analysis on that end point at the time of last known contact or 30 months, whichever was earlier. Secondary analyses included examining these same outcomes on all randomized subjects over the 21-month post-randomization period, the last three months of which subjects were not receiving randomized treatment, and hazards before and after study drug-discontinuation were assessed for qualitative differences.
For bleeding, the primary non-inferiority assessment was performed on randomized subjects treated with drug-eluting stents who completed at least 17 months of follow-up (the minimum allowable visit window for the 18 month post-randomization visit) or experienced a moderate or severe bleeding event. Bleeding event rates are presented as percentages. The moderate or severe bleeding hazard ratio is also presented as a post hoc descriptive analysis.
To account for missing data, we repeated the treatment comparisons including all randomized subjects using multiple imputation19 logistic regression modeling with baseline covariates (50 imputations) for missing data regarding the primary end points. We also assessed the consistency of the treatment effect on the primary end points for 14 pre-specified factors assessed at baseline.
Results
Study Population
Between Aug 13, 2009 and July 1, 2011, a total of 25,682 subjects from 452 sites in 11 countries were enrolled in the DAPT Study, of whom 22,866 received a drug-eluting stent. Among these subjects, 5,261 (23.0%) were not eligible for randomization after 12 months of follow-up, 7,644 (33.4%) were eligible but not randomized (Supplementary Appendix Table S2), and 9,961 (43.6%) were randomized (Figure 1). Among those who were eligible but not randomized, the most common reason was withdrawal of consent during the year between enrollment and randomization (76%).
Baseline characteristics of randomized subjects treated with drug-eluting stents were similar between treatment groups (Table 1). Overall, 26% presented with acute myocardial infarction, and 50.9% had at least one clinical or lesion-related risk factor for stent thrombosis (Supplementary Appendix Table S1). Rates of discontinuation of study drug were not different for the continued thienopyridine and placebo groups at 30 months (21.4% vs. 20.3%, respectively, P=0.18).
Table 1. Characteristics of Randomized Subjects Treated with Drug-Eluting Stents*.
| Measure | Continued Thienopyridine N=5020 Subjects |
Placebo N=4941 Subjects |
|---|---|---|
| Subject Characteristics | ||
|
| ||
| Age (years) | 61.8± 10.2 | 61.6 ± 10.1 |
| Female | 1242 (24.7%) | 1284 (26.0%) |
| Race-Non- White | 438/4918 (8.9%) | 419/4847 (8.6%) |
| Hispanic or Latino | 159/4924 (3.2%) | 159/4847 (3.3%) |
| Weight (kg) | 91.5± 19.7 (5009) | 91.5±19.4 (4931) |
| BMI (Kg/m2 ) | 30.5± 5.8 (4973) | 30.6±5.8 (4901) |
| Diabetes mellitus | 1556/5006 (31.1%) | 1481/4927 (30.1%) |
| Hypertension | 3796/5006 (75.8%) | 3649/4934 (74.0%) |
| Current cigarette smoker or within past year | 1222/4965 (24.6%) | 1210/4893 (24.7%) |
| Stroke/TIA | 155/5006 (3.1%) | 169/4931 (3.4%) |
| Congestive heart failure | 238/5001 (4.8%) | 223/4926 (4.5%) |
| Peripheral arterial disease | 284/4937 (5.8%) | 284/4857 (5.8%) |
| Prior PCI | 1518/4995 (30.4%) | 1529/4928 (31.0%) |
| Prior CABG | 568/5012 (11.3%) | 581/4930 (11.8%) |
| Prior MI | 1092/4953 (22.0%) | 1026/4870 (21.1%) |
| Positive stress test** | 1487/3916 (38.0%) | 1485/3843 (38.6%) |
| Indication for PCI | ||
| STEMI | 534 (10.6%) | 511 (10.3%) |
| NSTEMI | 776 (15.5%) | 767 (15.5%) |
| Unstable angina*** | 838 (16.7%) | 825 (16.7%) |
| Stable angina | 1882 (37.5%) | 1870 (37.8%) |
| Other | 990 (19.7%) | 968 (19.6%) |
| Region | ||
| North America | 4502 (89.7%) | 4416 (89.4%) |
| Europe | 402 (8.0%) | 405 (8.2%) |
| Australia/New Zealand | 116 (2.3%) | 120 (2.4%) |
|
| ||
| Treatment Characteristics | ||
|
| ||
| Clopidogrel | 3275 (65.24%) | 3230 (65.37%) |
| Prasugrel | 1745 (34.76%) | 1711 (34.63%) |
| DES type at index procedure | ||
| Everolimus-eluting | 2345 (46.71%) | 2358 (47.72%) |
| Paclitaxel-eluting | 1350 (26.89%) | 1316 (26.63%) |
| Zotarolimus-eluting† | 642 (12.79%) | 622 (12.59%) |
| Sirolimus-eluting | 577 (11.49%) | 541 (10.95%) |
| >1 DES type | 106 (2.11%) | 104 (2.10%) |
| Number of treated lesions | 1.30±0.55 | 1.29±0.54 |
| Number of treated vessels | 1.11±0.33 | 1.12±0.34 |
| Number of stents | 1.47±0.75 | 1.45±0.75 |
| Minimum stent diameter | ||
| <3 | 2341 (46.63%) | 2293 (46.41%) |
| =3 | 1593 (31.73%) | 1595 (32.28%) |
| >3 | 1086 (21.63%) | 1053 (21.31%) |
| Total stent length | 27.70±16.77 | 27.43±17.02 |
|
| ||
| Lesion Characteristics | N=6594 Lesions | N=6413 Lesions |
|
| ||
| Treated vessel(s) | ||
| Native coronary | 6396/6586 (97.12%) | 6204/6407 (96.83%) |
| Left main | 55/6586 (0.84%) | 55/6407 (0.86%) |
| LAD | 2715/6586 (41.22%) | 2586/6407 (40.36%) |
| RCA | 2153/6586 (32.69%) | 2057/6407 (32.11%) |
| Circumflex | 1473/6586 (22.37%) | 1506/6407 (23.51%) |
| Venous graft | 154/6586 (2.34%) | 173/6407 (2.70%) |
| Arterial graft | 36/6586 (0.55%) | 30/6407 (0.47%) |
| Modified ACC/AHA lesion class B2 or C‡ | 2754/6335 (43.47%) | 2643/6137 (43.07%) |
There were no significant differences between the two groups except for hypertension (P=0.03).
Denominators are shown when they differed from the total number in the treatment group.
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; BMI, body mass index; CABG, coronary artery bypass graft; LAD, left anterior descending; MI, myocardial infarction; NSTEMI, non-ST elevation MI; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST-elevation MI; TIA, transient ischemic attack.
For most variables, 0-3% of patients had missing values; 6% of patients were missing lesion class; 22% of patients were missing positive stress test due to not being collected in one contributing study.
Without reported cardiac enzyme elevation.
Endeavor stent.
Stent Thrombosis and Major Adverse Cardiovascular and Cerebrovascular Events
Over the primary analysis period for all randomized subjects, the continued thienopyridine group experienced a significantly lower cumulative incidence of stent thrombosis (0.4% vs. 1.4%, hazard ratio 0.29, 95% CI 0.17-0.48, P<0.001) and major adverse cardiovascular and cerebrovascular events (4.3% vs. 5.9%, hazard ratio 0.71, 95% CI 0.59-0.85, P<0.001), compared with the placebo group (Table 2a and Figures 2 and 3). Continued thienopyridine was associated with a lower cumulative incidence of myocardial infarction than placebo (2.1% vs. 4.1%, hazard ratio 0.47, P<0.001, Supplementary Appendix Figure S1), of which non-stent thrombosis-related myocardial infarction comprised 55% of the treatment benefit (1.8% vs. 2.9% hazard ratio 0.59, P<0.001). Rates of cardiac mortality (0.9% vs. 1.0%, P=0.98), vascular mortality (0.1% vs. 0.1%, P=0.98), and stroke (0.8% vs 0.9%, P=0.32) were similar between groups. Total mortality rates were 2.0% and 1.5%, respectively (hazard ratio 1.36, 95% CI 1.00-1.85, P=0.052, Supplementary Appendix Figure S2). The results using multiple imputation were consistent (hazard ratio for stent thrombosis 0.27, P<0.001; hazard ratio for major adverse cardiovascular and cerebrovascular events 0.77, P=0.002, Supplementary Appendix Table S3a), as were the findings when the analysis period included the three months after study drug discontinuation (Supplementary Appendix Table S4).
Table 2a. Stent Thrombosis and Major Adverse Cardiovascular and Cerebrovascular Events (MACCE).
Subjects were randomized to 18 months of either continued thienopyridine or placebo plus aspirin 12 months after receiving a drug-eluting stent. Data are presented according to intention-to-treat. The primary analysis period was 12-30 months after enrollment, and the study co-primary effectiveness end points were stent thrombosis and MACCE. Hazard ratios are presented as continued thienopyridine vs. placebo.
| Outcome | Continued Thienopyridine N=5020 |
Placebo N=4941 |
Stratified Hazard Ratio (95% CI) |
Stratified Log-rank P Value |
|---|---|---|---|---|
| Stent thrombosis | 19 (0.4%) | 65 (1.4%) | 0.29 (0.17, 0.48) | <0.001 |
| ARC definite | 15 (0.3%) | 58 (1.2%) | 0.26 (0.14, 0.45) | <0.001 |
| ARC probable | 5 (0.1%) | 7 (0.1%) | 0.71 (0.22, 2.23) | 0.55 |
| MACCE (death, MI, or stroke) | 211 (4.3%) | 285 (5.9%) | 0.71(0.59, 0.85) | <0.001 |
| Death | 98 (2.0%) | 74 (1.5%) | 1.36 (1.00, 1.85) | 0.052 |
| Cardiac | 45 (0.9%) | 47 (1.0%) | 1.00 (0.66, 1.52) | 0.98 |
| Vascular | 5 (0.1%) | 5 (0.1%) | 0.98 (0.28, 3.39) | 0.98 |
| Non-cardiovascular | 48 (1.0%) | 22 (0.5%) | 2.23 (1.32, 3.78) | 0.002 |
| MI | 99 (2.1%) | 198 (4.1%) | 0.47 (0.37, 0.61) | <0.001 |
| Stroke | 37 (0.8%) | 43 (0.9%) | 0.80 (0.51, 1.25) | 0.32 |
| Ischemic | 24 (0.5%) | 34 (0.7%) | 0.68 (0.40, 1.17) | 0.16 |
| Hemorrhagic | 13 (0.3%) | 9 (0.2%) | 1.20 (0.50, 2.91) | 0.68 |
| Type uncertain | 0 (0.0%) | 1 (0.0%) | 0 (--, --) | 0.32 |
Abbreviations: ARC, Academic Research Consortium; ASA, aspirin; MACCE, major adverse cardiovascular and cerebrovascular events; MI, myocardial infarction; ST, stent thrombosis.
Figure 2. Cumulative Incidence of Stent Thrombosis, According to Treatment Group.
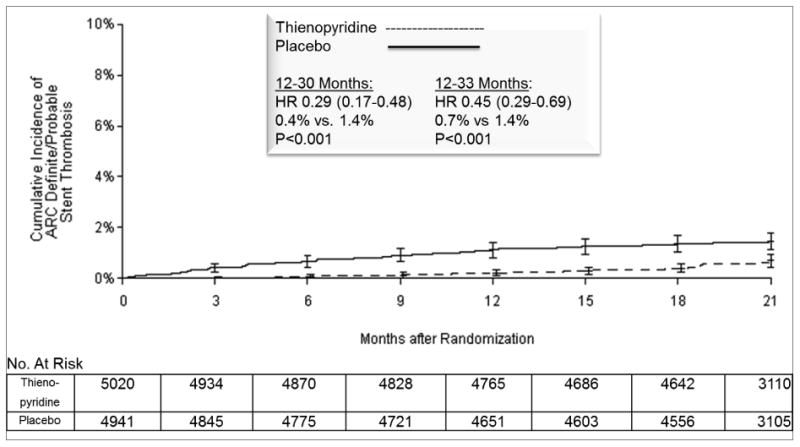
Cumulative incidence curve is shown for the primary effectiveness outcome of stent thrombosis in the intention-to-treat analysis population. Randomization occurred at 12 months after stenting. The primary analysis period was 12-30 months after percutaneous coronary intervention, i.e. the 18 months after randomization over which subjects were treated with study drug. Subjects were followed for an observational period of an additional three months, off study drug and off open label thienopyridine treatment, to a total of 33 months, i.e. 21 months post randomization. P values were calculated with stratified log-rank test. The number at risk is defined as the number of subjects without the event of interest and available for subsequent follow-up. The numbers at risk at the start of final 33 month visit (i.e. 20 months post randomization) were 4,438 vs. 4,362 for continued thienopyridine vs. placebo, respectively.
Abbreviations: ARC, Academic Research Consortium; HR, hazard ratio; MACCE, major adverse cerebral and cardiovascular events.
Figure 3. Cumulative Incidence of Major Adverse Cardiovascular and Cerebrovascular Events (MACCE) According to Treatment Group.
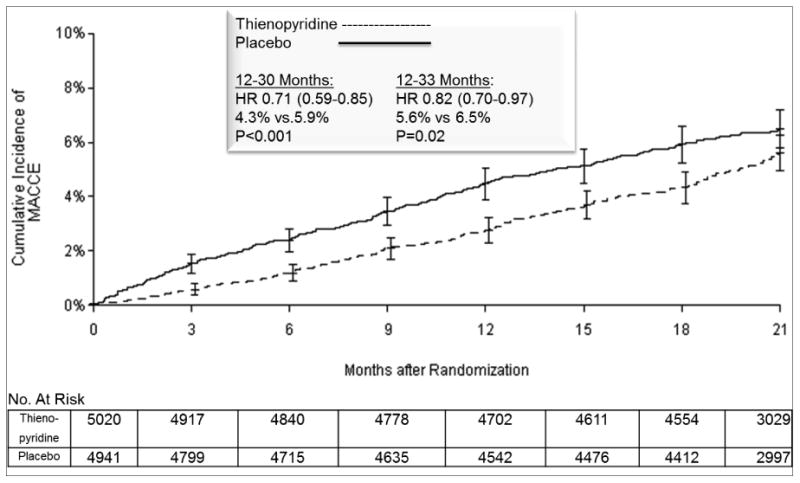
Cumulative incidence curve is shown for the primary effectiveness outcome of MACCE in the intention-to-treat analysis population. Randomization occurred at 12 months after stenting. The primary analysis period was 12-30 months after percutaneous coronary intervention, i.e. the 18 months after randomization over which subjects were treated with study drug. Subjects were followed for an observational period of an additional three months, off study drug and off open label thienopyridine treatment, to a total of 33 months, i.e. 21 months post randomization. P values were calculated with stratified log-rank test. Error The number at risk is defined as the number of subjects without the event of interest and available for subsequent follow-up. The numbers at risk at the start of final 33-month visit (i.e. 20 months post randomization) were 4,336 vs. 4,217 for continued thienopyridine vs. placebo, respectively.
Abbreviations: ARC, Academic Research Consortium; HR, hazard ratio; MACCE, major adverse cerebral and cardiovascular events.
Bleeding
Rates of moderate or severe bleeding for the primary analysis period were significantly higher in the continued thienopyridine group (2.53% vs. 1.57%, hazard ratio 1.61, 95% CI 1.21-2.16, P=0.001), and did not meet the pre-specified definition of non-inferiority versus placebo (P=0.70, Table 2b). There was not a significant difference between randomized treatments in GUSTO severe bleeding (0.81% vs. 0.56%, P=0.15) or in BARC fatal bleeding (type 5, 0.15% vs. 0.09%, P=0.38), in the continued thienopyridine vs. placebo groups, respectively. More details of bleeding results by BARC subtype are shown in Supplementary Appendix Table S5. The results using multiple imputation were consistent (risk difference for moderate or severe bleeding 0.98%, P=0.73 for non-inferiority, Supplementary Appendix Table S3b), as were the findings when the analysis period included the three months after study drug discontinuation (Supplementary Appendix Table S6).
Table 2b. Bleeding Outcomes at 12-30 Months.
The primary safety end point was GUSTO Moderate or Severe bleeding. The one-sided test of non-inferiority (based on a delta of 0.8%) was calculated according to the Farrington-Manning approach. Only evaluable subjects were included in this analysis, e.g. subjects whose last contact date was ≥ 510 days post randomization or who experienced any adjudicated bleeding outcome at or before 540 days. The secondary analysis of BARC results by subtype are shown in Supplementary Appendix Table S5.
| Bleeding Complications | Continued Thienopyridine N=4713 |
Placebo N=4650 |
Risk Difference (95% CI) |
Two-sided P Value for Difference | One-sided P Value for Non-Inferiority |
|---|---|---|---|---|---|
| GUSTO Severe/Moderate | 119 (2.53%) | 73 (1.57%) | 0.96% (0.38%, 1.53%) | 0.001 | 0.70 |
| GUSTO Severe | 38 (0.81%) | 26 (0.56%) | 0.25% (-0.09%, 0.58%) | 0.15 | |
| GUSTO Moderate | 81 (1.72%) | 48 (1.03%) | 0.69% (0.22%, 1.16%) | 0.004 | |
| BARC Types 2, 3, or 5 | 263 (5.58%) | 137 (2.95%) | 2.64% (1.82%, 3.45%) | <0.001 | |
| BARC Type 2 | 145 (3.08%) | 72 (1.55%) | 1.53% (0.92%, 2.14%) | <0.001 | |
| BARC Type 3 | 122 (2.59%) | 68 (1.46%) | 1.13% (0.56%, 1.70%) | <0.001 | |
| BARC Type 5 | 7 (0.15%) | 4 (0.09%) | 0.06% (-0.08%, 0.20%) | 0.38 |
Abbreviations: BARC, Bleeding Academic Research Consortium; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries.
Mortality
Over the primary analysis period of 12 to 30 months, the rates of all-cause mortality in the continued thienopyridine and placebo groups were 2.0% and 1.5%, respectively (hazard ratio 1.36, P=0.052). During the secondary analysis period of 12 to 33 months, all-cause mortality was 2.3% vs. 1.8% (hazard ratio 1.36, P=0.04, Supplementary Appendix Figure S2 and Table S4), the difference accounted for by non-cardiovascular death (1.1% vs. 0.6%, hazard ratio 1.80, P=0.01). Among these non-cardiovascular deaths, bleeding-related death (N=11 vs. 3, P=0.057) was mainly related to fatal trauma (N=7 vs. N=2, P=0.07). Cancer-related death differed between groups (N=31 vs. 14, P=0.02), and was mediated by bleeding in a few cases (N=3 vs. 0, Supplementary Appendix Table S7).
There were 22 more subjects with a history of cancer at baseline in the thienopyridine group (Supplementary Appendix Table S8), and blinded review of cancer-related deaths identified an imbalance in the number of randomized subjects where the cancer had been diagnosed prior to enrollment (8 vs. 1, Supplementary Appendix Table S9). When these subjects were excluded in a post hoc sensitivity analysis, differences in mortality were no longer significant (Supplementary Appendix Table S10).
Additional Analyses
The effect of continued thienopyridine versus placebo on the primary endpoints and on myocardial infarction was consistent across most subgroups (Supplementary Appendix Figure S3). Hazards after thienopyridine discontinuation are presented in the Supplementary Appendix.
Discussion
Among patients receiving drug-eluting coronary stents, continued thienopyridine and aspirin beyond one year reduced the risk of stent thrombosis and major adverse cardiovascular and cerebrovascular events compared with aspirin alone. This treatment benefit was driven by concurrent reductions in stent thrombosis and in myocardial infarction. Longer thienopyridine administration was also associated with more bleeding, although severe or fatal bleeding was uncommon and not significantly different between study groups.
The DAPT Study included a large proportion of subjects with stent thrombosis risk factors, including many with preceding myocardial infarction. Across almost all subjects and lesion types, continued thienopyridine was associated with reductions in the risk of both co-primary end points. Different stents20,21 and P2Y12 inhibitors22 have been associated with varied rates of stent thrombosis and myocardial infarction in previous reports, yet in this study, thienopyridine use beyond one year reduced the risks of both outcomes across all stent and drug types. Although results from prior studies vary with regard to risk of thienopyridine discontinuation after 6 months, 10-13,23,24 the current study detected an increased risk of myocardial infarction (both stent- and non-stent related) during the first three months after discontinuation in both treatment groups. Future evaluation of thienopyridine therapy to suppress risks of cardiovascular events beyond the duration of this study may be warranted.
An unexpected finding was that all-cause mortality during the treatment period was numerically higher for the continued thienopyridine group, driven by an increase in non-cardiovascular deaths. While the diagnosis of cancer did not differ after randomization, cancer-related death was more frequent in subjects treated with continued thienopyridine and may reflect a chance imbalance in subjects with known cancer prior to enrollment. While one study comparing long-term thienopyridine therapy to placebo in subjects with lacunar stroke identified an unexpected increase in mortality,25 other large randomized studies in subjects with coronary artery disease have not identified either increased or decreased risks of mortality.26-28
Several limitations of the study should be considered. First, only drug-compliant subjects who did not have major adverse cardiovascular and cerebrovascular events, stent thrombosis or moderate or severe bleeding in the first year were randomized, which may have selected for subjects at lower risk for late adverse events. Second, while we did not quantify the net impact of ischemic and bleeding events; decision analysis suggests that small absolute differences in cardiovascular event rates may be sufficient29 to counterbalance bleeding risks. Third, while four different metal platform durable polymer drug-eluting stents and two platelet P2Y12 inhibitors were included, whether the treatment benefits observed will be generalizable to other stent types30,31 or non-thienopyridine P2Y12 inhibitors32,33 is unknown. Additionally, since stent and drug type were not randomized, direct comparisons of different stent or drug types may be confounded, and within-subgroup estimates of treatment effect may be underpowered.
In conclusion, in patients treated with drug-eluting stents, continuation of thienopyridine plus aspirin beyond one year reduced the risks of ischemic events compared with aspirin alone. Reduction in risk of ischemic events was consistent across drug and stent types, and was evident regardless of risk of stent thrombosis. The clinical benefit of extended thienopyridine treatment was tempered by an increase in bleeding events.
Supplementary Material
Acknowledgments
We wish to acknowledge Joanna Suomi for assistance editing and formatting this manuscript and Wen-Hua Hsieh for assistance with statistical analysis.
Sponsored by Harvard Clinical Research Institute. Funded by Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Inc., Bristol-Myers Squibb Company/Sanofi Pharmaceuticals Partnership, Eli Lilly and Company, and Daiichi Sankyo Company Limited and the US Department of Health and Human Services (1RO1FD003870-01).
Footnotes
The full list of study investigators and study committee members is available in the Appendix
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Togni M, Balmer F, Pfiffner D, Maier W, Zeiher AM, Meier B. Percutaneous coronary interventions in Europe 1992-2001. Eur Heart J. 2004;25:1208–13. doi: 10.1016/j.ehj.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Mauri L, Hsieh WH, Massaro JM, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–9. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 5.Cutlip DE, Chhabra AG, Baim DS, et al. Beyond restenosis: five-year clinical outcomes from second-generation coronary stent trials. Circulation. 2004;110:1226–30. doi: 10.1161/01.CIR.0000140721.27004.4B. [DOI] [PubMed] [Google Scholar]
- 6.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 7.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 8.Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159–68. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 9.Valgimigli M, Campo G, Monti M, et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–26. doi: 10.1161/CIRCULATIONAHA.111.071589. [DOI] [PubMed] [Google Scholar]
- 10.Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362:1374–82. doi: 10.1056/NEJMoa1001266. [DOI] [PubMed] [Google Scholar]
- 11.Feres F, Costa RA, Abizaid A, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310:2510–22. doi: 10.1001/jama.2013.282183. [DOI] [PubMed] [Google Scholar]
- 12.Collet JP, Silvain J, Barthelemy O, et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)60612-7. [DOI] [PubMed] [Google Scholar]
- 13.Mauri L, Kereiakes DJ, Normand SL, et al. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J. 2010;160:1035–41. 41 e1. doi: 10.1016/j.ahj.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 14.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 15.An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med. 1993;329:673–82. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 16.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–8. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 18.Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med. 1990;9:1447–54. doi: 10.1002/sim.4780091208. [DOI] [PubMed] [Google Scholar]
- 19.Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley; 1987. [Google Scholar]
- 20.Stone GW, Rizvi A, Sudhir K, et al. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. J Am Coll Cardiol. 2011;58:19–25. doi: 10.1016/j.jacc.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Wijns W, Steg PG, Mauri L, et al. Endeavour zotarolimus-eluting stent reduces stent thrombosis and improves clinical outcomes compared with cypher sirolimus-eluting stent: 4 year results of the PROTECT randomized trial. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu318. [DOI] [PubMed] [Google Scholar]
- 22.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 23.Mehran R, Baber U, Steg PG, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714–22. doi: 10.1016/S0140-6736(13)61720-1. [DOI] [PubMed] [Google Scholar]
- 24.Ho PM, Peterson ED, Wang L, et al. Incidence of death and acute myocardial infarction associated with stopping clopidogrel after acute coronary syndrome. JAMA. 2008;299:532–9. doi: 10.1001/jama.299.5.532. [DOI] [PubMed] [Google Scholar]
- 25.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–25. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 27.Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 28.Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–33. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 29.Garg P, Galper BZ, Cohen DJ, Yeh RW, Mauri L. Balancing the Risks of Bleeding and Stent Thrombosis: A Decision Analytic Model to Compare Duration of Dual Antiplatelet Therapy after Drug-Eluting Stents. Am Heart J. 2014 doi: 10.1016/j.ahj.2014.11.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)61455-0. [DOI] [PubMed] [Google Scholar]
- 31.Meredith IT, Verheye S, Dubois CL, et al. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J Am Coll Cardiol. 2012;59:1362–70. doi: 10.1016/j.jacc.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 33.Bonaca MP, Bhatt DL, Braunwald E, et al. Design and rationale for the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. Am Heart J. 2014;167:437–44. e5. doi: 10.1016/j.ahj.2013.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


