Abstract
Purpose/Objective(s)
Current guidelines for esophageal cancer contouring are derived from traditional two-dimensional fields based on bony landmarks, and do not provide sufficient anatomical detail to ensure consistent contouring for more conformal radiotherapy techniques such as intensity-modulated radiation therapy (IMRT). Therefore, we convened an expert panel with the specific aim to derive contouring guidelines and generate an atlas for the clinical target volume (CTV) in esophageal or gastroesophageal junction (GEJ) cancer.
Methods and Materials
Eight expert academically-based gastrointestinal radiation oncologists participated. Three sample cases were chosen: a GEJ cancer, a distal esophageal cancer, and a mid-upper esophageal cancer. Uniform CT simulation datasets and an accompanying diagnostic PET-CT were distributed to each expert, and he/she was instructed to generate gross tumor volume (GTV) and CTV contours for each case. All contours were aggregated and subjected to quantitative analysis to assess the degree of concordance between experts and generate draft consensus contours. The panel then refined these contours to generate the contouring atlas.
Results
Kappa statistics indicated substantial agreement between panelists for each of the three test cases. A consensus CTV atlas was generated for the three test cases, each representing common anatomic presentations of esophageal cancer. The panel agreed on guidelines and principles to facilitate the generalizability of the atlas to individual cases.
Conclusions
This expert panel successfully reached agreement on contouring guidelines for esophageal and GEJ IMRT and generated a reference CTV atlas. This atlas will serve as a reference for IMRT contours for clinical practice and prospective trial design. Subsequent patterns of failure analyses of clinical datasets utilizing these guidelines may require modification in the future.
INTRODUCTION
Radiotherapy (RT) has an important role in the treatment of esophageal and gastro-esophageal junction (GEJ) cancer, both in the definitive and preoperative settings. Definitive concurrent chemoradiotherapy can achieve long-term survival in a subset of patients. [1] When used as preoperative therapy, chemoradiation improves rates of margin-negative resection, pathologic complete response, and long-term survival.[2] Traditionally, RT fields have been designed based on two-dimensional planning, using esophagrams to identify the primary lesion and utilizing simple geometric expansions and bony landmarks to shape radiation fields. To encompass subclinical disease extension and regional nodal spread, typical field borders were designated by 5cm expansions proximally and distally beyond apparent tumor along the length of the esophagus, and 2cm laterally, with these guidelines referring to distance to field or block edge.[3,4] In modern radiotherapy practice, treatment volumes are more commonly defined based on the ICRU definitions of clinical target volume (CTV) and planning target volume (PTV).
Intensity-modulated radiation therapy (IMRT) and other highly conformal techniques, including volumetric arc therapy (VMAT) and proton therapy, represent an important advance in radiation therapy. These techniques allow for greater sparing of normal tissues, particularly the lungs and heart. [5-8] However, highly conformal radiation techniques require the radiation oncologist to define target volumes with greater specificity, utilizing CT-derived images and anatomy. Although traditional guidelines for field design still govern contouring for IMRT in a broad sense, they are not likely to provide sufficient detail to ensure consistent delineation of target volumes between practitioners and patients.
Current prospective trials involving radiotherapy and esophageal cancer generally require three-dimensional conformal RT (3D-CRT), and may allow the use of IMRT. However, no consensus reference contouring guidelines or atlas is available to guide target delineation for patients on these trials. A remedy for this gap in clinical practice is important since it has been demonstrated that variation in target volume delineation may impact outcome of therapy and can be improved with atlases that serve as teaching aids in GI tract neoplasms.[9,10] To develop standardized contouring guidelines and to ensure adequacy of the CTV for ongoing and future clinical trials of radiotherapy for esophageal and GEJ cancers, we convened a panel of expert gastrointestinal radiation oncologists to generate a reference atlas for modern-day contouring. Since conformal techniques such as IMRT are also increasingly used in general practice to reduce radiation dose to organs at risk, such guidelines can serve as best practice surrogates for the clinician treating patients outside of a clinical trial setting.
MATERIALS AND METHODS
Expert Panel and Test Case Selection
An expert panel of academic radiation oncologists with expertise in gastrointestinal malignancies was convened, representing multiple NCI-designated cancer centers throughout the United States. Because the esophagus spans different anatomic regions in the body, and regional nodal volumes can differ depending on the location of primary tumor, three sample cases were selected (see Supplementary Material). Case 1 was a T3N0, Siewert II lesion spanning the GEJ, Case 2 was a T3N1 lesion of the distal esophageal cancer without overt involvement of the GEJ, and the Case 3 was a T3N1 proximal esophageal lesion at and above the level of the carina. A simulation computed tomography (CT) dataset from each case was distributed to each panelist and imported into his or her institutional treatment planning system. The panelists were instructed to contour each case assuming RT in a single course to 5040cGy in 180cGy fractions, with concurrent chemotherapy.
Because positron emission tomography (PET) imaging is now standard in the initial evaluation of esophageal cancer, and because fluorodeoxyglucose (FDG) avidity is a useful method to localize primary tumor and adenopathy on cross-sectional imaging, we distributed diagnostic PET-CT images for each test case to the panelists, along with a clinical vignette on each case which provided other pertinent information such as esophagoduodenoscopy and endoscopic ultrasound findings. The primary tumors had SUVmax values of 12.6, 10.0, and 13.2, respectively.
Contour Generation
The panelists were first instructed to contour the gross tumor volume (GTV) on each case, based on a free-breathing simulation CT, clinical information, and PET-CT images, so that the degree of consensus in GTV contouring could also be assessed.
The panelists were then instructed to utilize a reference GTV as the basis for their CTV to ensure that all panelists were using the same GTV to construct the CTV. Panelists were to define the CTV according to the basic instructions of the CALGB 80803 trial (see Supplementary Material). These specified a 3-4cm superior/inferior and 1-1.5cm radial margin from GTV, inclusion of peri-esophageal nodes, mediastinal and supraclavicular nodes in proximal tumors, and celiac nodes in distal/GEJ tumors.
Statistical Analysis
Panelists’ contours were imported from DICOM files and merged onto a single scan for each test case, and imported into the Computational Environment for Radiotherapy Research (CERR) for statistical analysis. Kappa statistics were calculated to characterize the level of agreement between physicians; a Kappa value of −1 represents complete disagreement, 0 represents agreement only at the level expected by chance, and 1 represents perfect agreement.[11]
The Simultaneous Truth and Performance Level Estimation (STAPLE) algorithm, implemented in the CERR software, was used to generate preliminary consensus contours, as previously described.[12] This algorithm considers a collection of contours and calculates a probabilistic estimate of the “true” contour.[13] STAPLE contours with a 95% confidence level were utilized.
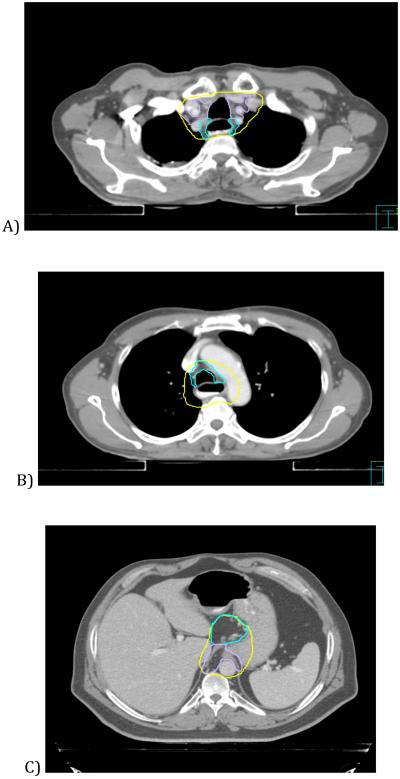
Based on review of the STAPLE contours and comparison of the submitted contours, the panel then discussed areas of significant variability or uncertainty, and arrived at a set of contouring guidelines to accompany the reference atlas. To enhance their generalizability, the consensus contours were referenced to existing consensus radiographic definitions of nodal levels in the neck, thorax and abdomen. (See figure 1) [14-16]
Figure 1.
Examples of consensus contours encompassing defined nodal regions. A: CTV contour (yellow) encompasses level 3 retrotracheal (blue) and level 2 upper paratracheal (purple) nodes. B: CTV encompasses level 4 lower paratracheal (blue) and level 8 periesophageal nodes. C: CTV encompasses lesser curvature/gastrohepatic ligament (blue) and paracardial (purple) nodes. D) CTV encompasses para-aortic (blue) and celiac (purple) nodes.
RESULTS
GTV Contours
GTV and CTV contours were successfully obtained from the eight panelists. Summary statistics on GTV and CTV contours are listed in Table 1. Kappa statistics indicated substantial agreement among the panelists, with values of 0.651, 0.790,and 0.623 for Cases 1, 2 and 3, respectively. (Kappa values between 0.61 and 0.80 are considered “substantial” agreement).
Table 1.
GTV and CTV Structure Measures
| Structure Measure (cc) |
Case 1 GTV |
Case 2 GTV |
Case 3 GTV |
Case 1 CTV |
Case 2 CTV |
Case 3 CTV |
|---|---|---|---|---|---|---|
| Minimum Volume | 36.94 | 41.96 | 14.51 | 397.89 | 364.72 | 265.28 |
| Maximum Volume | 106.18 | 68.28 | 67.20 | 563.21 | 712.12 | 496.65 |
| Mean Volume | 73.54 | 54.13 | 53.16 | 467.67 | 489.89 | 384.21 |
| Volume Standard Deviation |
21.57 | 8.17 | 18.48 | 64.98 | 112.71 | 92.34 |
| Intersection Volume | 27.10 | 34.05 | 12.13 | 219.15 | 178.45 | 110.50 |
| Union Volume | 133.20 | 88.15 | 102.91 | 790.01 | 979.83 | 835.49 |
| STAPLE Volume | 74.28 | 55.93 | 68.81 | 477.48 | 569.14 | 441.79 |
| Kappa (scale of −1 to 1) | 0.651 | 0.790 | 0.623 | 0.683 | 0.663 | 0.609 |
CTV Contours
Kappa statistics again indicated substantial agreement in all three cases, with values of 0.683, 0.663, and 0.609 for cases 1, 2 and 3, respectively. STAPLE contours were generated for each case with volumes of 477, 569, and 442cc, respectively.
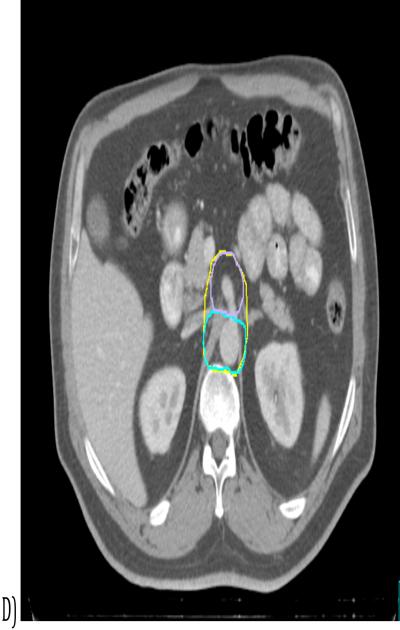
These preliminary consensus contours were then reviewed and edited to smooth out the contours and resolve areas where, due to averaging, the algorithm had generated anatomically illogical contours. (See figure 2).
Figure 2.
Example of consensus contour generation. Top: Superimposed panelists’ contours relative to the reference GTV (red). Bottom: STAPLE consensus contour (green) and final consensus contour (yellow).
CTV Contouring Guidelines
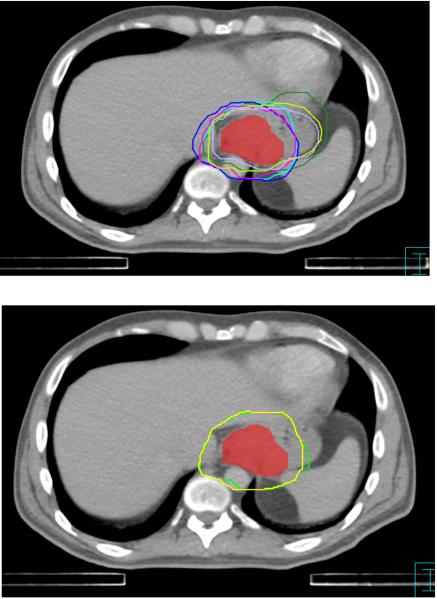
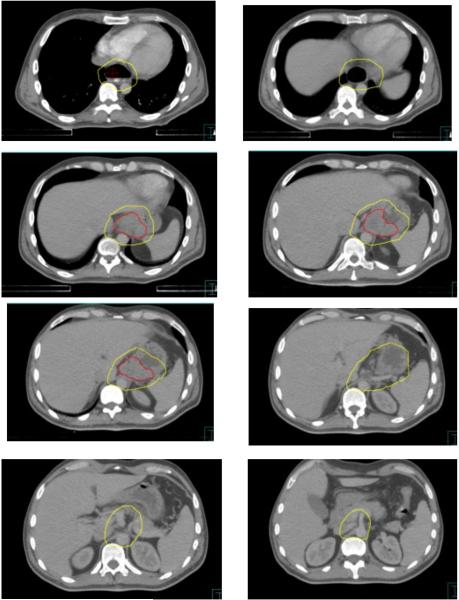
Based on the high degree of agreement among the panelists’ contours, the panel proceeded to establish the following guidelines for CTV contouring in esophageal cancer (See figures 3-5):
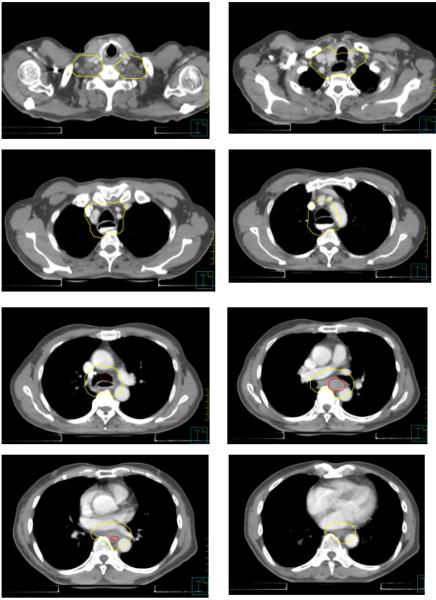
Figure 3.
Consensus contours for case 1 (T3N0, Siewert II gastroesophageal junction cancer. GTV in red)
Figure 5.
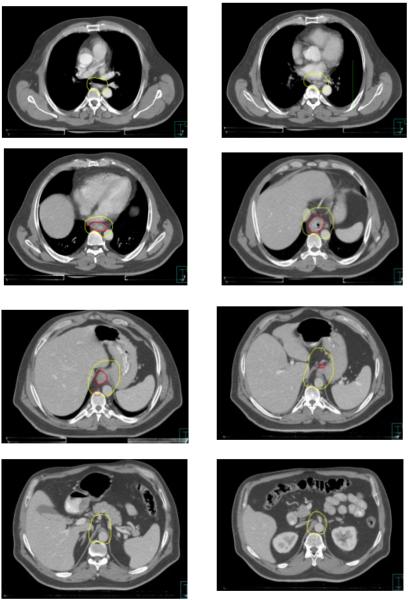
Consensus contours for T3N1 proximal esophageal cancer.
Proximal Border
3-4cm margin above the proximal edge of the GTV, or 1cm above any grossly involved periesophageal nodes, whichever is more cephalad. This margin should be oriented along the esophageal mucosa, instead of being a simple geometric expansion. For very proximal tumors, the upper border should not extend above the level of the cricoid cartilage unless there is gross disease at that level.
Distal Border
For proximal or mid-esophageal tumors, a 3-4cm margin below the proximal edge of the GTV, oriented along the esophageal mucosa. For distal esophageal or GE junction tumors, a 4cm geometric margin distally for all cases would extend well below the GE junction and include unacceptably large volumes of stomach or other abdominal viscera when treating to 5040cGy. Therefore for this situation, at least a 2cm margin along clinically uninvolved gastric mucosa was recommended. If treating to lower, preoperative-intent doses (≤4500cGy), a 4cm or greater gastric margin may be appropriate, particularly for tumors with significant gastric extension. Siewert III lesions, and lesions extending more than 5cm into the stomach, fall outside the scope of this atlas and may be contoured using gastric cancer-specific guidelines.
Radial Borders
In general, the CTV should include the GTV (including any grossly involved nodes) with at least a 1cm margin in all directions. A 1cm radial margin from the outer esophageal wall was recommended to encompass the peri-esophageal lymph nodes (level 8 in the International Association for the Study of Lung Cancer (IASLC) system). Unless the GTV was located at the esophagus/heart interface, it was recommended that the CTV expansion be limited to 0.5cm into cardiac tissue (including pericardium), given concern for excessive cardiac dose and the unlikelihood of microscopic extension into the myocardium in the absence of gross invasion. Similarly, the CTV expansion can be limited to 0.5cm into uninvolved liver. Excluding the liver and heart from the CTV entirely is reasonable if robust motion management techniques, such as respiratory gating or an ITV approach, are employed to minimize the possibility that a CTV border based on a static simulation scan is transgressed during radiation treatment due to tumor or organ motion. It was also recommended that the vertebral bodies be entirely excluded from the CTV in the absence of gross invasion.
Regional nodal volumes
For distal tumors involving or approaching the GE junction, the CTV should be extended inferiorly to the level of the origin of the celiac axis, in order to cover the celiac lymph nodes, which normally are located at the level of the T12 vertebral body. Typically, the celiac nodal CTV will be bounded by the lateral aspect of the vertebral body (usually T12) on the right, 0.5-1cm beyond the lateral aspect of the aorta on the left, the vertebral body posteriorly, and the pancreatic body anteriorly. The kidneys should be excluded from the CTV.
In the upper abdomen, between the level of the GE junction and the celiac nodes, it was recommended that para-aortic and gastrohepatic ligament (often classified as lesser curvature or left gastric) nodes be included in the CTV. In this region, the liver will typically bound the CTV on the right. On the left, the border will typically be the stomach. Anteriorly, the CTV typically includes the fatty space between the lesser curvature and the liver that contains the paracardial and gastrohepatic ligament nodes. The splenic hilar nodes are not considered regional nodes for esophageal cancer and do not need to be specifically included in the CTV, though they may be incidentally covered if the tumor extends significantly into the stomach. However, with Siewert Type II GE junction tumors, given a higher risk of lymph node involvement, the panel agreed that inclusion of some or all nodes in the splenic hilum and greater curvature region can be at the discretion of the treating physician if using lower doses, depending on the patient’s clinical and pathologic features. For tumors above the level of the carina, it was recommended that the bilateral supraclavicular nodal basins be included. The recommended borders of the supraclavicular nodes are analogous to Level IV nodes in head and neck cancer[16], in which the cranial border is the level of the cricoid cartilage, and the anterior, posterior, and lateral borders correspond to the borders of the sternocleidomastoid muscles, with the inferior border extending into the thoracic inlet.
A significant area of discrepancy exists regarding the extent to which mediastinal nodal stations should be explicitly included in the CTV. The 7th edition AJCC staging classification only considers nodes located in the para-esophageal region to be regional.[17] Routine inclusion of all mediastinal node stations in the CTV will result in treatment volumes significantly larger than traditional fields based on 1-2cm radial expansions from the esophagus, and result in significantly greater and potentially excessive radiation dose to the lungs. Therefore, for distal tumors in which the CTV extends superiorly to the mediastinum only in order to respect the 3-4cm proximal margin on gross tumor, the panel did not consider it mandatory to deliberately include superior mediastinal nodal stations electively, other than would be encompassed by a 1cm radial expansion of the esophagus.
For proximal tumors, in which the supraclavicular nodes are already at risk and lung dose is less of a limitation given the decreased cranio-caudal extent of CTV into the thorax and the smaller relative volume of lung tissue at the apices, the panelists favored a more generous CTV to encompass mediastinal lymph nodes in addition to the peri-esophageal nodes. Above the carina, the CTV will therefore typically encompass the entire trachea and extend radially in order to encompass the lower and upper paratracheal nodal stations, which correspond to levels 2 and 4 in the IASLC staging map[15]. Above the aortic arch, the anterior border of the CTV can be extended towards the sternum and clavicular heads in order to encompass the prevascular nodes (IASLC level 3) and create a smoother transition between the thoracic CTV and the supraclavicular nodal CTV. Above the level of the thoracic inlet, the trachea should be excluded from the CTV except insofar as the 1cm radial margin on the normal esophagus requires it.
PTV Guidelines
With respect to PTV delineation, the panel recommended expanding the CTV by 0.5-1cm in all directions, depending on institutional guidelines and the frequency of portal imaging. For tumors involving the distal esophagus and GE junction, it is critical that respiratory motion be taken into account when using highly conformal techniques such as IMRT. This should include, at a minimum, fluoroscopic or 4-dimensional CT imaging to estimate the degree of superior-inferior motion due to respiration, which can then be incorporated into the PTV expansion. For situations where respiratory motion is observed to be in excess of 1cm, the panel additionally recommends the use of techniques such as respiratory gating or abdominal compression. Variations in gastric filling may lead to significant intrafraction differences in the location of perigastric nodes, and dose to normal stomach. To mitigate this, most panelists recommended keeping patients NPO for 2-3 hours before simulation and each treatment. However, treating patients at a consistent interval after meals also appears to result in reproducible gastric positioning, and may be more comfortable for some patients.[18]
DISCUSSION
Though IMRT and other highly conformal radiotherapy techniques provide greater target volume conformality and dose homogeneity, as well as increased ability to control dose to adjacent normal structures, they also place a greater responsibility on the radiation oncologist to appropriately delineate the GTV, CTV and PTV at each axial level. Recent data indicates that patients found to have microscopic disease extending beyond CTV margins have inferior outcomes, underscoring the importance of accurate CTV delineation.[19] Existing guidelines have provided sufficient direction to design two-dimensional fields and blocks, but leave significant potential for uncertainty and variability when defining target volumes on cross-sectional imaging. This analysis indicates that expert gastrointestinal radiation oncologists can achieve a reasonable degree of consensus in contouring practice when starting from the same basic parameters (as outlined in CALGB 80803). Based on quantitative and qualitative analysis of each panelist’s contours for each test case, a contouring atlas and consensus guidelines could be generated.
A significant limitation of our guidelines is that they are based on expert consensus, and not directly on patient data regarding patterns of disease extension or recurrence relative to radiation treatment fields. Translating extant data on pathologic node involvement and patterns of failure directly into radiotherapy CTV guidelines is not straightforward, given the significant heterogeneity that exists in histology, anatomic site, T stage, and other factors that appear to modify the risk and location of lymph node involvement.
However, existing literature provides some support for our consensus guidelines. With respect to CTV margins on primary tumor, pathologic analysis of microscopic extension in resected tumor specimens indicates that proximal and distal mucosal margins of 3cm may be sufficient for the majority of cases to encompass submucosal spread of disease.[20] Clinicopathologic correlation of RT volumes with residual tumor location after surgery has also indicated that generous CTV margins are necessary to encompass the actual tumor within the RT field, since preoperative GTV delineation is frequently inaccurate.[19] A retrospective study of local relapse patterns after definitive chemoradiation also indicated that CTV margins on the order of 3cm appeared adequate.[21] To allow for variations in clinical judgment and potential uncertainty when using PET avidity to define GTV, we felt that a CTV range of 3-4cm was appropriate to insure adequate coverage of the primary tumor and any subclinical spread. Note that after adding a PTV margin of 0.5-1cm, and accounting for penumbra, this expansion is consistent with the traditional practice of expanding the GTV 5cm to block edge.
With respect to nodal target volumes, there is retrospective and limited prospective data suggesting no clear benefit to elective nodal irradiation. [22-24] However, these datasets are based on squamous cell carcinomas in Asian populations, which may not be fully applicable to the distal adenocarcinomas that are the typical subject of current multicenter trials. A recent analysis of nodal involvement in a large series of resected squamous cell carcinoma patients also supports the concept of elective mediastinal and supraclavicular node coverage in locally advanced proximal tumors.[25] Regarding celiac nodal coverage, a contemporary series of mostly adenocarcinomas treated with definitive chemoradiation indicated a significant enough failure rate to justify elective coverage, particularly given modest rates of associated toxicity with celiac irradiation.[26] A large series of resected GE junction adenocarcinomas also indicated a rate of celiac nodal involvement of approximately 20%, which is at or above the threshold that many practitioners use consider to justify elective coverage.[27]
Pathologic data also indicate that the most commonly involved nodes in GE junction cancers include the lesser curvature and paracardial regions, which are encompassed in our guidelines describing coverage of the gastrohepatic space.[27,28] These studies also indicate, as expected, that there is a significant rate of involvement of the paraesophageal nodes. For the case of GE junction tumors in particular, there is also a lower but still substantial risk of pathologic involvement of additional abdominal nodal sites such as the greater curvature and splenic hilum nodes.[27] Because inclusion of these sites will significantly expand the CTV volume and increase dose to the stomach and left kidney, the panel did not recommend routinely treating these regions to a dose of 5040cGy, but felt that this could be considered on a case-by-case basis if treating preoperatively to 4500cGy or lower. We note that the evidence for benefit from routine elective nodal irradiation of any kind remainsinconclusive, and if further study demonstrates that it can be safely omitted in distal esophageal and GEJ cancers, the therapeutic ratio of radiotherapy should improve.
Until more data are available, ideally prospective data on patterns of failure in esophageal and GEJ adenocarcinoma, expert consensus guidelines will remain the most useful aids to promote optimal radiotherapy technique in clinical trials and general practice. The EORTC has published radiotherapy guidelines for GEJ cancers that are broadly concurrent with our guidelines, but no contouring atlas is provided, and esophageal cancers proximal to the GEJ are not addressed.[29] To our knowledge, no other peer-reviewed, consensus guidelines for cross-sectional delineation of esophageal cancers exist. Besides CALGB 80803, the other major ongoing North American trial of esophageal cancer chemoradiation is RTOG 1010, which provides similarly basic guidelines that are insufficiently detailed to ensure consistent CTV definition.
Due to the anatomic heterogeneity of esophageal cancer, in which the primary tumor and involved lymph nodes can present in multiple disparate regions in the body, it is impossible to provide one or a few reference contours that will cover every possible presentation of esophageal or GE junction cancer. Therefore, these atlas contours and guidelines should not be a substitute for clinical judgment based on individualized analysis of each patient.
CONCLUSIONS
A reference contouring atlas and contouring guidelines have been generated for thoracic esophageal and gastro-esophageal junction (Siewert I/II) cancers. This atlas will serve as a reference within the treatment planning process for patients being treated on prospective trials of radiotherapy in esophageal cancer, and can also be used for patients being treated with IMRT or other highly conformal techniques in routine clinical practice.
Supplementary Material
SUMMARY.
Standardized, cross-sectional contouring guidelines are needed due to the increasing use of highly conformal radiotherapy techniques in esophageal and gastroesophageal junction cancer. Eight academic gastrointestinal radiation oncologists each generated contours for three representative cases; these were analyzed to generate a consensus atlas. The panel also reached agreement on general guidelines and principles for contouring, depending on the location of the primary lesion within the esophagus.
Figure 4.
Consensus contours for T3N1 distal esophageal cancer
Acknowledgments
The authors would like to thank Cesar Della-Bianca and Stephen McNamara for their technical assistance on this project.
Supported by grant U24CA81647 and U24 CA180803 from the National Cancer Institute.
Appendix 1. CALGB 80803 protocol treatment volumes (section 8.3.3)
GTV
The GTV is based on the pre-chemotherapy extent of disease using the initial PET/CT scan, endoscopy report, and CT scan. The entire esophageal wall, including any disease that has extended through the wall should be contoured as the GTV as well as any PET/CT-avid or enlarged lymph nodes.
CTV
The intent of pre-operative treatment is to include the tumor bed plus the nodal groups at risk (whether clinically positive or negative). The clinical target volume (CTV) should encompass the peri-esophageal lymph nodes, mediastinal lymph nodes for mid- and upper- thoracic esophageal tumors, and the submucosal spread longitudinally along the esophagus. This is generally a 3-4 cm expansion on the GTV superiorly and inferiorly and 1.0 cm expansion radially. For distal esophageal tumors and GE junction tumors, the CTV should include the celiac lymph nodes. For tumors above the carina, the supraclavicular lymph nodes should be included in the CTV.
Appendix 2. Clinical vignettes for sample cases
Patient 1 (GE junction case)
36 year-old man diagnosed with uT3N0, Siewert 2 adenocarcinoma with squamous features, starting 41cm from incisors and extending into the GE junction and cardia.
Patient 2 (distal esophagus case)
59 year-old man diagnosed with uT3N1 adenocarcinoma of the distal esophagus, 33-39cm from incisors, with involved gastrohepatic node.
Patient 3 (proximal esophagus case)
78 year-old man diagnosed with uT3N1 squamous cell carcinoma, 25-30cm from incisors, with involved upper paratracheal node.
Instructions to panelists
1) Referring to the provided diagnostic PET-CT images (and ignoring the provided GTV), contour what you would consider GTV.
2) Then, using the provided GTV, contour your CTV using the guidelines from the CALGB 80803 protocol. Assume that all patients will receive 5040cGy in 28 fractions with concurrent chemotherapy (either carboplatin/paclitaxel or FOLFOX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented, in part, at the 55th Annual Meeting of the American Society of Radiation Oncology (ASTRO), Atlanta, GA
REFERENCES
- 1.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr., Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (rtog 85-01). Radiation therapy oncology group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 2.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A, Group C. Preoperative chemoradiotherapy for esophageal or junctional cancer. The New England journal of medicine. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 3.Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. The New England journal of medicine. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 4.Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R. Phase iii trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: Calgb 9781. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra A, Guerrero TM, Liu HH, Tucker SL, Liao Z, Wang X, Murshed H, Bonnen MD, Garg AK, Stevens CW, Chang JY, Jeter MD, Mohan R, Cox JD, Komaki R. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2005;77:247–253. doi: 10.1016/j.radonc.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Kole TP, Aghayere O, Kwah J, Yorke ED, Goodman KA. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. International journal of radiation oncology, biology, physics. 2012;83:1580–1586. doi: 10.1016/j.ijrobp.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 7.Lin SH, Wang L, Myles B, Thall PF, Hofstetter WL, Swisher SG, Ajani JA, Cox JD, Komaki R, Liao Z. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. International journal of radiation oncology, biology, physics. 2012;84:1078–1085. doi: 10.1016/j.ijrobp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolini G, Ghosh-Laskar S, Shrivastava SK, Banerjee S, Chaudhary S, Agarwal JP, Munshi A, Clivio A, Fogliata A, Mancosu P, Vanetti E, Cozzi L. Volumetric modulation arc radiotherapy with flattening filter-free beams compared with static gantry imrt and 3d conformal radiotherapy for advanced esophageal cancer: A feasibility study. International journal of radiation oncology, biology, physics. 2012;84:553–560. doi: 10.1016/j.ijrobp.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 9.Fuller CD, Nijkamp J, Duppen JC, Rasch CR, Thomas CR, Jr., Wang SJ, Okunieff P, Jones WE, 3rd, Baseman D, Patel S, Demandante CG, Harris AM, Smith BD, Katz AW, McGann C, Harper JL, Chang DT, Smalley S, Marshall DT, Goodman KA, Papanikolaou N, Kachnic LA, Radiation Oncology Committee of the Southwest Oncology G Prospective randomized double-blind pilot study of site-specific consensus atlas implementation for rectal cancer target volume delineation in the cooperative group setting. International journal of radiation oncology, biology, physics. 2011;79:481–489. doi: 10.1016/j.ijrobp.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mavroidis P, Giantsoudis D, Awan MJ, Nijkamp J, Rasch CR, Duppen JC, Thomas CR, Jr., Okunieff P, Jones WE, 3rd, Kachnic LA, Papanikolaou N, Fuller CD, Southwest Oncology Group Radiation Oncology C Consequences of anorectal cancer atlas implementation in the cooperative group setting: Radiobiologic analysis of a prospective randomized in silico target delineation study. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2014 doi: 10.1016/j.radonc.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viera AJ, Garrett JM. Understanding interobserver agreement: The kappa statistic. Fam Med. 2005;37:360–363. [PubMed] [Google Scholar]
- 12.Allozi R, Li XA, White J, Apte A, Tai A, Michalski JM, Bosch WR, El Naqa I. Tools for consensus analysis of experts' contours for radiotherapy structure definitions. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2010;97:572–578. doi: 10.1016/j.radonc.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (staple): An algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 15.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, Members of ISC The iaslc lung cancer staging project: A proposal for a new international lymph node map in the forthcoming seventh edition of the tnm classification for lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2009;4:568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 16.Gregoire V, Levendag P, Ang KK, Bernier J, Braaksma M, Budach V, Chao C, Coche E, Cooper JS, Cosnard G, Eisbruch A, El-Sayed S, Emami B, Grau C, Hamoir M, Lee N, Maingon P, Muller K, Reychler H. Ct-based delineation of lymph node levels and related ctvs in the node-negative neck: Dahanca, eortc, gortec, ncic,rtog consensus guidelines. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2003;69:227–236. doi: 10.1016/j.radonc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Rice TW, Blackstone EH, Rusch VW. 7th edition of the ajcc cancer staging manual: Esophagus and esophagogastric junction. Annals of surgical oncology. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 18.Assessment of nonrespiratory stomach motion in healthy volunteers in fasting and postprandial states. Pract Radiat Oncol. 2014;4:288–293. doi: 10.1016/j.prro.2013.10.001. B. W, J. M, K. B, G. L, G. W, A. S, M.A. H, C. M, J. B, L.A. D, J. R. [DOI] [PubMed] [Google Scholar]
- 19.Muijs C, Smit J, Karrenbeld A, Beukema J, Mul V, van Dam G, Hospers G, Kluin P, Langendijk J, Plukker J. Residual tumor after neoadjuvant chemoradiation outside the radiation therapy target volume: A new prognostic factor for survival in esophageal cancer. International journal of radiation oncology, biology, physics. 2014;88:845–852. doi: 10.1016/j.ijrobp.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Gao XS, Qiao X, Wu F, Cao L, Meng X, Dong Z, Wang X, Gao G, Wu TT, Komaki R, Chang JY. Pathological analysis of clinical target volume margin for radiotherapy in patients with esophageal and gastroesophageal junction carcinoma. International journal of radiation oncology, biology, physics. 2007;67:389–396. doi: 10.1016/j.ijrobp.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Button MR, Morgan CA, Croydon ES, Roberts SA, Crosby TD. Study to determine adequate margins in radiotherapy planning for esophageal carcinoma by detailing patterns of recurrence after definitive chemoradiotherapy. International journal of radiation oncology, biology, physics. 2009;73:818–823. doi: 10.1016/j.ijrobp.2008.04.062. [DOI] [PubMed] [Google Scholar]
- 22.Hsu FM, Lee JM, Huang PM, Lin CC, Hsu CH, Tsai YC, Lee YC, Chia-Hsien Cheng J. Retrospective analysis of outcome differences in preoperative concurrent chemoradiation with or without elective nodal irradiation for esophageal squamous cell carcinoma. International journal of radiation oncology, biology, physics. 2011;81:e593–599. doi: 10.1016/j.ijrobp.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Ma JB, Song YP, Yu JM, Zhou W, Cheng EC, Zhang XQ, Kong L. Feasibility of involved-field conformal radiotherapy for cervical and upper-thoracic esophageal cancer. Onkologie. 2011;34:599–604. doi: 10.1159/000334194. [DOI] [PubMed] [Google Scholar]
- 24.Zhao KL, Ma JB, Liu G, Wu KL, Shi XH, Jiang GL. Three-dimensional conformal radiation therapy for esophageal squamous cell carcinoma: Is elective nodal irradiation necessary? International journal of radiation oncology, biology, physics. 2010;76:446–451. doi: 10.1016/j.ijrobp.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 25.Cheng J, Kong L, Huang W, Li B, Li H, Wang Z, Zhang J, Zhou T, Sun H. Explore the radiotherapeutic clinical target volume delineation for thoracic esophageal squamous cell carcinoma from the pattern of lymphatic metastases. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:359–365. doi: 10.1097/JTO.0b013e31827e1f6d. [DOI] [PubMed] [Google Scholar]
- 26.Amini A, Xiao L, Allen PK, Suzuki A, Hayashi Y, Liao Z, Hofstetter W, Crane C, Komaki R, Bhutani MS, Lee JH, Ajani JA, Welsh J. Celiac node failure patterns after definitive chemoradiation for esophageal cancer in the modern era. International journal of radiation oncology, biology, physics. 2012;83:e231–239. doi: 10.1016/j.ijrobp.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier I, Merkel S, Papadopoulos T, Sauer R, Hohenberger W, Brunner TB. Adenocarcinoma of the esophagogastric junction: The pattern of metastatic lymph node dissemination as a rationale for elective lymphatic target volume definition. International journal of radiation oncology, biology, physics. 2008;70:1408–1417. doi: 10.1016/j.ijrobp.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 28.Dresner SM, Lamb PJ, Bennett MK, Hayes N, Griffin SM. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery. 2001;129:103–109. doi: 10.1067/msy.2001.110024. [DOI] [PubMed] [Google Scholar]
- 29.Matzinger O, Gerber E, Bernstein Z, Maingon P, Haustermans K, Bosset JF, Gulyban A, Poortmans P, Collette L, Kuten A. Eortc-rog expert opinion: Radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;92:164–175. doi: 10.1016/j.radonc.2009.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.