Abstract
Since arsenic trioxide (As3+) has been successfully used in the treatment of acute promyelocytic leukemia (APL), its adverse effects on patients have been problematic and required a solution. Considering the good therapeutic potency and low toxicity of tetraarsenictetrasulfide (As4S4) in the treatment of APL, we investigated the effects of combining As4S4 and As3+ on the apoptosis and differentiation of NB4 and primary APL cells. As4S4, acting similarly to As3+, arrested the G1/S transition, induced the accumulation of cellular reactive oxygen species, and promoted apoptosis. Additionally, low concentrations of As4S4 (0.1–0.4 μM) induced differentiation of NB4 and primary APL cells. Compared with the As4S4- or As3+-treated groups, the combination of As4S4 and As3+ obviously promoted apoptosis and differentiation of NB4 and primary APL cells. Mechanistic studies suggested that As4S4 acted synergistically with As3+ to down-regulate Bcl-2 and nuclear factor-κB expression, up-regulate Bax and p53 expression, and induce activation of caspase-12 and caspase-3. Moreover, the combination of low concentrations of As4S4 and As3+ enhanced degradation of the promyelocytic leukemia-retinoic acid receptor α oncoprotein. In summary, As4S4 and As3+ synergistically induce the apoptosis and differentiation of NB4 and primary APL cells.
Introduction
Acute promyelocytic leukemia (APL) is an M3 subtype of acute myeloid leukemia [1]. The typical characteristic of APL is the specific chromosomal translocation t(15;17) (q22;q21), which induces the expression of the promyelocytic leukemia-retinoic acid receptor α (PML-RARα) oncoprotein [1–3]. Two drugs, all-trans retinoic acid and arsenic trioxide (As3+), have hitherto been successfully used in the treatment of APL [4–6]. At high concentrations (0.5–2.0 μM), As3+ triggers apoptosis, and at low concentrations (0.1–0.5μM) it induces partial differentiation of APL cells [7]. Mechanistic studies have suggested that As3+ promotes apoptosis and differentiation of APL cells by inducing degradation of the PML-RARα oncoprotein [8]. However, As3+ organ injury, especially to the liver and kidneys, causes significant pain to patients [9,10]. Methylation of As3+ can induce the accumulation of reactive oxygen species (ROS) and generate more toxic monomethylarsonous and dimethylarsinous acids [11–14]. Currently, combination therapy is widely used in cancer treatment. Therefore, combination therapy for APL treatment can enhance As3+ therapeutic potency and reduce its adverse effects.
In addition to As3+, realgar is another inorganic form of arsenic that has been used in traditional Chinese medicine for many years [15,16]. Compared with As3+, realgar has a positive therapeutic reputation and reduced toxicity [17]. Lu et al. have reported that when used alone, the major constituent of realgar, tetraarsenic tetrasulfide (As4S4), showed high efficiency and safety in all stages of APL [18]. Wang et al. showed that the combination of As4S4, indigo and naturalis can promote the differentiation of APL cells, induce degradation of the PML-RARα fusion protein, and arrest the cell cycle at G1/G0 [19]. However, the molecular mechanism of As4S4 potency in APL treatment is unclear. Moreover, realgar is a mixture that contains up to approximately 10% trivalent arsenicals [18], and both bivalent and trivalent arsenicals may contribute to the therapeutic potency of realgar. Clarifying the mechanism of action of As4S4 and As3+ combination on the apoptosis and differentiation of APL cells is necessary.
Apoptosis is the major pathway for drug-induced cancer cell death [20]. Mitochondria-mediated intrinsic apoptosis, death receptor-mediated extrinsic apoptosis and endoplasmic reticulum stress-mediated apoptosis are the three predominant apoptosis pathways and are regulated by a series of apoptotic factors [21]. Of these factors, Bcl-2 family members [22], nuclear factor-κB (NFκB) [23], p53 tumor suppressor [24], caspase-12 and caspase-3 play key roles in As3+-induced apoptosis [25]. Although the function of PML is unclear, degradation of the PML-RARα oncoprotein contributes to apoptosis and differentiation of APL cells [8,26]. These apoptotic factors, as well as the PML-RARα fusion protein, may be important for clarifying the mechanism of As4S4-induced apoptosis and differentiation in APL cells.
The NB4 cell line is a unique APL-derived cell line that expresses the PML-RARα oncoprotein [27]. In this work, we found that As4S4 and As3+ exerted synergistic effects on the apoptosis and differentiation of NB4 and primary APL cells. Multiple pathways were involved in As4S4 and As3+-induced apoptosis. As4S4 and As3+ acted synergistically to promote apoptosis of NB4 cells by up-regulating p53 expression, enhancing the mitochondria-mediated intrinsic pathway, enhancing the endoplasmic reticulum stress-mediated pathway, and inhibiting the NFκB signaling pathway. Moreover, low doses of As4S4 could be combined with As3+ to enhance degradation of the PML-RARα oncoprotein and promote NB4 and primary APL cell differentiation through the retinoic acid-signaling pathway.
Materials and Methods
Caution: Due to the potential risk of arsenic compounds, safeguards should be implemented [11–14].
Reagents
High purity As4S4 was obtained from Yiji industry (Shanghai, China). NaAsO2, bovine serum albumin (BSA), anti-PML rabbit mAb and anti-caspase-12 rabbit mAb were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-Bcl-2 (50E3) rabbit mAb, anti-Bax (D2E11) rabbit mAb, anti-NFκB p65 (D14E12) XP rabbit mAb, anti-caspase-3 (8G10) rabbit mAb and anti-p53 (7F5) rabbit mAb were obtained from Cell Signaling Technology (Boston, MA, USA). Anti-β-actin mouse mAb was purchased from Beyotime (Nantong, China). FITC anti-human CD11b antibody was obtained from BioLegend (San Diego, CA. USA).
Cell culture and growth assay
Human NB4 leukemia cells were purchased from SXBIO Biotech (Shanghai, China). Human primary APL cells were separated from the bone marrow of four primary APL patients acquired from DrumTower Hospital (Nanjing, China) by Ficoll-Hypaque density centrifugation as reported in the previously published article [28]. Written informed consent was obtained from individual subjects. For this case we did not seek approval of the Ethics Committee of Drum Tower Hospital and did not obtain a waiver from the Ethics Committee because the bone marrow was part of that acquired for clinical diagnosis and destroyed after this experiment. NB4 cells were cultured in RPMI-1640 (KeyGEN Biotech, China) with 10% fetal bovine serum (FBS) at 37°C under a 5% CO2 atmosphere. The fresh primary APL cells were cultured in RPMI-1640 with 15% FBS. Trypan blue exclusion was used to determine viability of NB4 and primary APL cells after 48 h and 96 h of culture [28]. The effects of As4S4 and As3+ on cell growth were determined using the WST-1 cell proliferation assay kit (KeyGEN Biotech, China). In brief, 4×104 cells/ml were seeded into a 96-well culture plate and treated with various concentrations of As4S4, As3+, or their combination for 48 h. Untreated cells served as controls [28,29].
Calculation of combination index (CI)
The CI values were calculated by equation (1): CI = [D]1/[Dx]1 + [D]2/[Dx]2 + α[D]1[D]2/[Dx]2[Dx]2 [30]. Here, [Dx]1 and [Dx]2 respectively represent the concentrations of As3+ and As4S4 alone, resulting in growth inhibition of NB4 and primary APL cells (in x%). [D]1 and [D]2 are the concentrations of As3+ and As4S4 when they are used in combination to inhibit the cell growth at same percentage (x%). When the two drugs are assumed to be non-exclusive, the value of α is 1; when the two drugs are assumed to be exclusive, the value of α is 0. CI = 1 indicate an additive effect; CI < 1indicate a synergistic effect; CI > 1 indicate an antagonism effect [30].
Analysis of apoptosis
The proportions of apoptotic cells were measured with the Annexin V-FITC and propidium iodide (PI) apoptosis detection kit (KeyGEN Biotech, China) using flow cytometry [28,29,31]. NB4 and primary APL cells were treated with 2.0 μM As4S4, 2.0 μM As3+, or 1.0 μM As4S4 and 1.0 μM As3+ for 48 h. After treatment, the cells were washed twice with Ca2+-free phosphate buffer (PBS), stained with Annexin V-FITC and PI in the dark at room temperature for 15 min, and detected using a BD LSRL Fortessa flow cytometer. The percentages of apoptotic cells were analyzed using the BD FACSDiva software.
Analysis of cell cycle distribution
The effects of As4S4 and As3+ on cell cycle distribution were determined using a PI cell cycle detection kit (KeyGEN Biotech, China) [28]. After treatment with As4S4 (2.0 μM), As3+ (2.0 μM), or a combination of 1.0 μM As4S4 and 1.0 μM As3+ for 48 h, NB4 and primary APL cells were collected, washed with Ca2+-free PBS and fixed with 70% ethanol at 4°C for 16 h. The fixed cells were then digested in PBS containing 0.5 mg/ml RNase (Sigma-Aldrich, USA) at 37°C for 30 min and stained with 0.05 mg/ml PI in the dark at room temperature for 30 min. Data on the cell cycle distribution were determined using the ModFit LT 3.3 software.
Analysis of cellular ROS levels using 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA)
Cellular ROS levels were assayed using DCFH-DA. DCFH-DA can be hydrolyzed to DCFH by esterases and oxidized to 2’,7’-dichlorofluorescein (DCF) by cellular ROS [32]. Thus, the DCF fluorescence intensity is positively correlated with cellular ROS levels. After treatment with As4S4 (0.5, 1.0 and 2.0 μM), As3+ (2.0 μM), or a combination of 1.0 μM As4S4 and 1.0 μM As3+ for 24 h and 36 h, NB4 and primary APL cells were washed twice with PBS and then incubated in RPMI-1640 medium containing 10.0 μM DCFH-DA at 37°C for 30 min. Excess probe was washed out with PBS, and the percentages of DCF-positive cells were detected by flow cytometry and analyzed using FlowJo.7.6.
Analysis of mRNA by RT-PCR
RNAiso Plus (Takara-Bio, Japan) was used to extract total RNA from NB4 and primary APL cells. The concentration and purity of the isolated total RNA were determined by trace nucleic acid protein measurement instrument (NanoDrop ND-1000) [28,29]. 2.0 μg of total RNA was reverse-transcribed to cDNA, and 2.0 μL of transcribed cDNA was used for PCR amplification with specific primers. After initial denaturing at 94°C for 5 min, thirty cycles of 30 s denaturation at 94°C; 30 s annealing at 52°C (β-actin), 57°C (heme oxygenase-1 (HMOX1), Bax, Bcl-2, and NFκB-3), or 51°C (caspase-3); and 30 s extension at 72°C were performed. The PCR products were separated on 1% agarose gels containing ethidium bromide. The separated bands were imaged on a Gel Doc XR System (Bio-Rad). The primer sequences are shown in Table 1.
Table 1. Primer sequences for the apoptosis factors in RT-PCR.
| Name | Primer sequence | |
|---|---|---|
| HMOX1 | sense | 5′-CTTTGAGGAGTTGCAGGAGC-3′ |
| antisense | 5′-TGTAAGGACCCATCGGAGAA-3′ | |
| Bax | sense | 5′-TGACGGCAACTTCAACTGGG-3′ |
| antisense | 5′-AGCACTCCCGCCACAAAGA-3′ | |
| Bcl-2 | sense | 5′-GGGAGGATTGTGGCCTTCTT-3′ |
| antisense | 5′-GGCCAAACTGAGCAGAGTCTTC-3′ | |
| NFκB-3 | sense | 5′-ACTACGAGGGACCAGCCAAGA-3′ |
| antisense | 5′-CGCAGCCGCACTATACTCA-3′ | |
| Caspase-3 | sense | 5′-GTGGAATTGATGCGTGATG-3′ |
| antisense | 5′-AACCAGGTGCTGTGGAGTA-3′ | |
| β-Actin | sense | 5′-GACCTGACTGACTACCTC-3′ |
| antisense | 5′-TCTTCATTGTGCTGGGTGC-3′ | |
Protein analysis by western-blot
After treatment with As4S4, As3+ or a combination, NB4 and primary APL cells were collected, washed twice with PBS, and then lysed in ice-cold RIPA cell lysis buffer (Beyotime, China) containing 1.0 mM PMSF for 60 min to extract total cellular protein [28,29]. The concentration of total protein was determined using the BCA protein quantification kit (Beyotime, China). 25.0 μg of total protein from each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a PVDF membrane (Millipore, USA).The membrane was then blocked with 5% skim milk at room temperature for 60 min and sequentially incubated with primary and secondary antibodies. Proteins on the PVDF membrane were visualized using chemiluminescent HRP substrate (Millipore, USA). The band intensities were corrected using the β-actin intensities. All experiments were repeated at least three times.
Analysis of cell differentiation
We analyzed NB4 and primary APL cell differentiation with an FITC anti-human CD11b antibody using flow cytometry [19]. After treatment with As4S4, As3+, or a combination for 96 h, cells were harvested, washed twice with PBS and counted. A total of 1.0×106 cells in 100 μl PBS were incubated with 20 μl FITC anti-human CD11b antibody at 4°C for 30 min. Excess antibody was washed out, and the percentages of FITC-CD11b-positive cells were analyzed using FlowJo.7.6.
Statistical analysis
Two-tailed Student’s t-tests were performed for comparisons of two groups, and P<0.05 was considered to be statistically significant.
Results
As4S4 enhances As3+-inhibition of NB4 and primary APL cell growth
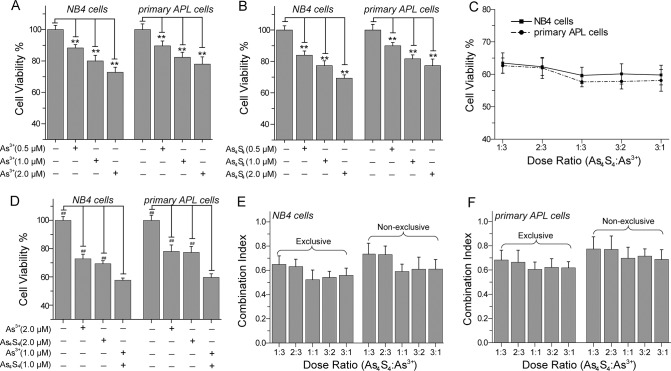
The viability of NB4 and primary APL cells was determined by trypan blue exclusion [28]. Following 48 h of treatment, 97.5% of NB4 cells and 97.0% of primary APL cells were viable. The effects of As4S4, As3+, or their combination on NB4 and primary APL cell proliferation were analyzed using the WST-1 cell proliferation assay. After 48 h of treatment, As3+ and As4S4 obviously inhibited the growth of NB4 and primary APL cells (Fig 1A and 1B). The inhibitory potency of As3+ and As4S4 on cell growth increased with the increasing of concentration from 0.5 μM to 2.0 μM (Fig 1A and 1B). Subsequently, we investigated the combination effects of As3+ (0.5–1.5 μM) and As4S4 (0.5–1.5 μM) at fixed dose ratio (As4S4/As3+ were 1/3, 2/3, 1/1, 3/2, and 3/1) on the growth of NB4 and primary APL cells (Fig 1C). In our tested range of dose ratios, combining As4S4 and As3+ obviously inhibited the growth of NB4 and primary APL cells (Fig 1C). Compared with 2.0 μM As3+ alone or 2.0 μM As4S4 alone treated group, the combination of 1.0 μM As3+ and 1.0 μM As4S4 markedly induced the death of NB4 and primary APL cells (Fig 1D). In order to clarify whether As3+ and As4S4 exerted synergistic effects on cell growth, we analyzed CI values of the two drugs at different dose ratios (Fig 1E and 1F). When the dose ratio of As4S4/As3+ was in the range of 1:3 to 3:1, the combination of As3+ and As4S4 yielded a moderate synergistic effect (0.6≤CI<0.8) on the growth of NB4 and primary APL cells (Fig 1E and 1F) [30].
Fig 1. The effects of combining As3+ and As4S4 on the growth of NB4 and primary APL cells.
(A) The effects of As3+ on cell viability. (B) The effects of As4S4 on cell viability. (C&D) The combined effects of As3+ and As4S4 on cell viability. (E) CI of concurrent treatment with As3+ and As4S4 in NB4 cells. (F) CI in primary APL cells. CI<1.0 indicated a synergistic effect. The viability of NB4 and primary APL cells were determined by WST-1 cell proliferation assay kit after 48 h of treatment. Error bars represent the S.D. from the mean of three separate experiments. **P<0.01 compared with the control. ##P<0.01 compared with As3+ and As4S4 combination treated cells.
As4S4 acts synergistically with As3+ to promote the apoptosis of NB4 and primary APL cells
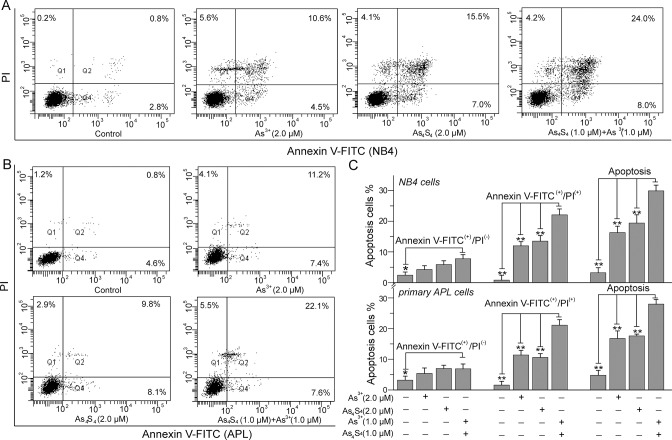
Cell death comprises two major pathways: apoptosis and necrosis [20]. We investigated the effects of As4S4 on As3+-induced apoptosis in NB4 and primary APL cells by flow cytometry. As shown in Fig 2, Q2 and Q4 represent the percentages of Annexin V-FITC(+)/PI(+) and Annexin V-FITC(+)/PI(-) cells, respectively. After 48 h of treatment, both As3+ and As4S4 obviously induced NB4 cell apoptosis (Fig 2A). Compared with the control, the percentage of apoptotic cells in the group treated with 2.0 μM As4S4 increased from 3.7±1.3% to 19.4±2.6%, and the percentage reached 16.3±2.0% in the 2.0 μM As3+-treated group. Subsequently, we investigated the effects of combining 1.0 μM As4S4 and 1.0 μM As3+ on NB4 cell apoptosis. Compared with the groups treated with either 2.0 μM As4S4 or 2.0 μM As3+, the combination of 1.0 μM As4S4 and 1.0 μM As3+ markedly increased the proportion of apoptotic cells to 29.9±1.9% (Fig 2A and 2C). Similarly, As4S4 acted synergistically effects with As3+ on primary APL cell apoptosis (Fig 2B). Compared with the control, 2.0 μM As4S4 increased the percentage of apoptotic cells from 4.8±1.6% to 17.6±0.7%, and 2.0 μM As3+ increased the percentage of apoptotic cells to 16.8±2.6%. Furthermore, 1.0 μM As4S4 and 1.0 μM As3+ acted synergistically to increase the percentage of apoptotic cells to 28.0±1.5% (Fig 2B and 2C). The combination of As4S4 and As3+ synergistically promoted NB4 and primary APL cell apoptosis.
Fig 2. The effects of combining As3+ and As4S4 on the apoptosis of NB4 and primary APL cells.
(A) The apoptosis of NB4 cells. (B) The apoptosis of primary APL cells. (C) The percentage of apoptotic cells in NB4 and primary APL cells. After 48 h of treatment, the cells were stained with Annexin V-FITC and PI. Q1 and Q3, represent the dead cells and living cells, respectively. Q2 and Q4 were used to calculate the proportion of apoptotic cells. Figures show a representative experiment of three independent experiments. *P<0.05 and **P<0.01 compared with As3+ and As4S4 combination treated cells.
As4S4 and As3+ act synergistically to arrest the cell cycle in G0/G1 phase
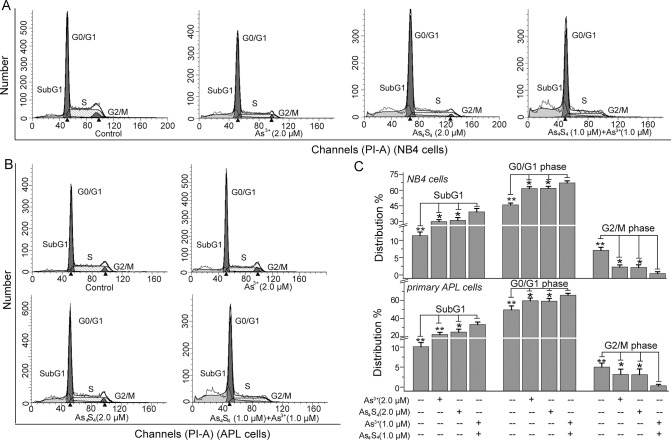
The cell cycle is a highly regulated process, and aberrations in cell cycle distribution can induce abnormal cell changes such as apoptosis and differentiation [33,34]. The SubG1 content represents the percentage of apoptotic cells [35]. In agreement with the results of the Annexin V-FITC and PI staining, As3+ and As4S4 obviously increased the SubG1 contents (Fig 3). Furthermore, As4S4 enhanced As3+-induced apoptosis in NB4 and primary APL cells (Fig 3A and 3B). Compared with the groups treated with either 2.0 μM As3+ or 2.0 μM As4S4, combined treatment with 1.0 μM As3+ and 1.0 μM As4S4 for 48 h resulted in the increasing of SubG1 content (Fig 3).
Fig 3. The effects of combining As3+ and As4S4 on cell cycle distribution.
(A) Cell cycle distribution in NB4 cells. (B) Cell cycle distribution in primary APL cells. (C) The percentage of cell cycle distribution in each phase. After 48 h of treatment, NB4 and primary APL cells were stained with PI and analyzed by flow cytometry. Figures show a representative experiment of three independent experiments. *P<0.05 and **P<0.01 compared with As3+ and As4S4 combination treated cells.
As3+ inhibited the G1/S and S/G2 transitions of NB4 and primary APL cells (Fig 3). In NB4 cells, As3+ increased the percentage of DNA at G0/G1 phase from 45.9±1.85% to 61.5±1.9%, and the percentage at G2/M phase decreased from 7.1±0.9% to 2.3±0.6% (Fig 3A and 3C). In primary APL cells, As3+ increased the percentage of DNA at G0/G1 phase from 45.9±1.85% to 61.9±1.8%, and the percentage at G2/M phase decreased from 7.1±0.9% to 2.1±0.8% (Fig 3B and 3C). As4S4, acting similarly to As3+, increased the percentage of DNA at G0/G1 phase and decreased the percentage at G2/M phase (Fig 3). Compared with 2.0 μM As3+ alone or 2.0 μM As4S4 alone treated group, the combination of 1.0 μM As4S4 and 1.0 μM As3+ obviously blocked the G1/S transition, as the DNA content at G0/G1 phase respectively reached 67.0±2.1% and 65.9±2.0% in NB4 and primary APL cells (Fig 3).
As4S4 has no obvious effects on As3+-induced ROS accumulation
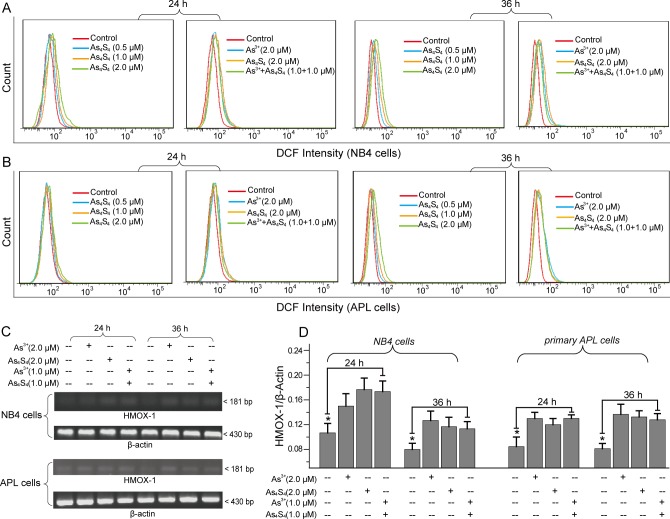
As3+-induced apoptosis is related to cellular ROS accumulation [7,24]. To investigate whether As4S4-induced apoptosis in NB4 and primary APL cells is related to ROS generation, we detected cellular ROS with DCFH-DA fluorescence probe by flow cytometry. The results suggested that As4S4 obviously increased the percentage of DCF-positive cells, indicating an increase in cellular ROS levels (Fig 4A and 4B) [32]. Compared with the 2.0 μM As3+- or 2.0 μM As4S4-treated groups, the combination of 1.0 μM As3+ and 1.0 μM As4S4 did not obviously increase the percentage of DCF-positive cells (Fig 4A and 4B). We also analyzed the expression of HMOX1, a key oxidative stress response enzyme, to examine the effects of combining As3+ and As4S4 on cellular ROS [36]. RT-PCR analysis showed that 2.0 μM As3+ and 2.0 μM As4S4 up-regulated HMOX1 expression (Fig 4C). However, HMOX1 expression was not significantly different between cells treated with 2.0 μM As3+ and cells treated with both 1.0 μM As3+ and 1.0 μM As4S4 (Fig 4C and 4D). Notably, As4S4 induced ROS accumulation in NB4 and primary APL cells but did not affect As3+-induced ROS accumulation.
Fig 4. The effects of combining As3+ and As4S4 on cellular ROS accumulation.
(A) Cellular ROS were determined with DCFH-DA fluorescence probe in NB4 cells. (B) Cellular ROS in primary APL cells. (C) The effects of As4S4 and As3+ on HMOX1 expression. (D) The percentage of relative HMOX1 intensity obtained by RT-PCR. Error bars represent the S.D. from the mean of three independent experiments. *P<0.05 compared with As3+ and As4S4 combination treated cells.
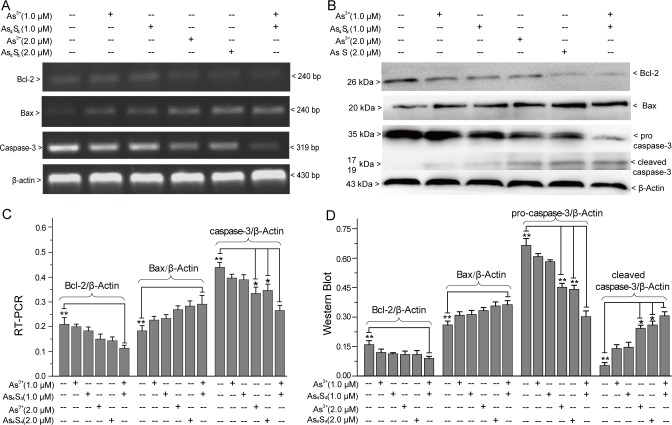
As4S4 and As3+ regulate apoptotic factor expression
Bax, Bcl-2, caspase-3, NFκB, p53 and caspase-12 are the six factors that play key roles in As3+-induced cell apoptosis [21–25]. To clarify the mechanism of apoptosis, we investigated the effects of combining As4S4 and As3+ on the expression of these factors. RT-PCR and western-blot analysis suggested that both As4S4 and As3+ up-regulated the expression of pro-apoptotic factor Bax, down-regulated the expression of anti-apoptotic factor Bcl-2, and induced the activation of caspase-3 (Fig 5A and 5B). Compared with 2.0 μM As3+ alone or 2.0 μM As4S4 alone treated group, the combination of 1.0 μM As4S4 and 1.0 μM As3+ enhanced caspase-3 activation. However, their combined effects on Bax and Bcl-2 expression were not obvious (Fig 5C and 5D).
Fig 5. The effects of combining As4S4 and As3+ on mitochondria-mediated apoptosis.
(A) RT-PCR analysis of Bax, Bcl-2 and caspase-3 expression. (B) Western-blot analysis of Bax, Bcl-2 and caspase-3 expression. (C) Relative intensity of expression obtained by RT-PCR. (D) Relative intensity expression obtained by western-blot. Error bars represent the S.D. from the mean of three separate experiments. *P<0.05 and **P<0.01 compared with As3+ and As4S4 combination treated cells.
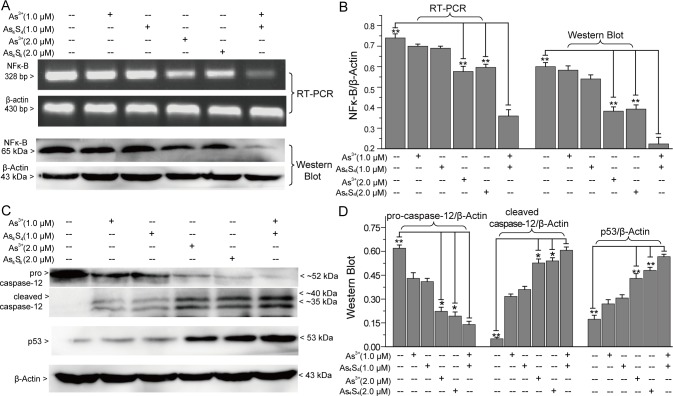
Subsequently, we investigated the effects of As4S4 and As3+ on NFκB, caspase-12 and p53 expression (Fig 6). As4S4 inhibited NFκB expression similarly to As3+ (Fig 6A and 6B). Moreover, As4S4 induced caspase-12 activation and promoted p53 tumor suppressor expression (Fig 6C and 6D). Compared with 2.0 μM As3+ alone or 2.0 μM As4S4 alone treated group, the addition of 1.0 μM As4S4 obviously promoted the regulation of 1.0 μM As3+ on NFκB, caspase-12 and p53 (Fig 6).
Fig 6. The effects of combining As4S4 and As3+ on NFκB, caspase-12 and p53 expression.
(A) RT-PCR and Western-blot analysis of NFκB expression. (B) Relative NFκB intensity obtained by RT-PCR and western-blot. (C) The effects of As4S4 and As3+ on caspase-12 and p53 expression. (D) Relative intensities of caspase-12 and p53 obtained by western-blot. Error bars represent the S.D. from the mean of three separate experiments. *P<0.05 and **P<0.01 compared with As3+ and As4S4 combination treated cells.
Low concentrations of As4S4 promote As3+-induced cell differentiation
Previous research suggested that As4S4 could induce NB4 cell differentiation [19]. However, the effects of As4S4 on As3+-induced NB4 cell differentiation have not been clarified. Herein, we investigated the effects of combining As4S4 and As3+ on NB4 and primary APL cell differentiation. As shown in Fig 7A, 0.1–0.4 μM As4S4 induced NB4 cell differentiation. When the concentration of As4S4 was greater than 0.3 μM, the percentage of CD11b-positive cells did not increase with the increasing of As4S4 concentration any more (Fig 7A). Compared with 0.4 μM As3+ alone- or 0.4 μM As4S4 alone-treated groups, the combination of 0.2 μM As4S4 and 0.2 μM As3+ obviously promoted NB4 cell differentiation (Fig 7B). Similarly, 0.1–0.4 μM As4S4 induced primary APL cell differentiation. Compared with the 0.4 μM As3+ and 0.4 μM As4S4 treatments, the percentage of CD11b-positive cells was obviously increased in the group treated with both 0.2 μM As4S4 and 0.2 μM As3+; however, the percentage of CD11b-positive cells was not increased in the group treated with 0.4 μM As4S4 and 0.4 μM As3+ (Fig 7C and 7D). Further investigation suggested that 0.1–0.25 μM As3+ and 0.1–0.25 μM As4S4 synergistically promoted the differentiation of NB4 and primary APL cells (data not show). The formation of the PML-RARα oncoprotein blocks NB4 and primary APL cell differentiation [8]. Therefore, we studied the effects of combining As4S4 and As3+ on PML-RARα expression by western-blot. After 96 h of treatment, 0.2 and 0.4 μM As4S4 induced PML-RARα oncoprotein degradation. Furthermore, As4S4 enhanced the As3+-induced degradation of the PML-RARα fusion protein in NB4 and primary APL cells (Fig 7E).
Fig 7. As4S4 acts synergistically with As3+ to affect NB4 and primary APL cell differentiation.
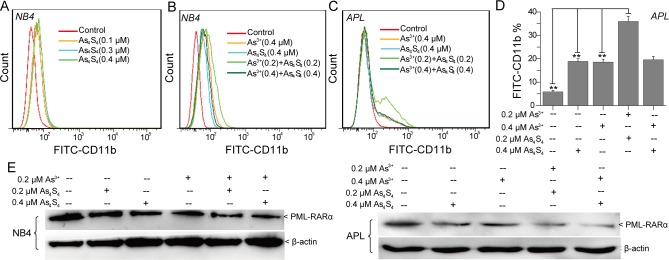
(A) The effects of As4S4 on CD11b expression in NB4 cells. (B) The effects of As4S4 on As3+-induced differentiation in NB4 cells. (C) The effects of As4S4 on As3+-induced differentiation in primary APL cells. (D) The percentage of FITC-CD11b-positive primary APL cells. (E) Western-blot analysis of PML-RARα expression in NB4 and primary APL cells. The figures show a representative experiment of three independent experiments. **P<0.01 compared with 0.2 μM As3+ and 0.2 μM As4S4 combination treated cells.
Discussion
As4S4, the major constituent of realgar, is another arsenic drug that has been used in traditional Chinese medicine for many years [16]. Since As3+ has been successfully used in APL treatment, the therapeutic potency of As4S4 in APL treatment has also been investigated [18,19]. The toxicity of ATO to organs, especially the liver and kidney, causes pain to patients [9,10]. As4S4 has low toxicity and good therapeutic potential for APL treatment, but it could not replace As3+. Pastorek M et al. have reported that the combination of As4S4 nanoparticles and As3+ induced dose-dependent activation of autophagy and apoptosis in melanoma cell lines [37]. Thus, Low concentrations of As3+ (0.1–1.0 μM) in combination with As4S4 (0.1–1.0 μM) may enhance the therapeutic efficacy of As3+ and reduce its adverse effects in the treatment of APL. Herein, we found that As4S4 and As3+ induced the apoptosis and differentiation of NB4 and primary APL cells in dose-dependent manner: 0.5–1.0 μM As3+ and 0.5–1.0 μM As4S4 synergistically promoted cell apoptosis; 0.1–0.25 μM As3+ and 0.1–0.25 μM As4S4 exerted synergistic effects on induction of cell differentiation.
To investigate the mechanism of apoptosis, we examined the effects of combining As4S4 and As3+ on the accumulation of cellular ROS and the expression of Bcl-2, Bax, NFκB, p53, caspase-12 and caspase-3. Mitochondria- and endoplasmic reticulum stress-mediated apoptosis pathways are always triggered by the accumulation of ROS [38,39]. Bcl-2 family members are key in mitochondria-mediated intrinsic apoptosis and caspase-3 activation [38]. The other organelle-controlled apoptosis pathway, endoplasmic reticulum stress-mediated apoptosis, is caused by multiple stimulations [39]. In response to stress, the endoplasmic reticulum induces the activation of caspase-12 and caspase-3, and promotes apoptosis [39]. As4S4 and As3+, the two different forms of arsenic, induced the accumulation of ROS, up-regulated the expression of the pro-apoptotic factor Bax, down-regulated the expression of the anti-apoptotic factor Bcl-2, and induced the activation of caspase-12 and caspase-3. Moreover, the synergistic effect of As4S4 on As3+-induced activation of caspase-12 and caspase-3 was obvious. However, the effects of combining As4S4 and As3+ on cellular ROS accumulation and Bcl-2 and Bax expression were not obvious. In addition to ROS, the p53 tumor suppressor also contributes to mitochondria-mediated and endoplasmic reticulum stress-mediated apoptosis [40,41]. Additionally, the p53 tumor suppressor regulates the G1/S transition [24]. As3+ and As4S4 acted synergistically to arrest the cell cycle at G0/G1 and up-regulate p53 expression. Therefore, both ROS and p53 contributed to the apoptosis of NB4 and primary APL cells induced by As3+ and As4S4. We also studied the effects of combining As3+ and As4S4 on the extrinsic apoptosis pathway [23]. The results of RT-PCR and western-blot suggested that As4S4 and As3+ down-regulated NFκB expression, indicating that As4S4 and As3+ may promote NB4 cell apoptosis by inhibiting NFκB activation [23]. Above all, As4S4 and As3+ showed similar characteristics towards NB4 and primary APL cell apoptosis. Multiple pathways contributed to As4S4- and As3+-induced apoptosis. The combination of As4S4 and As3+ obviously promoted apoptosis by enhancing p53 expression, enhancing the endoplasmic reticulum stress-mediated pathway and inhibiting the NFκB signaling pathway.
Myeloid cell differentiation is regulated by many factors such as the CCAAT/enhancer-binding proteins, PU.1, and c-Myc [19]. NB4 and primary APL cells express the PML-RARα oncoprotein that prevents differentiation via the retinoic acid signaling pathway [19,42]. Moreover, As3+ was shown to control the fate of the PML-RARα oncoprotein through direct binding to the PML RING domain [8]. Therefore, we analyzed the effects of combining As4S4 and As3+ on the degradation of the PML-RARα oncoprotein in NB4 and primary APL cells. At low concentrations (0.1–0.4 μM), As4S4 promoted cell differentiation. The combination of 0.2 μM As3+ and 0.2 μM As4S4 obviously enhanced degradation of the PML-RARα oncoprotein and promoted differentiation of NB4 and primary APL cells via the retinoic acid signaling pathway.
Conclusions
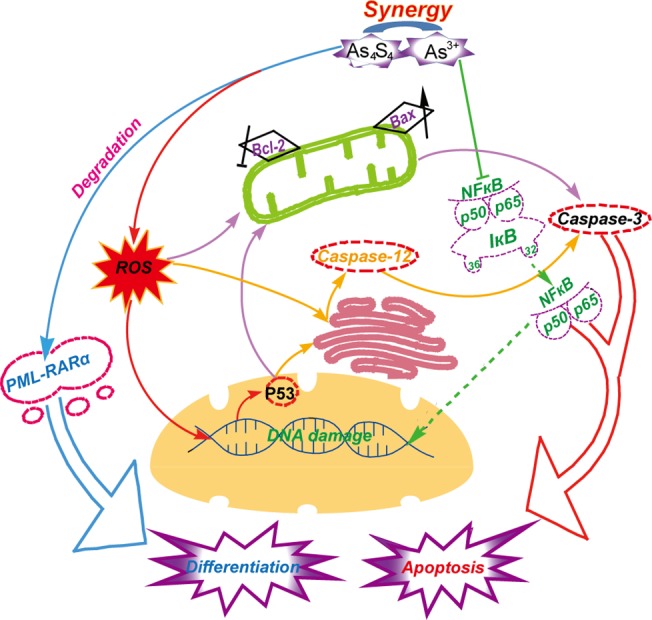
As4S4 acts synergistically with As3+ towards NB4 and primary APL cell apoptosis and differentiation (Fig 8). As4S4 and As3+ induced the accumulation of cellular ROS and up-regulated the expression of the p53 tumor suppressor. ROS and p53 promoted mitochondria- and endoplasmic reticulum stress-mediated apoptosis by regulating Bcl-2 and Bax expression and inducing activation of caspase-12 and caspase-3. Concomitantly, As4S4 and As3+ synergistically inhibited NFκB activation to promote apoptosis. Moreover, low concentrations of As4S4 interacted synergistically with As3+ to induce degradation of the PML-RARα oncoprotein and promote NB4 and primary APL cell differentiation via the retinoic acid signal pathway. In this work, we found that the combination of As4S4 and As3+ could act synergistically to promote NB4 and primary APL cell apoptosis and differentiation, which may be a better therapeutic avenue for APL treatment.
Fig 8. Mechanism for the synergistic effects of As4S4 and As3+ on apoptosis and differentiation of acute promyelocytic leukemia cells.
Acknowledgments
This research was supported by the National Basic Research Program of China (2013CB922102), the National Natural Science Foundation of China (21201101, 21275072 and 21475059) and the Scientific Research Innovation Project for Postgraduates of Jiangsu Province (CXZZ12-0038).
Data Availability
All relevant data are within the paper.
Funding Statement
ZW was supported by the National Basic Research Program of China (2013CB922102), (http://www.973.gov.cn/English/Index.aspx) and the National Natural Science Foundation of China (21275072 and 21475059) (www.nsfc.gov.cn). ZG was supported by the National Natural Science Foundation of China (21201101) (www.nsfc.gov.cn). SW was supported by the Scientific Research Innovation Project for Postgraduates of Jiansu Province (CXZZ12-0038) (http://www.ec.js.edu.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Degos L (2003) The history of acute promyelocytic leukaemia. Brit J Haematol 122: 539–553. [DOI] [PubMed] [Google Scholar]
- 2. De Thé H, Chomienne C, Lanotte M, Degos L, Dejean A (1990) The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature 347: 558–561. [DOI] [PubMed] [Google Scholar]
- 3. Wang ZY, Chen Z (2008) Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111: 2505–2515. 10.1182/blood-2007-07-102798 [DOI] [PubMed] [Google Scholar]
- 4. Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, et al. (1988) Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 72: 567–572. [PubMed] [Google Scholar]
- 5. Iland HJ, Seymour JF (2013) Role of arsenic trioxide in acute promyelocytic leukemia. Curr Treat Option On 14: 170–184. 10.1007/s11864-012-0223-3 [DOI] [PubMed] [Google Scholar]
- 6. Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu YM, et al. (2004) All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. P Natl Acad Sci USA 101: 5328–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, et al. (1997) Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood 89: 3345–3353. [PubMed] [Google Scholar]
- 8. Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, et al. (2010) Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science 328: 240–243. 10.1126/science.1183424 [DOI] [PubMed] [Google Scholar]
- 9. Ratnaike RN (2003) Acute and chronic arsenic toxicity. Postgrad Med J 79: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lengfelder E, Hofmann WK, Nowak D (2012) Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia 26: 433–442. 10.1038/leu.2011.245 [DOI] [PubMed] [Google Scholar]
- 11. Tapio S, Grosche B (2006) Arsenic in the aetiology of cancer. Mutat Res 612: 215–246. [DOI] [PubMed] [Google Scholar]
- 12. Wang SP, Geng ZR, Shi Nan, Li XL, Wang ZL (2014) The functions of crucial cysteine residues in the arsenite methylation catalyzed by recombinant human arsenic (III) methyltransferase. PloS One 9: e110924 10.1371/journal.pone.0110924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas DJ, Styblo M, Lin S (2001) The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharm 176: 127–144. [DOI] [PubMed] [Google Scholar]
- 14. Sharma VK, Sohn M (2009) Aquatic arsenic: toxicity, speciation, transformations, and remediation. Environ Int 35: 743–759. 10.1016/j.envint.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 15. Chen SJ, Zhou GB, Zhang XW, Mao JH, de Thé H, Chen Z (2011) From an old remedy to a magic bullet: molecular mechanisms underlying the therapeutic effects of arsenic in fighting leukemia. Blood 117: 6425–6437. 10.1182/blood-2010-11-283598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu J, Shao Y, Liu J, Chen G, Ho PC (2011) The medicinal use of realgar (As4S4) and its recent development as an anticancer agent. J Ethnopharmacol 135: 595–602. 10.1016/j.jep.2011.03.071 [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Liang SX, Lu YF, Miao JW, Wu Q, Shi JS (2011) Realgar and realgar-containing Liu-Shen-Wan are less acutely toxic than arsenite and arsenate. J Ethnopharmacol 134: 26–31. 10.1016/j.jep.2010.11.052 [DOI] [PubMed] [Google Scholar]
- 18. Lu DP, Qiu JY, Jiang B, Wang Q, Liu KY, Liu YR, et al. (2002) Tetra-arsenic tetra-sulfide for the treatment of acute promyelocytic leukemia: a pilot report. Blood 99: 3136–3143. [DOI] [PubMed] [Google Scholar]
- 19. Wang L, Zhou GB, Liu P, Song JH, Liang Y, Yan Xj, et al. (2008) Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. P Natl Acad Sci USA 105: 4826–4831. 10.1073/pnas.0712365105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Majno G, Joris I (1995) Apoptosis, oncosis, and necrosis, an overview of cell death. Am J Pathol 146: 3–15. [PMC free article] [PubMed] [Google Scholar]
- 21. Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar S, Yedjou CG, Tchounwou PB (2014) Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J Exp Clin Cancer Res 33: 42 10.1186/1756-9966-33-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mathas S, Lietz A, Janz M, Hinz M, Jundt F, Scheidereit C, et al. (2003) Inhibition of NF-κB essentially contributes to arsenic-induced apoptosis. Blood 102: 1028–1034. [DOI] [PubMed] [Google Scholar]
- 24. Liu Q, Hilsenbeck S, Gazitt Y (2003) Arsenic trioxide-induced apoptosis in myeloma cells: p53-dependent G1 or G2/M cell cycle arrest, activation of caspase-8 or caspase-9, and synergy with APO2/TRAIL. Blood 101: 4078–4087. [DOI] [PubMed] [Google Scholar]
- 25. Chen J, Wei H, Xie B, Wang B, Cheng J, Cheng J (2012) Endoplasmic reticulum stress contributes to arsenic trioxide-induced apoptosis in drug-sensitive and-resistant leukemia cells. Leuk Res 36: 1526–1535. 10.1016/j.leukres.2012.08.018 [DOI] [PubMed] [Google Scholar]
- 26. Guo A, Salomoni P, Luo J, Shih A, Zhong S, Gu W, et al. (2000) The function of PML in p53-dependent apoptosis. Nat Cell Biol 2: 730–736. [DOI] [PubMed] [Google Scholar]
- 27. Lanotte M, Martin-Thouvenin V, Najman S, Balerini P, Valensi F, Berger R (1991) NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 77: 1080–1086. [PubMed] [Google Scholar]
- 28.Wang SP, Geng ZR, Shi N, Li XL, Wang ZL (2014) Dose-dependent effects of selenite (Se4+) on arsenite (As3+)-induced apoptosis and differentiation in acute promyelocytic leukemia cells. Cell Death Disease 10.1038/cddis.2014.563 [DOI] [PMC free article] [PubMed]
- 29. Wang SP, Shi N, Geng ZR, Li XL, Hu X, Wang ZL (2014) Inhibitory mechanism of dimercaptopropanesulfonic acid (DMPS) in the cellular biomethylation of arsenic. Biochimie 106: 167–174. 10.1016/j.biochi.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 30. Chou TC, Motzer RJ, Tong Y, Bosl GJ (1994) Computerized quantitation of synergism and antagonism of Taxol, topotecan and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. J Natl Cancer Inst 86: 1517–1524. [DOI] [PubMed] [Google Scholar]
- 31. Zhang G, Gurtu V, Kain SR, Yan G (1997) Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques 23: 525–531. [DOI] [PubMed] [Google Scholar]
- 32. Eruslanov E, Kusmartsev S (2010) Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol 594: 57–72. 10.1007/978-1-60761-411-1_4 [DOI] [PubMed] [Google Scholar]
- 33. Jakoby M, Schnittger A (2004) Cell cycle and differentiation. Curr Opin Plant Biol 7: 661–669. [DOI] [PubMed] [Google Scholar]
- 34. Darzynkiewicz Z, Zhao H, Halicka HD, Rybak P, Dobrucki J, Woldkowic D (2011) DNA damage signaling assessed in individual cells in relation to the cell cycle phase and induction of apoptosis. Crit Rev Cl Lab Sci 49: 199–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. He Y, Xu K, Keiner B, Zhou JF, Czudai V, Li TX, et al. (2010) Influenza A virus replication induces cell cycle arrest in G0/G1 phase. J Virol 84: 12832–12840. 10.1128/JVI.01216-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morse D, Choi AM (2005) Heme oxygenase-1: from bench to bedside. Am J Resp Crit Care 172: 660–670. [DOI] [PubMed] [Google Scholar]
- 37. Pastorek M, Gronesova P, Cholujova D, Hunakova L, Bujnakova Z, Balaz P, et al. (2014) Realgar (As4S4) nanoparticles and arsenic trioxide (As2O3) induced autophagy and apoptosis in human melanoma cells in vitro. Neoplasma 61: 700–709. 10.4149/neo_2014_085 [DOI] [PubMed] [Google Scholar]
- 38. Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Gene Dev, 13: 1899–1911. [DOI] [PubMed] [Google Scholar]
- 39. Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC (2003) Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 22: 8608–8618. [DOI] [PubMed] [Google Scholar]
- 40. Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. (2003) p53 Has a Direct Apoptogenic Role at the Mitochondria. Mol Cell 11: 577–590. [DOI] [PubMed] [Google Scholar]
- 41. Li J, Lee B, Lee AS (2006) Endoplasmic Reticulum Stress-induced Apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem 281: 7260–7270. [DOI] [PubMed] [Google Scholar]
- 42. Cai X, Shen YL, Zhu Q, Jia PM, Yu Y, Zhou L, et al. (2000) Arsenic trioxide-induced apoptosis and differentiation are associated respectively with mitochondrial transmembrane potential collapse and retinoic acid signaling pathways in acute promyelocytic leukemia. Leukemia 14: 262–270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.