Abstract
Because of its limited healing capacity, treatments for articular cartilage injuries are still challenging. Since the first report by Brittberg, autologous chondrocyte implantation has been extensively studied. Recently, as an alternative for chondrocyte-based therapy, mesenchymal stem cell–based therapy has received considerable research attention because of the relative ease in handling for tissue harvest, and subsequent cell expansion and differentiation. This review summarizes latest development of stem cell therapies in cartilage repair with special attention to scaffold-free approaches.
Keywords: mesenchymal stem cells, synovial cells, articular cartilage, animal models, biomaterials
Introduction
Articular cartilage injuries leading to overt lesions occur frequently and are quite common. In a prospective study of 1,000 knee arthroscopies, focal chondral or osteochondral defects were found in 19% of the patients.1 Articular cartilage does not usually heal spontaneously because of its avascular and aneural surroundings, as well as its relatively unique matrix organization. Therefore, a variety of approaches have been tested to improve cartilage healing over the past few decades.2,3
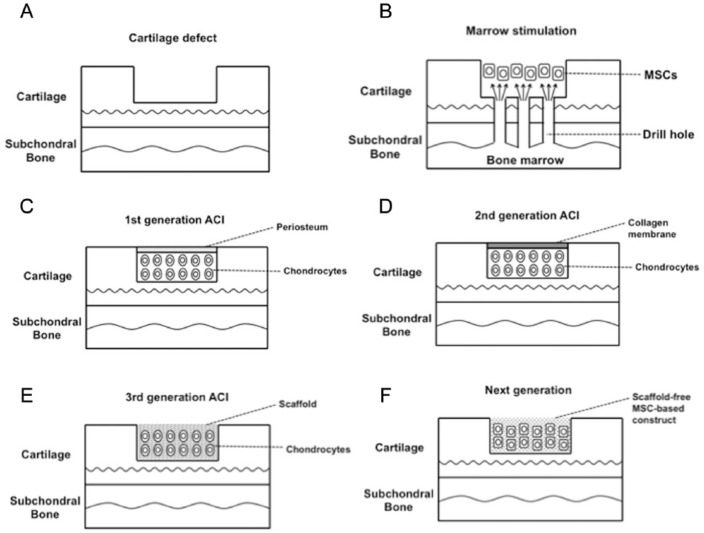
In the 1980s, bone marrow stimulation techniques, including subchondral bone microfracture were introduced (Fig. 1A and 1B). In this technique, multiple drill holes are made in the damaged cartilage lesion to allow for an influx of bone marrow cells that stimulates repair with formation of fibrocartilage in the lesions. Most studies have shown initial fibrocartilage formation with deterioration of this newly formed tissue over time.4-6 Similarly, several authors have reported a significant decrease in clinical outcome at longer follow-up relative to short-term high satisfaction.7-10 To overcome such unsatisfactory clinical results, a recent study has shown an improvement in repair tissue quality by enhancing microfracture with a biomaterial.11
Figure 1.
Schematic representation of cell-based cartilage repair. (A) Typical cartilage defect. (B) Marrow stimulation technique. Subchondral bone penetration to release bone marrow mesenchymal stem cells (MSCs) that form a stem cell–rich clot into the cartilage defect. (C) First generation of autologous chondrocyte implantation (ACI). Chondrocytes isolated from a biopsy of a non-weightbearing location are culture-expanded and subsequently implanted under a periosteal cover. (D) Second generation of ACI. A cover of a collagen membrane replaces the periosteal cover of the first generation of ACI. (E) Third generation of ACI. Autologous chondrocytes are delivered into the defect using biomaterial scaffolds. (F) Next generation cartilage repair using scaffoldless MSC-based technique. In vitro generated scaffold-free 3-dimensional tissue-engineered construct (TEC) that is composed of MSCs derived from synovium and the extracellular matrices (ECM) synthesized by the cells is implanted into cartilage defect.
In 1994, the first results on autologous chondrocyte implantation (ACI) were published by Brittberg et al.12 In the first generation of this ACI technique, autologous chondrocytes were isolated from a biopsy of cartilage in a non-weightbearing location in the knee. The cells were culture-expanded and subsequently implanted back into the chondral defects under a periosteal cover (Fig. 1C). The same group reported that good to excellent long-term results were considered to occur in 89% of the patients, and 8 of 12 biopsy specimens showed findings consistent with formation of hyaline cartilage–like tissue.13 On the other hand, another group reported that 62 patients improved, 6 reported no change, and 19 worsened at 5-year follow-up.14 Reasons for failure with the first generation ACI technique included separation of the periosteal flap from the surrounding cartilage and hypertrophy in the periosteal flap that required subsequent shaving. In the second generation of ACI, a cover of collagen or a resorbable biofilm replaced the periosteal cover, a modification that does not require harvesting or suturing of an autologous periosteal flap (Fig. 1D).15-17 A recent study showed that the use of a collagen membrane in combination with autologous chondrocytes leads to superior clinical long-term outcome compared to the first generation ACI methodology.15 In the third generation ACI technique, autologous chondrocytes were delivered into the defects using scaffolds made of either synthetic or natural polymers as the biomaterials (Fig. 1E).18,19 This technique is robust and easy for surgeons to handle and significantly improves the healing of cartilage defects. Interestingly, a recent systematic review indicated that either the second or third generation ACI methodology provided better clinical results than did the first generation, but with weak evidence.16 While the third generation is technically attractive, further long-term studies are likely required before the technique can be widely adopted.
As mentioned above, chondrocyte-based therapies have been extensively studied over the past decades since the first report of successful ACI.20-22 However, these procedures may have limitations including the sacrifice of undamaged cartilage within the same joint, as well as potential alterations associated with the in vitro expansion of the cells. Furthermore, because of the degenerative changes in cartilage that can accompany aging, the availability of cells may be limited in elderly individuals, both quantitatively and qualitatively.23
To overcome such potential problems, stem cell therapies have become a focus to facilitate regenerative tissue repair. Mesenchymal stem cells (MSCs) have the capability to differentiate into a variety of connective tissue cells including bone, cartilage, tendon, muscle, and adipose tissue.24 These cells can be isolated from various tissues such as bone marrow, skeletal muscle, synovial membrane, adipose tissue, and umbilical cord blood,24-29 as well as synovial fluid.30 Pluripotent cells isolated from synovium may be well suited for cell-based therapies for cartilage because of the relative ease of harvest and their strong capability for chondrogenic differentiation.26 Synovium-derived cells are reported to exhibit the greatest chondrogenic potential among the other mesenchymal tissue–derived cells examined.27 As other options for a cell source, allogeneic MSCs31,32 or induced pluripotent stem (iPS) cells33, 34 may also be considered. However, there have not been much evidence using these cells forth coming in terms of preclinical and clinical safety, and thus further studies with such cells are likely necessary.
In addition to selection of a cell source, effective local delivery of cells to chondral lesions has been another area of concern and the focus of additional research. It is widely accepted that an appropriate 3-dimensional (3D) environment is important to optimize cell proliferation and chondrogenic differentiation.35 Therefore, a 3D scaffold, which is seeded with cells, is usually utilized to enhance repair of the defects. Such scaffolds generally consist of synthetic polymers such as poly(l-lactide) (PLLA), poly(glycolide) (PGA), poly (dl-lactide-co-glycolide) (PLGA), alginate,36-39 or of biological materials such as collagen, fibrin, hyaluronan, and chitosan.40-44 Various scaffolds have been approved for clinical use by some governmental institutions.45 However, there are still several issues associated with the long-term safety and efficacy of these materials. Synthetic polymers may have potential problems regarding retention and degradation in situ.46,47 Biological materials potentially carry the risk of transmission of infectious agents and initiating immunnological reactions.48,49 Taken together, in order to minimize unknown risk, such materials should ideally be excluded throughout the treatment procedure, and in this regard, a scaffold-free cell delivery system would be an excellent alternative.
Recently, several scaffold-free approaches have been tested. Ebihara et al.50 used layered chondrocyte sheets prepared on a temperature-responsive culture dish. With this technique, cultured cells can be harvested noninvasively from the dishes by reducing only temperature.51 Moreover, because the harvest does not need enzymatic digestion, differentiated cell phenotypes are retained. Nakamura et al.52 injected synovial MSCs into the cartilage defect, and kept the knee immobilized for 10 minutes before wound closure. They showed that leaving cell suspension in cartilage defects for 10 minutes made it possible for cells to adhere in the defect. These techniques would potentially be an attractive cell-delivery system. However, the delivered cells do not contain an extracellular matrix (ECM) and it may be difficult to effectively cover a large chondral defect due to the availability of a limited number of cells.
To address several of these issues, we have developed a novel scaffold-free 3D tissue engineered construct (TEC) that is composed of either human or porcine MSCs derived from synovium and an ECMs synthesized by the cells. Such a new, scaffoldless, MSC-based technique could be considered as the next-generation vehicle for cartilage repair (Fig. 1F).53 In the present review, the suitability and effectiveness of the TEC methodology for cartilage repair and regeneration will be discussed.
Characterization of Cultured Cells Derived from Human Synovium
The cultured cells isolated from human synovium displayed a long-term self–renewal capacity and expanded over at least 10 passages in basal medium with consistent growth kinetics (unpublished observations). The cell surface phenotypic marker analyses showed that the cells were consistently positive (>80%) for CD13, CD44, and CD90, weakly positive (3% to 80%) for CD29, CD34, CD54, CD105, and CD166, and negative (<3%) for CD14, CD31, and CD45. Although there were slight changes in expression levels of markers between cells at passages 4 and 7, such profiles are generally similar to those of MSCs from various tissues such as bone marrow, adipose tissue, and synovial membrane,27,54,55 except that the expression of CD105 was somewhat lower in these studies. The differentiation capacity of the cultured human cells to chondrogenic, osteogenic, and adipogenic lineages at several passages was confirmed by in vitro differentiation assays (Fig. 2). Based on these findings, the cultured cells derived from human synovium were considered to be MSCs.
Figure 2.
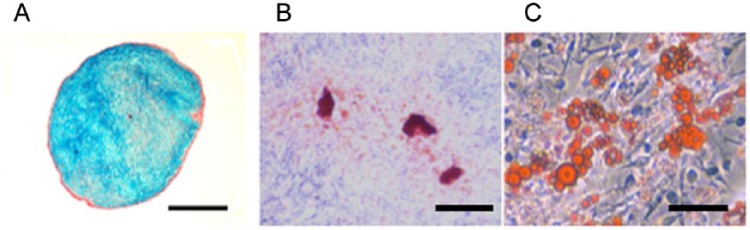
Pluripotency of the synovial cells.
(A) Alcian blue staining of the cultured synovial cells under a pellet culture system in chondrogenic medium. There is intense blue staining observed. Bar = 500 μm. (B) Alizarin red staining of the synovial cells (at passage 5) under osteogenic medium. These synovial cells form a mineralized matrix as evidenced by Alizarin red staining. Bar = 100 μm. (C) Oil-red O staining of synovial cells (at passage 5) after exposure to an adipogenic medium. Morphological changes in cells, as well as the formation of neutral lipid vacuoles are noticeable. Bar = 100 μm.
In Vitro Development of the Basic TEC Configuration
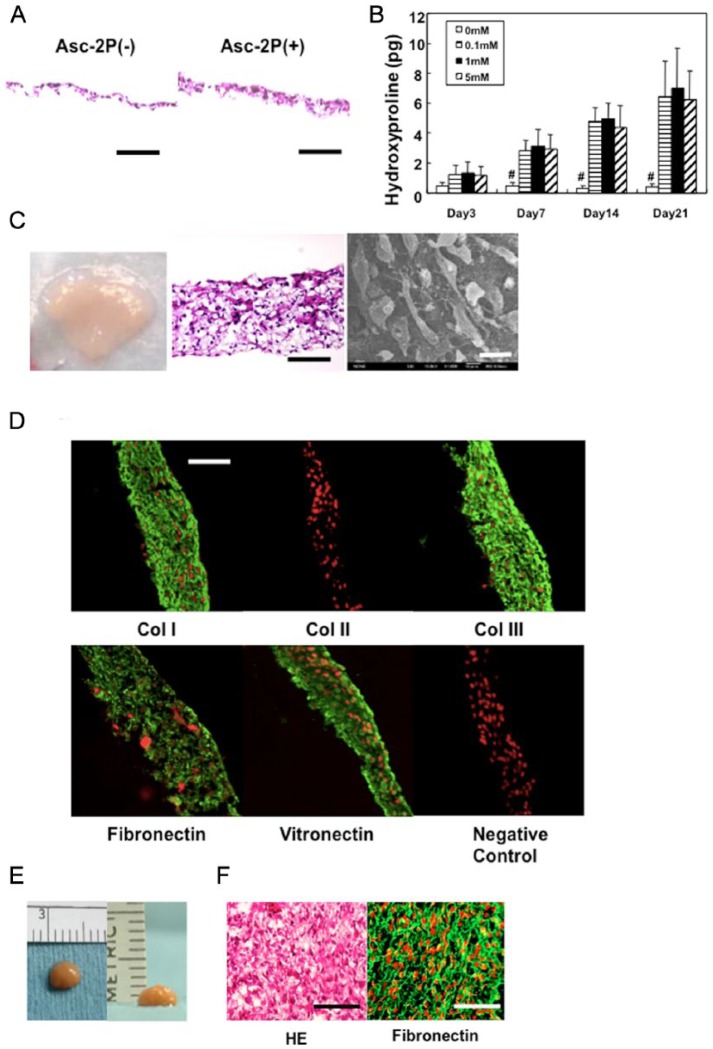
When synovium-derived MSCs were cultured to confluence in the basic growth medium, they did not synthesize an abundant collagenous matrix. In contrast, in the presence of >0.1 mM ascorbic acid-2 phosphate (Asc-2P), collagen synthesis significantly increased with time in culture (Fig. 3A and 3B). Subsequently, the monolayer cell-matrix complex cultured in Asc-2P became a stiff sheet-like structure, a structure that could be readily detached from the substratum by exerting mild shear stress at the cell-substratum interface using gentle pipetting. After detachment, the monolayer sheet immediately began to actively contract and evolved into a thick 3D tissue (Fig. 3C). Histology and scanning electron microscope (SEM) assessment of this 3D tissue indicated that the cells and the corresponding ECM were 3 dimensionally integrated together at high cell density. Immunohistochemical analysis showed that the TEC was rich in collagen I and III. In contrast, there was no detectable expression of collagen II within the TEC. However, fibronectin and vitronectin were also abundant in the TEC (Fig. 3D). Notably, all the molecules detected were diffusely distributed throughout the matrix and there was no overt polarity to the matrix organization within the TEC. As the TEC developed when the matrix folded and contracted, it was apparent that the layers were integrated into each other. When complete, the folding a process which led to development of one spherical body several millimeters thick (Fig. 3E and 3F). This contracted tissue was termed a tissue-engineered construct (TEC) derived from MSCs.
Figure 3.
Development of the tissue-engineered construct (TEC). (A) Photomicrograph of monolayer culture in the absence (left) or presence (right) of 0.2 mM ascorbic acid 2-phosphate (Asc-2P). Bar = 100 μm. (B) The hydroxyproline contents of the TEC (1.6 × 106 cells/12-well culture plate) cultured in the growth medium in the absence or presence of Asc-2P (0.1, 1, and 5 mM). There is a significant increase in collagen synthesis when Asc-2P is added at the concentration of more than 0.1 mM over 7 days (N = 4, #P < 0.001, compared with 0 mM). There is no significant dose effect of Asc-2P at more than 0.1 mM. In the presence of Asc-2P, collagen synthesis was significantly increased with time-dependency (P < 0.001). (C) Macroscopic view (left, bar = 1 cm), photomicrograph (middle, bar = 100 μm), and scanning electron microscopic view (right, bar = 20 μm) of the TEC. (D) Immunohistochemical analysis of the TEC stained with type I collagen (Col I), type II collagen (Col II), type III collagen (Col III), fibronectin, vitronectin, and negative IgG (control). Red are nuclei and green is target antibody. Adhesion molecules such as fibronectin and vitronectin are diffusely distributed within the TEC. Bar = 100 μm. (E) Macroscopic view of the TEC (8.0 × 106 cells/6-cm dish, 14 days culture) that was integrated to one spherical body. The diameter of this TEC was 5 mm and the thickness was 2 mm. (F) Hematoxylin and eosin staining (left), and fibronectin staining (right) of the TEC that was integrated to one spherical body with additional 7 days culture. Bar = 100 μm.
The Basic Human TEC Has Adhesive Properties That Facilitate Association and Adhesion to a Cartilage Matrix
As mentioned above, because of its unique matrix organization, articular cartilage exhibits antiadhesive properties and therefore, integration of the implanted tissue to the adjacent cartilage normal matrix has been an issue in the treatment of chondral injuries.3 To overcome this problem, most implantation procedures to repair chondral lesions have required an enzyme treatment of the surface of the cartilage matrix,56 reinforcement of the initial fixation by suturing,57,58 or by the use of absorbable pins.54 However, an animal study revealed that a suture track in the surrounding articular cartilage remained unhealed, and thus becomes a defect, which could potentially be a trigger site for subsequent degradation of matrix around the margin between the implant and the adjacent cartilage tissue.57 Therefore, to avoid such potential complications, an implantable tissue that possesses highly adhesive properties to cartilage tissue is likely advantageous for secure tissue integration.
To test the adhesive property of TEC to an established intact cartilage matrix, basic human TECs were placed on the injured surfaces of thawed fresh-frozen human chondral fragments. Within 5 minutes, the TEC had adhered to the chondral fragments. When the TEC-chondral complexes were further cultured for 7 days, they remained stably associated for the entire time. Histology at day 7 showed close adhesion of the TEC to the injured surface of the chondral fragments (Fig. 4A). Immunohistochemistry showed that fibronectin (Fig. 4B) and vitronectin (data not shown) were localized at the interface between the TEC and the injured surfaces of chondral fragments.
Figure 4.
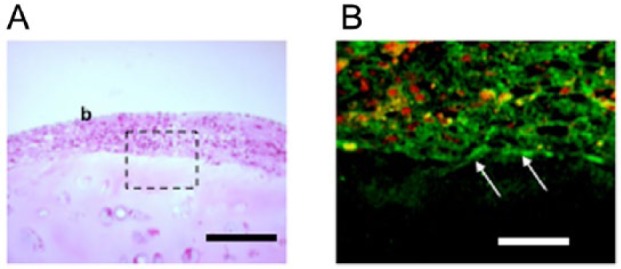
Tissue-engineered constructs (TECs) exhibit adhesiveness to a normal cartilage matrix. (A) Photomicrograph (hematoxylin and eosin staining) of a cultured chondral fragment for 7 days after the implantation of a TEC on the injured surface. As can be seen, the bioengineered tissue is closely attached to the injured surface. Bar =200 μm. (B) Immunohistochemical analysis staining for fibronectin in area enclosed by dotted rectangle in A. Bar = 50 μm.
Chondrogenic Differentiation Capacity of Human TECs
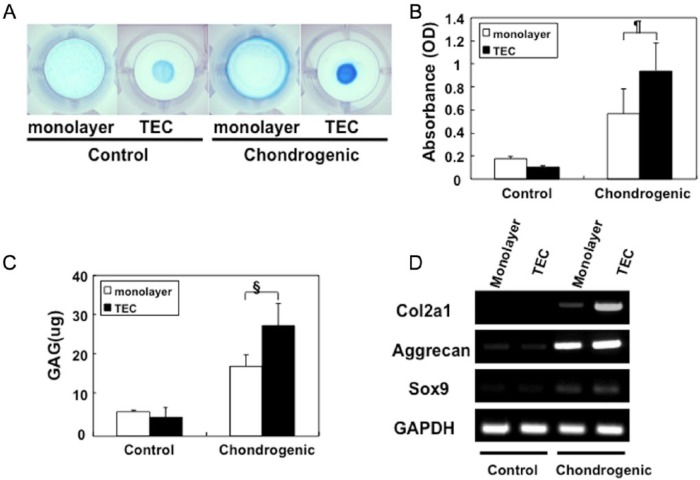
TEC derived from human MSC were cultured in a chondrogenic medium containing BMP-2 showed increased glycosaminoglycan (GAG) synthesis and deposition as evidenced by intense Alcian blue staining (Fig. 5A). The quantification of GAGs indicated that GAG synthesis was significantly higher in the TEC exposed to the chondrogenic medium compared to those generated in the absence of such components (Fig. 5B and 5C). Detection of cartilage-specific markers, collagen II (Col2a1), aggrecan, and sox9 messenger RNA (mRNA) by semiquantitative reverse transcription–polymerase chain reaction (RT-PCR) confirmed the cartilage-like phenotype of the TEC exposed to the differentiation medium. Untreated basic TEC, as well as monolayer cell cultures, showed only weak expression of these cartilage-specific markers (Fig. 5D).
Figure 5.
Chondrogenesis of the tissue-engineered construct (TEC). (A) Alcian blue staining of a monolayer of cultured synovial cells, a TEC in control medium or in chondrogenic medium for 14 days, respectively. (B, C) The quantification of Alcian blue staining (B) and glycosaminoglycan (GAG) contents (C) of a monolayer culture complex, or a TEC in control medium or chondrogenic medium, respectively. GAG synthesis is significantly higher in the TEC cultured in chondrogenic medium (N = 8, ¶P = 0.047, §P = 0.016). (D) Semiquantitative reverse transcription–polymerase chain reaction (RT-PCR) analysis for chondrogenic marker genes, type II collagen (Col2a1), aggrecan, Sox 9, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Basic Porcine TECs Can Effectively Repair Chondral Defects In Vivo and Inhibit the Progression of Chondral Defects to Overt Osteoarthritis in Both Skeletally Mature and Immature Animals
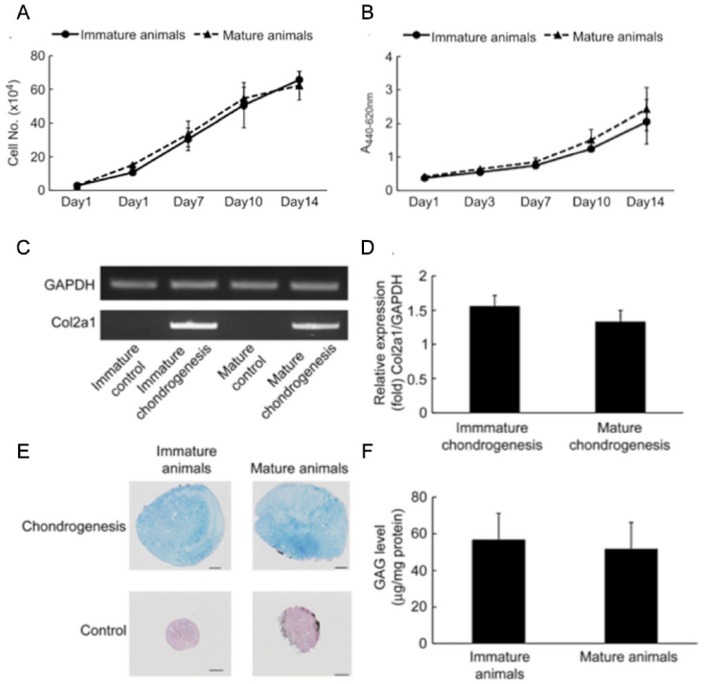
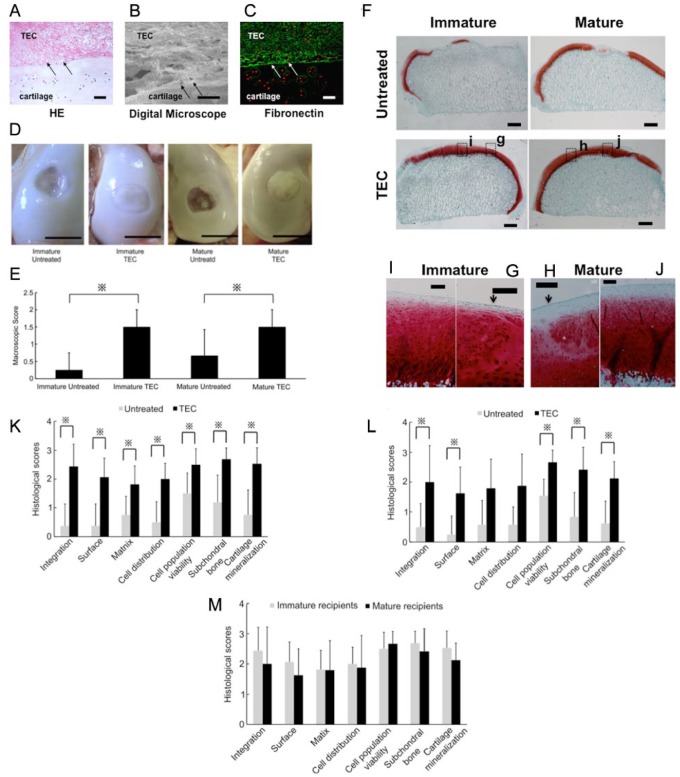
One of the crucial factors that may affect the results of cell-based therapies is the age of the donors and the recipients. Regarding the cell proliferation and differentiation capacities of MSCs, it is controversial as to whether they are age-dependent59-62 or not.26,63-66 In terms of the host tissue reaction, natural healing responses of osteochondral defects has been compared between immature and mature animals using rabbit models, and in this species, the studies demonstrated better healing responses in immature animals.67-70 On the other hand, there have been no studies which compared the results of cell-based repair of chondral defects between immature and mature animal models. Regarding the use of a clinically relevant animal model for cartilage repair, it is difficult to create a chondral injury that does not breach the subchondral bone in small animals such as rabbits, rats, and mice because of the limited thickness of their articular cartilage, and therefore, these conditions may not be as clinically relevant as use of a larger animal. Thus, in consideration of clinical relevance, it is preferable to utilize a large animal model to investigate the influence of maturity on the results of cell-based therapies to repair chondral lesions. Therefore, in order to assess the efficacy of the TEC in an in vivo model, a porcine model was chosen as the physiology of the pig is similar to that of humans in many respects,71 and porcine articular cartilage of the knee is sufficiently thick as to allow creation of a chondral defect without damaging the subchondral bone. Prior to performing such studies, a preliminary characterization of the ability of porcine-derived MSC from synovium to generate a TEC comparable to that discussed above for the human MSC was undertaken. To that end, we compared the in vitro characteristics of cell proliferation and chondrogenic capacity in porcine MSCs isolated from skeletally immature animals (3-4 months old) with mature animals (12 months old). Cell number assessments, as well as WST-1 assays, demonstrated that there were no significant differences in the proliferation capacity of porcine synovial MSCs derived from immature or mature animals (Fig. 6A and 6B). In addition, there were no significant differences in chondrogenic capacities between MSCs isolated from immature and mature animals, based on the results of collagen II mRNA expression levels detected by RT-PCR, GAG synthesis, or Alcian blue staining using a pellet culture system (Fig. 6C-6F). To test the feasibility of using the porcine TEC approach for a wide range of recipient ages without chondrogenic manipulation to repair a chondral injury, immature as well as mature porcine chondral injury models were utilized in experiemental studies. After implantation, the TEC firmly adhered to the injured joint surface without suturing. To confirm the early adhesion mode of the TEC to the injured surfaces, histology at day 7 was assessed. The TEC were tightly adhered to the injured chondral surfaces (Fig. 7A). Higher magnification revealed that the adhesion was mediated by matrix-to-matrix interaction (Fig. 7B) and, as shown in the in vitro culture study, fibronectin was localized to the interface between the TEC and the surface of the defects (Fig. 7C). At 6 months postimplantation, regardless of starting age untreated lesions exhibited no evidence for repair or only partial tissue coverage, while the defects treated with a basic TEC were totally or primarily covered with repair tissue (Fig. 7D). The mean macroscopic scores for the TEC groups (1.50 ± 0.50, immature group, and 1.50 ± 0.50, mature group) were significantly higher than those for the untreated groups (0.25 ± 0.50, immature group, and 0.67 ± 0.75, mature group) (P = 0.017 and P = 0.034, respectively) (Fig. 7E). In this situation, a lower score is suggestive of a failure to resolve the injury and progression toward overt osteoarthritis. Histologically, the chondral lesions in the nontreatment control groups showed evidence of osteoarthritic changes, with loss of cartilage and destruction of subchondral bone in both skeletally immature and mature animals (Fig. 7F). Conversely, when treated with a TEC, the defects were filled with repair tissue exhibiting good integration to the adjacent cartilage and the restoration of a smooth surface, regardless of age at the time of implantation (Fig. 7F). Higher magnification views indicated that there was good tissue integration to the adjacent cartilage obtained when the TEC were implanted in both immature and mature animals (Fig. 7G and 7H, arrows). The repair tissue exhibited predominantly spindle-shaped fibroblast-like cells in the superficial zone of the repair tissue, while the majority of the remaining repair matrix contained round-shaped cells in lacuna (Fig. 7I and 7J). Following implantation, no histological findings were obtained that suggested either central necrosis of the implanted TEC or that an abnormal inflammatory macrophage and lymphocyte response consistent with some form of immunological rejection had occurred in this allogenic situation, regardless of the age.of the pigs. Using histological scoring, the TEC groups exhibited significantly higher scores than did the control group in all criteria categories in the immature animals (Fig. 7K). However, in mature animals, the TEC group had significantly higher scores than did the corresponding control group in all categories except the “Matrix” and “Cell Distribution” categories (Fig. 7L). Comparing the repair tissues developing following TEC implantation in immature and mature animals, no significant differences were detected (Fig. 7M).
Figure 6.
Cell proliferation assay and chondrogenic potential of porcine mesenchymal stem cells (MSCs) derived from immature and mature animals. The cell proliferation assay was assessed by cell counting (A) and the WST-1 method (B). There were no significant differences in proliferative capacity between immature (N = 3) and mature porcine synovial MSCs (N = 3). Chondrogenic potential of porcine MSCs derived from immature and mature animals assessed by reverse transcription–polymerase chain reaction (RT-PCR) for collagen II expression (C, D), Alcian blue staining (E), and glycosaminoglycan (GAG) synthesis (F). Bar = 200 mm. There were no significant differences detected between immature-cell pellets (N = 3) and mature-cell pellets (N = 3) by RT-PCR analysis (D) or GAG synthesis (F).
Figure 7.
Macroscopic and histologic assessment of tissue-engineered construct (TEC) implanted in vivo on porcine chondral defects. (A-C) Photomicrograph (hematoxylin and eosin [HE] staining; A), the interface view using a digital microscope (B), and fibronectin staining (C) of porcine chondral defects treated with a TEC at day 7. Arrows indicate interface between the TEC and the cartilage defect. Bar = 50 μm. (D) Macroscopic view of immature or mature porcine chondral lesions treated with a TEC or left untreated at 6 months after surgery. Bar = 10 mm. (E) Macroscopic score of chondral lesions treated with a TEC (immature animals, N = 8; mature animals, N = 6) or left untreated (immature animals, N = 4; mature animals, N = 6) at 6 months postsurgery. Regardless of age, the TEC group showed significantly higher scores than did the untreated group. ※P < 0.05. (F) Safranin-O staining of untreated chondral lesions or lesions repaired with a TEC. Bar = 1 mm. (G-J) Higher magnification view at the TEC/normal cartilage boundary area (G, H) and the central area (I, J) of TEC-mediated repair tissue. Bar = 200 mm. Regardless of age, the defects treated with a TEC were completely filled with Safranin-O-positive repair tissue (I, J) with good integration to normal cartilage (G, H, arrow). (K-M) Modified International Carticlage Repair Society (ICRS) score for repair cartilage in immature (K) and mature animals (L). The TEC group (N = 8) exhibited significantly higher scores than did the untreated control group (N = 4) in all the criteria categories in the immature animals. ※P < 0.05. Likewise, the TEC group (N = 6) exhibited significantly higher scores than did the untreated control group (N = 6) in all the criteria categories except for the “Matrix” and “Cell Distribution” categories in the mature recipients. ※P < 0.05. (M) As to the quality of the repair cartilage mediated by the TEC, there were no significant differences observed in any criteria category between the immature (N = 8) and mature animals (N = 6).
The Mechanical Properties of Porcine Chondral Defects Treated with a Porcine-Derived TEC Approximates Those of Normal Cartilage at 6 Months Postimplantation
It is accepted that articular cartilage is a biphasic viscoelastic material which exhibits strain-rate dependent mechanical behavior.72 It means that the viscoelasticity of cartilage which retains interstitial water might be mainly reflected in faster compression test, while the matrix viscoelasticity without interstitial water could be mainly reflected in slower compression tests.
In the tissue localized in the defects of the untreated control group, the tangent modulus (defined as the slope of the curve at 5 % strain) in immature animals was significantly lower than that for normal cartilage at a compression rate of either 4 μm/s (Fig. 8A) or 100 μm/s (Fig. 8B). In contrast, there were no significant differences detected between the tangent modulus for the repair tissue resulting from implantation of a TEC and that for normal cartilage at either 4 μm/s (Fig. 8A) or 100 μm/s (Fig. 8B) in immature animals. Similarly, the mean tangent modulus in the untreated mature animals was significantly lower than that for normal cartilage at a compression rate of 4 μm/s (Fig. 8A), while there were no significant differences detected between the tangent modulus for repair tissue in mature recipients treated with a TEC and that for normal cartilage at either 4 μm/s (Fig. 8A) or 100 μm/s (Fig. 8B). These results suggest that the viscoelastic properties of the tissue in defects repaired by TEC implantation are likely similar to those of normal cartilage, regardless of age at the time of implantation.
Figure 8.
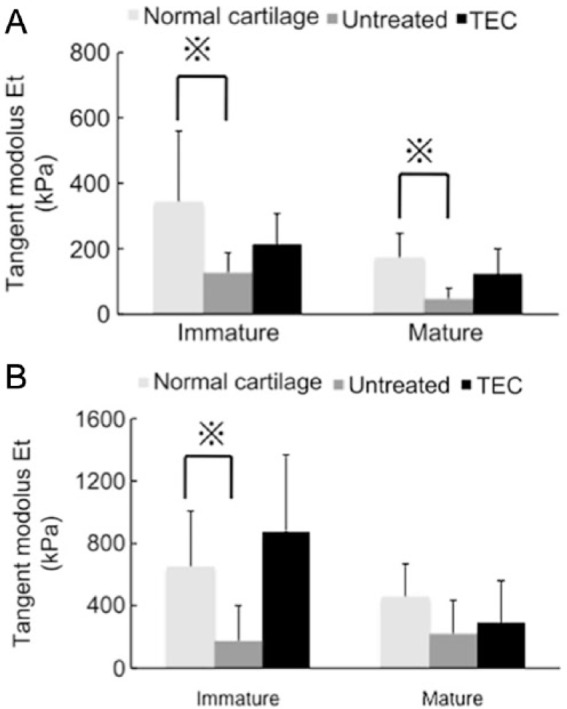
Mechanical assessment of in vivo implanted tissue-engineered construct (TEC) in porcine chondral defect model. (A, B) The results of compression tests at slower compression speed (4 μm/s) (A) and at faster compression speed (100 μm/s) (B). (Immature animals: normal cartilage, N = 11, TEC, N = 7, untreated, N = 4. Mature animals: normal cartilage, N = 5, TEC, N = 5, untreated, N = 5.) Regardless of age, there were no significant differences detected in the tangent modulus of the repair tissue mediated by a TEC compared with normal cartilage at either the slower or faster compression speed. Conversely, the untreated cartilage defects, whether immature or mature, showed significantly lower tangent modulus than did normal cartilage at either the slower or faster compression speed. ※P < 0.05.
Comparison of Zonal Structure and Mechanical Properties of Repair Cartilage Resulting from Implantation of a Porcine-Derived TEC
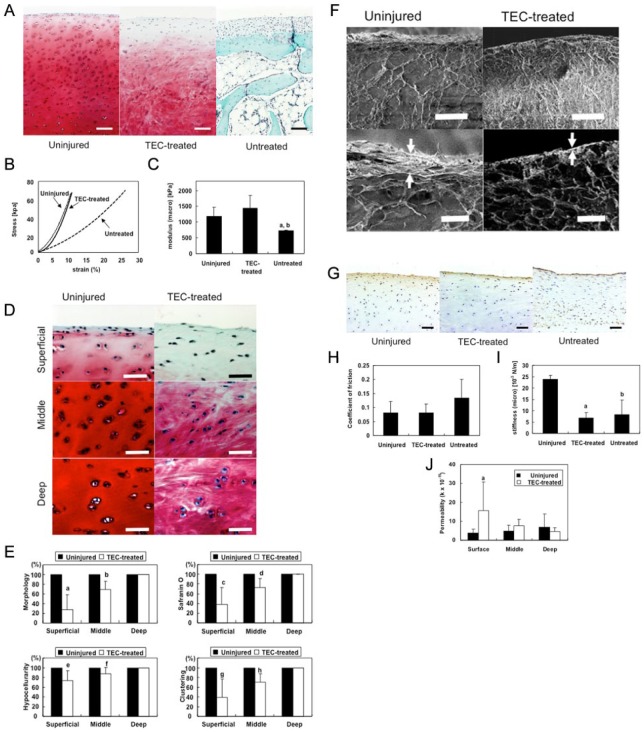
As mentioned above, following implantation for 6 months, TEC efficiently led to the repair of chondral defects by mediating the development of a cartilage-like tissue without the involvement of any detectable immunologic reaction, regardless of skeletal maturity.31 However, more detailed observations revealed that more spindle-shaped fibroblast-like cells were dominant in the superficial area of the repair tissue and this was not observed in normal cartilage.31,73,74 Therefore, we investigated the quality of the repair cartilage generated from implantation of a TEC derived from synovial mesenchymal stem cells at 6 months postimplantation in more detail, especially in the superficial zone location. This repair material was then compared with that from uninjured cartilage using the same porcine chondral defect model discussed above. TEC-mediated repair tissue was cartilaginous with Safranin O staining (Fig. 9A), and had macroscale compressive properties comparable to uninjured cartilage (Fig. 9B and 9C). However, morphological assessments revealed that the superficial zone of the TEC-mediated repair tissue was more fibrocartilage-like, in contrast to the middle or deep zones that were more hyaline cartilage-like with Safranin O staining (Fig. 9D). Histological scoring of the TEC-mediated repair tissue indicated it was significantly compromised in the superficial zone compared to the middle and deep zones (Fig. 9E). Scanning electron microscopy showed a thick tangential bundle of collagen fibers at the most superficial layer of uninjured cartilage, while no such corresponding structures were detected at the surface of TEC-mediated repair tissues (Fig. 9F). Interestingly, immunohistochemical analysis revealed that the lubricating molecule PRG4 was localized to the superficial area of uninjured cartilage, as well as the TEC-mediated repair tissue (Fig. 9G). Friction testing showed that the lubrication properties of the 2 tissues were similar (Fig. 9H). However, microindentation analysis revealed that the surface stiffness of the TEC-repair tissue was significantly lower than that of uninjured cartilage (Fig. 9I). Permeability testing indicated that the TEC-mediated repair tissue exhibited lower water retention capacity than did uninjured cartilage, specifically at the superficial zone (Fig. 9J). Taken together, cartilage defects, repaired by implantation of a scaffold-free TEC derived from synovial mesenchymal stem cells, becomes a cartilaginous tissue that exhibits macroscale compressive properties similar to uninjured cartilage. However, the TEC-mediated repair cartilage lacks the lamina splendens, as well as the superficial tangential zone, and exhibits inferior microscaled mechanical properties such as surface stiffness and water retention capacity. Further improvement of these surface structures will be required to optimize cartilage regeneration. As well, assessment of TEC-mediated repair tissue properties at longer time points post-implantation to determine whether the quality improves with time, or perhaps requires some biological assistance to improve the superficial zone location.
Figure 9.
Comparison of zonal structure and mechanical properties of repair cartilage generated from a porcine-derived tissue-engineered construct (TEC). (A) Safranin O staining of uninjured normal porcine articular cartilage and chondral lesions treated with or without a TEC at 6 months after implantation. Bar = 100 μm. (B) Typical stress–strain relationships of TEC-treated repair tissue compared with those of uninjured cartilage and defects left untreated. (C) Tangent modulus of uninjured cartilage (n = 10), repair tissue in chondral lesions of the group treated with a TEC (n = 6), and those in the untreated group (n = 3) at compression rate of 4 μm/s. aP < 0.05, compared with uninjured cartilage. bP < 0.05 compared with the TEC-treated group. There were no significant differences between the tangent modulus of TEC-mediated repair tissue and that of uninjured cartilage. (D) Safranin O staining of superficial, middle and deep zone of porcine chondral lesions 6 months after implantation of TECs and uninjured cartilage. Bar = 25 μm. (E) Zonal histological and histochemical grading scale of uninjured articular cartilage and TEC-mediated repair tissue (n = 8). a,c,gP < 0.001; b,d,hP < 0.01; e,fP < 0.05 compared with the uninjured cartilage. (F) Scanning electron microscopic (SEM) view of normal porcine cartilage and chondral lesions treated with a TEC at 6 months after implantation (upper pictures). Bar = 100 μm. Higher magnification SEM view of uninjured porcine cartilage and chondral lesions treated with a TEC (lower pictures). Bar = 25 μm. Arrow; the thickness of the superficial layer. (G) PRG4/Lubricin expression at the surface zone of uninjured porcine cartilage and chondral lesions treated with or without a TEC. (H) Frictional coefficient of uninjured cartilage (n = 11) and chondral lesions in the TEC-treated group (n = 7) at 60 seconds with the application of a compressive force of 1.76 N. There were no significant differences between the frictional coefficients of repair tissue following implantation of a TEC and those of normal cartilage. (I) The surface stiffness of normal cartilage, the repair tissue of chondral lesions in the TEC-treated group, and that in the untreated group. aP < 0.05, bp < 0.05 compared with normal cartilage. (J) Permeability of uninjured cartilage (n = 11) and repair tissue in chondral lesions of the TEC-treated group (n = 7) at the surface, middle, and deep zone. aP < 0.05, compared with normal cartilage.
Clinical Trials Using a TEC Derived from Human Synovial MSCs for Repair of an Isolated Cartilage Defect
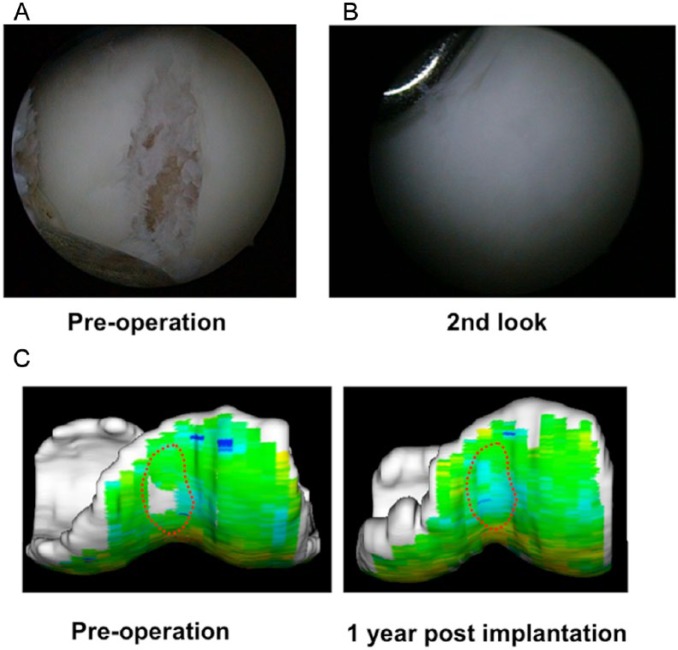
Based on the encouraging results of the preclinical studies discussed above, we have now proceeded clinical studies under the auspices of an approved first in human protocol.75 Patients with symptomatic chondral lesions of the knee, and who meet the inclusion criteria (isolated chondral lesion ≤ 5 cm2, 20 to 60 years of age, with normal alignment) have been enrolled (Fig. 10A). Under general or spinal anesthesia, approximately 1 g of synovial membrane is harvested from the knee joint, which is then subjected to the isolation and culture of MSC for their separation and expansion. Following 3 to 5 weeks post–tissue harvest, the TECs are prepared for autologous implantation. By mini-arthrotomy or arthroscopy, the chondral lesion is debrided so as to not breach the subchondral bone. Before implantation, the TEC is washed several times with sterile phosphate buffered saline to minimize calf serum–related protein contamination, followed by the adjustment of the TEC size to match that of the chondral defect. Implantation is completed within 5 to 10 minutes, without any reinforcement for fixation. The knee is immobilized in a brace for 2 weeks followed by the initiation of range-of-motion exercises and muscle exercises. Full weightbearing is allowed 6 to 8 weeks after implantation surgery. Return to strenuous activity is allowed approximately 12 months following implantation. The duration for follow-up is 1 year and the primary end point of this study is an analysis of adverse reactions. The secondary end point is the assessment of feasibility, which consists of subjective assessment (visual analog score [VAS] for pain, Knee Injury and Osteoarthritis Outcome Score [KOOS]), and structural assessment. For the structural assessment, histologic analysis of a biopsy specimen at 12 months and magnetic resonance imaging (conventional and quantitative such as T2-weighted mapping) at 3, 6, and 12 months are performed. The preliminary results indicate that this treatment restored normal joint function by completely covering the cartilage defect with cartilage-like repair tissue (Fig. 10B) with high T2-weighted mapping profile (Fig. 10C). This clinical study will be completed by March 2015.
Figure 10.
Arthroscopic and magnetic resonance imaging (MRI) analyses of repair tissue following implantation of a tissue-engineered construct (TEC) to repair human chondral defects in clinical trial. (A, B) Arthroscopic views of the preoperation defect and then 1 year after implantation of a TEC. The cartilage defect was completely covered with a cartilage-like repair tissue. (C) T2-weighted mapping of the lesion at the femoral groove. Left, before implantation; right, 1 year after implantation.
TEC Derived from Synovial MSCs: The Next-Generation Cell-Based Strategy to Regenerate Cartilage
The present review has demonstrated the feasibility of using a unique scaffold-free TEC generated from synovial MSCs for effective cell-based cartilage repair. The cultured synovium–derived cells had high self-renewal capacity and stable expression profiles for surface antigen characteristics through passage 4 to 7, similar to what has been observed for bone marrow–derived MSCs. Furthermore, these cells retained the capability for osteogenic, chondrogenic, and adipogenic differentiation as previously reported.26 Thus, the cultured synovium–derived cells have characteristics similar to those of MSCs derived from other sources, and consequently, the in vitro generated TEC could be regarded as an MSC-based 3D bioengineered tissue.
The development of a 3D tissue without an artificial scaffold is the crucial center of this tissue engineering technology, a technology that is based on the active contraction of a cultured monolayer cell/matrix complex. The phenomenon of active tissue contraction to form a TEC is somewhat similar to that observed with cells in collagen gels.76,77 As reported in previous collagen gel studies,78 negative regulators of the actin-cytoskeleton significantly inhibit the contraction of the monolayer sheet and generation of a contracted TEC (unpublished observations). Contractile forces generated within the actin-cytoskeleton of the cultured MSCs may be at least partially involved in this active tissue contraction. Importantly, the TEC develops without any exogenous scaffold and therefore, implantation of the TEC would have minimal risk of potential side effects induced by artificial or extrinsic biological materials contained in a scaffold. Furthermore, we confirmed that human serum is no less effective than bovine serum in promoting proliferation of synovium-derived MSCs without losing the differentiation potential of the cells (unpublished observations). Accordingly, with the use of autologous human serum, it is technically possible to develop the TEC in a xeno-free system, a set of circumstances which would minimize the risk of infectious agents, as well as immune reactivity developing to the TEC via any associated xeno-proteins.49
Since the active contraction of the monolayer cell/matrix complexes could be expected as a natural course when the culture conditions of the complex converts from a conventional adherent culture to a suspension culture, as has been reported in previous collagen gel contraction studies,76 the safe and reproducible detachment of the cultured monolayer cell/matrix complex is a crucial element of this tissue engineering technology. Previous studies have shown the feasibility of using a temperature sensitive culture dish to detach a cultured cell sheet from the substratum.79 Conversely, the present TEC method does not require any special equipment and thus could be an easier and more direct method to accomplish the same purpose.
A further structural advantage of the TEC is that the MSCs and the ECM synthesized by the cells are integrated together into a 3D structure with a uniform cellular distribution. Thus, there is no need to modify or adjust the cellular distribution within the TEC. It is also notable that the TEC possesses sufficiently self-supporting mechanical properties in spite of the fact that it does not contain an artificial scaffold. The tensile strength of the TEC, which is developed in the presence of Asc-2P for 14 or 21 days, is comparable to that of healing ligament tissue at 1 to 2 weeks after injury.80 Therefore, such TEC can be readily handled without causing overt damage to the matrix during implantation procedures.
Another important biological characteristic of the TEC described in this report is its tissue adhesiveness. This property contributes to the rapid and secure adhesion of the TEC to a natural cartilage matrix and thus, simple implantation procedures for the placement of the TEC into chondral lesions or defects could be expected to proceed without augmentation of the initial fixation. Moreover, such adhesiveness also enables rapid self-association internally with its own matrix, a factor that likely contributes to the tissue plasticity of the TEC. In reality, it is thus possible to develop a spherical-shaped tissue several millimeter thick by allowing the released monolayers from several dishes to fold in series. With such “plasticity,” it is possible to develop a TEC that matches the needed size and shape to repair a chondral defect more than several millimeters in thickness. Although we have not yet identified the crucial factor(s) that determine the tissue adhesiveness of the TEC, immunohistochemical analysis has shown that fibronectin and vitronectin are localized at the interface between the TEC and the base of the chondral lesions. Therefore, fibronectin and vitronectin may likely be, at least partially, involved in the adhesive properties of these in vitro generated TEC.
It is known that a 3D culture environment at high density, such as in micromass cultures81 or pellet cultures,82 is an important variable to promote chondrogenesis. However, these methods cannot be directly applied to most clinical situations because of limitations in the mass size of the materials.35 The present studies revealed that the TEC approach overcomes this problem of tissue size while providing a 3D and highly dense environment for the MSCs to differentiate toward a chondrogenic phenotype following implantation without leading to cell and tissue necrosis. The TEC originally does not contain chondrogenic marker molecules such as collagen II, and instead is rich in collagen I and III. However, following implantation in vivo, the basic TEC which did not receive ex vivo stimulation toward chondrogenic differentiation, appears to have responded to the endogenous environment and evolved a matrix composition to that of a chondrogenic tissue. The findings from the in vitro chondrogenesis experiments suggest that local biological and mechanical environment factors may facilitate degradation of the “old” matrix followed by the synthesis of a new chondrogenic matrix, thus leading to an overall phenotypic change of the matrix within the TEC during postimplantation chondrogenic differentiation in vivo.
The series of in vitro experiments reported suggest that the TEC are plastic, adhesive, and capable of chondrogenic differentiation, and a unique and promising implant for cartilage repair. This possibility was initially confirmed following assessment of TEC implanted in vivo into porcine chondral defects. The TEC firmly attached to the surface of injured cartilage at the initial stage of implantation, and thus a sutureless implantation was possible. Thereafter, the TEC maintained good tissue integration to the adjacent cartilage matrix and the repair tissue exhibited chondrogenic differentiation without any evidence of central necrosis out to 6 months after implantation. This biological integration was already evident 3 months after implantation. Using the modified ICRS histological score,83 it was shown that the repair tissue with the TEC implants was histologically superior compared with that of the control lesions in all aspects and implantation of the TEC appeared to prevent progression of the defects toward overt osteoarthritis. Biomechanical analysis also revealed that the tissue repaired with the TEC implant exhibited modulus and frictional properties similar to the properties of normal cartilage. To our knowledge, this study was the first demonstration of a successful MSC-based therapy for repair of a chondral injury without breaching the subchondral plate. Additionally, these results were equivalently observed in both immature and mature animals. Therefore, this study would support the clinical application of this strategy to promote cartilage repair and regeneration in patients over wide range of patient ages. This may be particularly relevant to older patients where autologous chondrocytes are limited in number and quality. Also, this procedure has been translated into clinical trials, and the preliminary clinical results indicate that this treatment restored normal joint functions by covering the cartilage defects with a predominantly hyaline-like cartilage repair tissue. Therefore, these studies would support the clinical application of this TEC strategy to promote cartilage repair and regeneration in appropriate patients with defined chondral lesions.
It is notable that the implantation of TEC without any pretreatment to promote a specific differentiation pathway resulted in tissue repair associated with an active chondrogenic differentiation response. However, the repair was not perfect, in that the repair tissue still contained some fibrous tissue, mainly at the surface or in the superficial zone. In the detailed biomechanical studies discussed above, the TEC-mediated repair cartilage still exhibited some compromised mechanical properties at the superficial zone, properties that likely need improvement in the future for maintenance of long term repair cartilage integrity.74
However, it should be noted that the implanted TEC did not exhibit any inappropriate phenotypic changes. While the mechanisms underlying the success obtained thus far are still not clearly delineated, it should be noted that the animal model used in the present study involved a chondral injury that did not breach the subchondral plate and thus, an environment relatively free of bleeding and bone marrow leakage at the site of the lesion. Such conditions might be involved in the specific chondrogenic differentiation observed in vivo rather than fibrocartilage development which is observed after breaching the subchondral bone barrier with the microfracture method. However, to attain a more extensive chondrogenic differentiation response including surface area, some biological manipulation of the TEC may be required before (or after) implantation to further optimize the rate and extent of repair, and this direction is the focus of some of our current research. In addition, the use of TEC in the repair of osteochrondral defects is currently underway.
In conclusion, we have elucidated many of the characteristics of a scaffold-free 3D synthetic tissue (TEC) derived from cultured synovium-derived MSCs as a unique and promising implant for cartilage repair. This was demonstrated in vivo using a preclinical model of a range of ages31,73,84 as well as more recently in clinical trials. Because of the scaffold-free nature of the in vitro–generated structure, implantation of the TEC could yield more long-term safety and efficacy than that derived from scaffold-based cell therapies. Being a collagen I–rich matrix, the basic TEC could also be potentially suitable for augmenting repair of compromised skin, or enhancing the repair of ligaments or tendons, which are also collagen I rich. Since the TEC also has osteogenic or adipogenic differentiation capacity, the basic TEC could likely also be used for other applications. Moreover, the TEC could be developed from MSCs derived from other tissues, such as adipose tissue which is an abundant source of MSC and readily obtained without entering the joint. Therefore, tissue engineering using the TEC technology could potentially provide a variety of therapeutic interventions in regenerative medicine for a number of tissue applications using MSC from different sources.
Footnotes
Acknowledgments and Funding: We thank Kiyoto Nagai, MS, Yoshihisa Fujishima, BS, Machiko Imura, BS, and Ryosuke Nansai, MS for mechanical testing. This work is supported by a grant from the New Energy and Industrial Technology Development Organization, Japan, and a Grant-in-Aid for Scientific Research, Japan Society for the Promotion of Science. DAH was supported by the Alberta Innovates Health Solutions Osteoarthritis Team Grant.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by our institutional review board.
References
- 1. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730-4. [DOI] [PubMed] [Google Scholar]
- 2. Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;(402):21-37. [DOI] [PubMed] [Google Scholar]
- 3. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432-63. [DOI] [PubMed] [Google Scholar]
- 4. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911-20. [DOI] [PubMed] [Google Scholar]
- 5. Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;(365):149-62. [DOI] [PubMed] [Google Scholar]
- 6. Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S. Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials. 2005;26:3617-29. [DOI] [PubMed] [Google Scholar]
- 7. Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005;13:213-21. [DOI] [PubMed] [Google Scholar]
- 8. Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22:1180-6. [DOI] [PubMed] [Google Scholar]
- 9. Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119-25. [DOI] [PubMed] [Google Scholar]
- 10. Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37:33-41. [DOI] [PubMed] [Google Scholar]
- 11. Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am. 2013;95:1640-50. [DOI] [PubMed] [Google Scholar]
- 12. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 13. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12. [DOI] [PubMed] [Google Scholar]
- 14. Browne JE, Anderson AF, Arciero R, Mandelbaum B, Moseley JB, Jr, Micheli LJ, et al. Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res. 2005;(436):237-45. [DOI] [PubMed] [Google Scholar]
- 15. Niemeyer P, Salzmann G, Feucht M, Pestka J, Porichis S, Ogon P, et al. First-generation versus second-generation autologous chondrocyte implantation for treatment of cartilage defects of the knee: a matched-pair analysis on long-term clinical outcome. Int Orthop. 2014;38:2065-70. [DOI] [PubMed] [Google Scholar]
- 16. Goyal D, Goyal A, Keyhani S, Lee EH, Hui JH. Evidence-based status of second- and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy. 2013;29:1872-8. [DOI] [PubMed] [Google Scholar]
- 17. Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy. 2007;23:381-7. [DOI] [PubMed] [Google Scholar]
- 18. Bright P, Hambly K. A systematic review of reporting of rehabilitation in articular-cartilage-repair studies of third-generation autologous chondrocyte implantation in the knee. J Sport Rehabil. 2014;23:182-91. [DOI] [PubMed] [Google Scholar]
- 19. Gobbi A, Kon E, Berruto M, Filardo G, Delcogliano M, Boldrini L, et al. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med. 2009;37:1083-92. [DOI] [PubMed] [Google Scholar]
- 20. Brittberg M, Peterson L, Sjogren-Jansson E, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am. 2003;85-A(Suppl 3):109-15. [DOI] [PubMed] [Google Scholar]
- 21. Ochi M, Adachi N, Nobuto H, Yanada S, Ito Y, Agung M. Articular cartilage repair using tissue engineering technique—novel approach with minimally invasive procedure. Artif Organs. 2004;28:28-32. [DOI] [PubMed] [Google Scholar]
- 22. Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;(435):96-105. [DOI] [PubMed] [Google Scholar]
- 23. Hickery MS, Bayliss MT, Dudhia J, Lewthwaite JC, Edwards JC, Pitsillides AA. Age-related changes in the response of human articular cartilage to IL-1α and transforming growth factor-β (TGF-β): chondrocytes exhibit a diminished sensitivity to TGF-β. J Biol Chem. 2003;278:53063-71. [DOI] [PubMed] [Google Scholar]
- 24. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-7. [DOI] [PubMed] [Google Scholar]
- 25. Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem cells. Gene Ther. 2002;9:642-7. [DOI] [PubMed] [Google Scholar]
- 26. De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928-42. [DOI] [PubMed] [Google Scholar]
- 27. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521-9. [DOI] [PubMed] [Google Scholar]
- 28. Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003;(412):196-212. [DOI] [PubMed] [Google Scholar]
- 29. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669-75. [DOI] [PubMed] [Google Scholar]
- 30. Ando W, Kutcher JJ, Krawetz R, Sen A, Nakamura N, Frank CB, et al. Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage. Cytotherapy. 2014;16:776-88. [DOI] [PubMed] [Google Scholar]
- 31. Shimomura K, Ando W, Tateishi K, Nansai R, Fujie H, Hart DA, et al. The influence of skeletal maturity on allogenic synovial mesenchymal stem cell-based repair of cartilage in a large animal model. Biomaterials. 2010;31:8004-11. [DOI] [PubMed] [Google Scholar]
- 32. Dashtdar H, Rothan HA, Tay T, Ahmad RE, Ali R, Tay LX, et al. A preliminary study comparing the use of allogenic chondrogenic pre-differentiated and undifferentiated mesenchymal stem cells for the repair of full thickness articular cartilage defects in rabbits. J Orthop Res. 2011;29:1336-42. [DOI] [PubMed] [Google Scholar]
- 33. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-76. [DOI] [PubMed] [Google Scholar]
- 34. Tsumaki N, Okada M, Yamashita A. iPS cell technologies and cartilage regeneration. Bone. 2015;70:48-54. [DOI] [PubMed] [Google Scholar]
- 35. De Bari C, Dell’Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50:142-50. [DOI] [PubMed] [Google Scholar]
- 36. Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130-8. [DOI] [PubMed] [Google Scholar]
- 37. Andriano KP, Tabata Y, Ikada Y, Heller J. In vitro and in vivo comparison of bulk and surface hydrolysis in absorbable polymer scaffolds for tissue engineering. J Biomed Mater Res. 1999;48:602-12. [DOI] [PubMed] [Google Scholar]
- 38. Guo JF, Jourdian GW, MacCallum DK. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 1989;19:277-97. [DOI] [PubMed] [Google Scholar]
- 39. Masuda K, Takegami K, An H, Kumano F, Chiba K, Andersson GB, et al. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21:922-30. [DOI] [PubMed] [Google Scholar]
- 40. Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221:1-22. [DOI] [PubMed] [Google Scholar]
- 41. Homminga GN, Buma P, Koot HW, van der Kraan PM, van den Berg WB. Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand. 1993;64:441-5. [DOI] [PubMed] [Google Scholar]
- 42. Brun P, Cortivo R, Zavan B, Vecchiato N, Abatangelo G. In vitro reconstructed tissues on hyaluronan-based temporary scaffolding. J Mater Sci Mater Med. 1999;10:683-8. [DOI] [PubMed] [Google Scholar]
- 43. Lahiji A, Sohrabi A, Hungerford DS, Frondoza CG. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res. 2000;51:586-95. [DOI] [PubMed] [Google Scholar]
- 44. Shimomura K, Moriguchi Y, Murawski CD, Yoshikawa H, Nakamura N. Osteochondral tissue engineering with biphasic scaffold: current strategies and techniques. Tissue Eng Part B Rev. 2014;20:468-76. [DOI] [PubMed] [Google Scholar]
- 45. O’Grady JE, Bordon DM. Global regulatory registration requirements for collagen-based combination products: points to consider. Adv Drug Deliv Rev. 2003;55:1699-721. [DOI] [PubMed] [Google Scholar]
- 46. Daniels AU, Andriano KP, Smutz WP, Chang MK, Heller J. Evaluation of absorbable poly(ortho esters) for use in surgical implants. J Appl Biomater. 1994;5:51-64. [DOI] [PubMed] [Google Scholar]
- 47. van der Elst M, Klein CP, de Blieck-Hogervorst JM, Patka P, Haarman HJ. Bone tissue response to biodegradable polymers used for intra medullary fracture fixation: a long-term in vivo study in sheep femora. Biomaterials. 1999;20:121-8. [DOI] [PubMed] [Google Scholar]
- 48. Yang C, Hillas PJ, Báez JA, Nokelainen M, Balan J, Tang J, et al. The application of recombinant human collagen in tissue engineering. BioDrugs. 2004;18:103-19. [DOI] [PubMed] [Google Scholar]
- 49. Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228-32. [DOI] [PubMed] [Google Scholar]
- 50. Ebihara G, Sato M, Yamato M, Mitani G, Kutsuna T, Nagai T, et al. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials. 2012;33:3846-51. [DOI] [PubMed] [Google Scholar]
- 51. Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T. Temperature-responsive culture dishes allow nonenzymatic harvest of differentiated Madin-Darby canine kidney (MDCK) cell sheets. J Biomed Mater Res. 2000;51:216-23. [DOI] [PubMed] [Google Scholar]
- 52. Nakamura T, Sekiya I, Muneta T, Hatsushika D, Horie M, Tsuji K, et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy. 2012;14:327-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nakamura N, Horibe S, Iwahashi T, Kawano K, Shino K, Yoshikawa H. Healing of a chondral fragment of the knee in an adolescent after internal fixation. A case report. J Bone Joint Surg Am. 2004;86-A:2741-6. [DOI] [PubMed] [Google Scholar]
- 55. De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267-70. [DOI] [PubMed] [Google Scholar]
- 56. Hunziker EB, Rosenberg LC. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am. 1996;78:721-33. [DOI] [PubMed] [Google Scholar]
- 57. Lee CR, Grodzinsky AJ, Hsu HP, Spector M. Effects of a cultured autologous chondrocyte-seeded type II collagen scaffold on the healing of a chondral defect in a canine model. J Orthop Res. 2003;21:272-81. [DOI] [PubMed] [Google Scholar]
- 58. Dorotka R, Bindreiter U, Macfelda K, Windberger U, Nehrer S. Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthritis Cartilage. 2005;13:655-64. [DOI] [PubMed] [Google Scholar]
- 59. Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704-13. [DOI] [PubMed] [Google Scholar]
- 60. Quarto R, Thomas D, Liang CT. Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif Tissue Int. 1995;56:123-9. [DOI] [PubMed] [Google Scholar]
- 61. Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996;11:568-77. [DOI] [PubMed] [Google Scholar]
- 62. Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Oreffo RO, Bennett A, Carr AJ, Triffitt JT. Patients with primary osteoarthritis show no change with ageing in the number of osteogenic precursors. Scand J Rheumatol. 1998;27:415-24. [DOI] [PubMed] [Google Scholar]
- 64. Leskela HV, Risteli J, Niskanen S, Koivunen J, Ivaska KK, Lehenkari P. Osteoblast recruitment from stem cells does not decrease by age at late adulthood. Biochem Biophys Res Commun. 2003;311:1008-13. [DOI] [PubMed] [Google Scholar]
- 65. De Bari C, Dell’Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85-95. [DOI] [PubMed] [Google Scholar]
- 66. Scharstuhl A, Schewe B, Benz K, Gaissmaier C, Buhring HJ, Stoop R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 2007;25:3244-51. [DOI] [PubMed] [Google Scholar]
- 67. Rudert M. Histological evaluation of osteochondral defects: consideration of animal models with emphasis on the rabbit, experimental setup, follow-up and applied methods. Cells Tissues Organs. 2002;171:229-40. [DOI] [PubMed] [Google Scholar]
- 68. Bos PK, Verhaar JA, van Osch GJ. Age-related differences in articular cartilage wound healing: a potential role for transforming growth factor beta1 in adult cartilage repair. Adv Exp Med Biol. 2006;585:297-309. [DOI] [PubMed] [Google Scholar]
- 69. Yamamoto T, Wakitani S, Imoto K, Hattori T, Nakaya H, Saito M, et al. Fibroblast growth factor-2 promotes the repair of partial thickness defects of articular cartilage in immature rabbits but not in mature rabbits. Osteoarthritis Cartilage. 2004;12:636-41. [DOI] [PubMed] [Google Scholar]
- 70. Wei X, Gao J, Messner K. Maturation-dependent repair of untreated osteochondral defects in the rabbit knee joint. J Biomed Mater Res. 1997;34:63-72. [DOI] [PubMed] [Google Scholar]
- 71. Vodicka P, Smetana K, Jr, Dvorankova B, Emerick T, Xu YZ, Ourednik J, et al. The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci. 2005;1049:161-71. [DOI] [PubMed] [Google Scholar]
- 72. Huang CY, Soltz MA, Kopacz M, Mow VC, Ateshian GA. Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage. J Biomech Eng. 2003;125:84-93. [DOI] [PubMed] [Google Scholar]
- 73. Ando W, Tateishi K, Hart DA, Katakai D, Tanaka Y, Nakata K, et al. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials. 2007;28:5462-70. [DOI] [PubMed] [Google Scholar]
- 74. Ando W, Fujie H, Moriguchi Y, Nansai R, Shimomura K, Hart DA, et al. Detection of abnormalities in the superficial zone of cartilage repaired using a tissue engineered construct derived from synovial stem cells. Eur Cell Mater. 2012;24:292-307. [DOI] [PubMed] [Google Scholar]
- 75. Nakamura N, Hui J, Koizumi K, Yasui Y, Nishii T, Lad D, et al. Stem cell therapy in cartilage repair—culture-free and cell culture–based methods. Oper Tech Orthop. 2014;24:54-60. [Google Scholar]
- 76. Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76:1274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sheetz MP, Felsenfeld DP, Galbraith CG. Cell migration: regulation of force on extracellular-matrix-integrin complexes. Trends Cell Biol. 1998;8:51-4. [DOI] [PubMed] [Google Scholar]
- 78. Yanase M, Ikeda H, Matsui A, Maekawa H, Noiri E, Tomiya T, et al. Lysophosphatidic acid enhances collagen gel contraction by hepatic stellate cells: association with rho-kinase. Biochem Biophys Res Commun. 2000;277:72-8. [DOI] [PubMed] [Google Scholar]
- 79. Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials. 1995;16:297-303. [DOI] [PubMed] [Google Scholar]
- 80. Provenzano PP, Hayashi K, Kunz DN, Markel MD, Vanderby R., Jr. Healing of subfailure ligament injury: comparison between immature and mature ligaments in a rat model. J Orthop Res. 2002;20:975-83. [DOI] [PubMed] [Google Scholar]
- 81. Denker AE, Nicoll SB, Tuan RS. Formation of cartilage-like spheroids by micromass cultures of murine C3H10T1/2 cells upon treatment with transforming growth factor-β 1. Differentiation. 1995;59:25-34. [DOI] [PubMed] [Google Scholar]
- 82. Lennon DP, Haynesworth SE, Arm DM, Baber MA, Caplan AI. Dilution of human mesenchymal stem cells with dermal fibroblasts and the effects on in vitro and in vivo osteochondrogenesis. Dev Dyn. 2000;219:50-62. [DOI] [PubMed] [Google Scholar]
- 83. Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85-A(Suppl 2):45-57. [PubMed] [Google Scholar]
- 84. Ando W, Tateishi K, Katakai D, Hart DA, Higuchi C, Nakata K, et al. In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: biological and mechanical properties and further chondrogenic potential. Tissue Eng Part A. 2008;14:2041-9. [DOI] [PubMed] [Google Scholar]