Abstract
Introduction:
Osteochondral allograft (OCA) transplantation is a suitable treatment option for large osteochondral defects. Magnetic resonance imaging (MRI) is an objective, reproducible, noninvasive monitoring tool for postoperative assessment after cartilage surgery.
Objective:
To correlate Osteochondral Allograft MRI Scoring System (OCAMRISS) in patients undergoing OCA transplantation in the knee with clinical outcomes and determine interobserver agreement of this scoring system.
Methods:
Fifteen patients underwent OCA transplantation in the knee and received a postoperative MRI. Four examiners read each MRI and completed an OCAMRISS. Interobserver agreement and intraclass correlation coefficients (ICCs) were assessed. Clinical outcomes were evaluated. Correlation between the OCAMRISS and clinical outcomes was calculated using Spearman’s correlation coefficients.
Results:
Interobserver agreement on individual features of the OCAMRISS was superior (κ = 0.81-1.0) in 65% of comparisons, substantial (κ = 0.61-0.8) in 14%, moderate (κ = 0.41-0.6) in 18%, and fair (κ = 0.21-0.4) in 3%. Agreement among readers was very strong for the cartilage, bone, ancillary, and total scores with 96% of comparisons having an ICC >0.80. International Knee Documentation Committee (IKDC) function scores were correlated with OCAMRISS cartilage score (ρ = 0.53, P = 0.044) and total score (ρ = 0.67, P = 0.006). The Knee injury and Osteoarthritis Outcome Score (KOOS) sports/recreation subscale was correlated with OCAMRISS ancillary score (ρ = 0.58, P = 0.049) and total score (ρ = 0.64, P = 0.024). No correlation was observed with subchondral bone features of OCAMRISS and any of the outcome scores.
Conclusions:
The recently described OCAMRISS is a reproducible grading system for in vivo evaluation after osteochondral allograft transplantation.
Keywords: knee, cartilage repair, osteochondral allograft transplantation, magnetic resonance imaging
Introduction
Osteochondral allograft (OCA) transplantation is indicated for selected cases of cartilage injuries, especially in young patients with large lesions with subchondral bone involvement.1-3 Osteochondritis dissecans,4 degenerative and posttraumatic cartilage injuries,5 bipolar cartilage injuries,1 and osteonecrosis1-3 are the most common causes of large cartilage injuries suitable for osteochondral allografting. OCA transplantation replaces both cartilage and underlying subchondral bone.6
Magnetic resonance imaging (MRI) is an objective and reproducible monitoring tool for postoperative assessment after cartilage repair procedures.7-10 As a noninvasive and accurate method, MRI can assess cartilage repair morphology, cartilage volume, peripheral integration, and subchondral bone changes.11-13 Although not widely used on clinical scanners, recent advancements in specific sequences of MRI such as delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), T1 rho, T2-mapping and diffusion-weighted imaging can assess biochemical details of cartilage tissue.14 The Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score is a semiquantitative scale used for monitoring tissue after cartilage repair surgeries such as autologous chondrocyte implantation and microfracture.15,16 However, recent studies have shown a lack of correlation between clinical outcomes and the MRI.17 Welsch et al.13 noted the importance of subchondral bone after cartilage repair and introduced a subchondral interface feature in the 3D MOCART score, but no specific detail was provided for the use of this score after OCA transplantation. Therefore, our group developed a comprehensive Osteochondral Allograft MRI Scoring System (OCAMRISS) and validated this score in an experimental model with histopathologic and micro–computed tomography (μCT) reference standards.18
The OCAMRISS is an attractive score for OCA transplantation because of the inclusion of 4 features addressing the subchondral bone: subchondral bone plate congruity, subchondral bone marrow signal, presence of subchondral cystic changes, and osseous integration. This recently developed scoring system has not yet been used for the evaluation of OCA transplantation outcome in human knees.
The objective of this study was to validate the OCAMRISS for clinical use by evaluating the interobserver variability and determining the radiological features that best correlate with clinical outcome.
Methods
Study Population
In this retrospective study, 15 patients were identified who had MRI exams after OCA transplantation in an institutional review board–approved clinical database. Patient demographics and clinical characteristics are detailed in Table 1.
Table 1.
Patient Demographics and Clinical Characteristics of the Osteochondral Allografts.
| Age, years, mean ± SD | 31.2 ± 11.8 |
| Diagnosis, n (%) | |
| Osteochondritis dissecans | 9 (60.0) |
| Degenerative chondral lesion | 4 (26.7) |
| Tibial plateau fracture | 1 (6.7) |
| Failed osteochondral allograft | 1 (6.7) |
| Male/female, n | 11/4 |
| Body mass index, kg/m2, mean ± SD | 25.2 ± 3.9 |
| Location of the graft, n (%) | |
| Medial femoral condyle | 7 (46.7) |
| Lateral femoral condyle | 4 (26.7) |
| Trochlea | 2 (13.3) |
| Patella | 1 (6.7) |
| Lateral tibial plateau | 1 (6.7) |
| Allograft size, cm2, mean ± SD | 8.2 ± 6.7 |
The fresh OCAs were processed and obtained from the Joint Restoration Foundation (Centennial, CO). The OCA transplantations were performed through a medial or lateral parapatellar arthrotomy for substitution of the injured cartilage in the femoral condyle, trochlea, tibial plateau, and patella.19 The size of the lesion was recorded and the nonviable tissue found was debrided and prepared down in a geometric format to a depth of 5 to 7 mm. For lesions smaller than 10 cm2, a dowel technique was employed.19 For lesions larger than 10 cm2, a shell allograft technique was chosen.19 In order to decrease the immunogenicity of the graft, the immunogenic marrow elements from the osseous surface was washed out with pulsatile lavage. The graft was trimmed into a shape matching the lesion and trial fittings were performed to ensure a well-positioned graft. The grafts were fixed either by press fit fixation or with the use of bioabsorbable pins (OrthoSorb; DePuy Synthes, Warsaw, IN).
Imaging and Image Analysis
The examinations followed a standard clinical protocol for MRI musculoskeletal injuries (Figs. 1-3). Imaging was performed on a 1.5T Magnetom Avanto system (Siemens, Erlangen, Germany). MRI protocols incorporated coronal and sagittal fast spin-echo (FSE) intermediate-weighted, coronal and sagittal FSE intermediate-weighted with fat suppression, and axial FSE intermediate-weighted with fat suppression (Table 2). No quantitative cartilage imaging sequence was employed.
Figure 1.
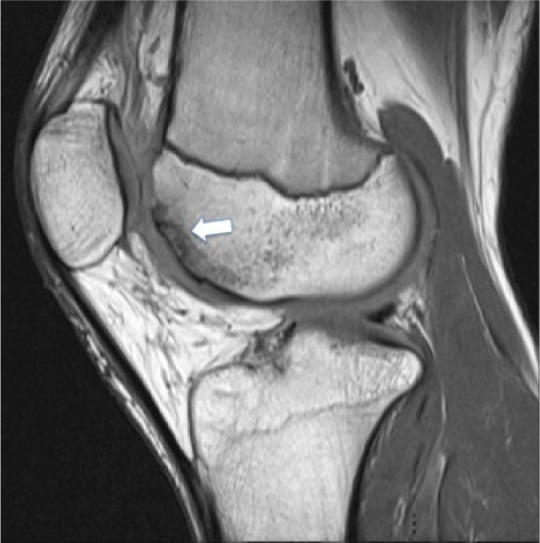
Sagittal intermediate-weighted magnetic resonance image of a patient after osteochondral allograft (OCA) transplantation in the femoral trochlea (arrow). OCA cartilage signal, “fill,” and surface congruity are normal compared with adjacent host cartilage. Subchondral bone plate is incongruent and subchondral bone marrow signal is abnormal, but the host-graft junction demonstrates some osseous incorporation.
Figure 2.
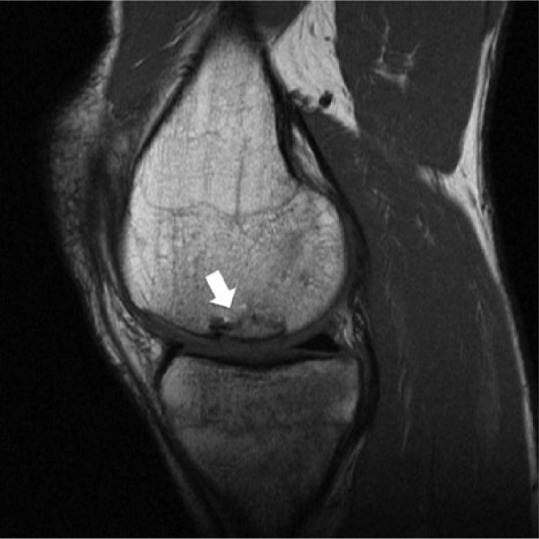
Sagittal intermediate-weighted magnetic resonance image of a patient after osteochondral allograft (OCA) transplantation in the medial femoral condyle (arrow). OCA cartilage signal, “fill,” and surface congruity are normal compared with adjacent host cartilage. Subchondral bone plate is incongruent, but subchondral bone marrow signal is preserved and the host-graft junction demonstrates some osseous incorporation.
Figure 3.
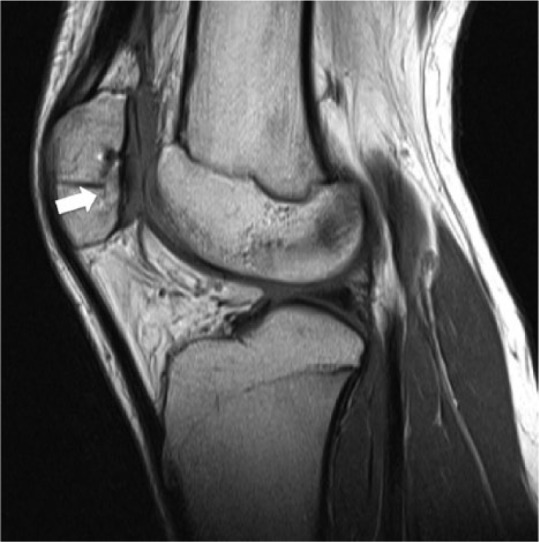
Sagittal intermediate-weighted magnetic resonance image of a patient after osteochondral allograft (OCA) transplantation in the patella (arrow). OCA cartilage signal, “fill,” and surface congruity are normal compared with adjacent host cartilage. Subchondral bone plate is congruent, subchondral bone marrow signal is preserved, and the host-graft junction demonstrates osseous incorporation.
Table 2.
Magnetic Resonance Imaging (MRI) Protocol.
| MRI Protocol | Repetition Time (TR), ms/Echo Time (TE), ms | Echo-Train Length (ETL) | Slice Thickness, mm | Interslice Gap, mm | Matrix | Field of View, cm | Signal Average |
|---|---|---|---|---|---|---|---|
| Coronal and sagittal fast spin-echo (FSE) intermediate-weighted | 1040/23 | 3 | 3.5 | 1 | 320 × 192 | 14 | 1 |
| Coronal and sagittal fast spin-echo (FSE) intermediate-weighted with fat suppression | 2730/47 | 7 | 3.5 | 1 | 256 × 205 | 14 | 1 |
| Axial fast spin-echo (FSE) intermediate-weighted with fat suppression | 2590/44 | 7 | 4.0 | 1 | 256 × 192 | 14 | 2 |
For MRI assessment of osteochondral allograft transplantation, we used the previously published comprehensive OCAMRISS.18 The original scoring system included 9 primary features of the graft (5 cartilage and 4 subchondral bone) and 4 ancillary features of the joint (Table 3). Since ultrashort echo (UTE) sequences necessary for the evaluation of the integrity of the calcified cartilage layer20 are not yet available on clinical scanners, this feature was not included in our analysis. All available images and planes are utilized to formulate the OCAMRISS score. Furthermore, for the binary subscores (Features 6 through 13), if the appearance is normal on one slice and abnormal on an adjacent slice, the abnormal score should be used.
Table 3.
Osteochondral Allograft Magnetic Resonance Imaging Scoring System (OCAMRISS) Scheme.
| MRI Feature | MRI Score | |
|---|---|---|
| Cartilage features | 1. Cartilage signal of graft | 0: Normal |
| 1: Altered intensity (either hypointense or hyperintense, but not fluid) | ||
| 2: Fluid signal intensity on all sequences | ||
| 2. Cartilage “fill” of graft (percentage of volume) | 0: 76% to 100% | |
| 1: 51% to 75% or >100% | ||
| 2: <50% | ||
| 3. Cartilage edge integration at host- graft junction | 0: No discernible boundary | |
| 1: Discernible boundary | ||
| 2: Discernible fissure >1 mm | ||
| 4. Cartilage surface congruity of graft and host-graft junction | 0: Flush | |
| 1: <50% offset of host cartilage | ||
| 2: >50% offset of host cartilage | ||
| 5. Calcified cartilage integrity of graft | 0: Intact, thin, and smooth | |
| 1: Altered (disrupted, thickened, or blurred) | ||
| Bone features | 6. Subchondral bone plate congruity of graft and host-graft junction | 0: Intact and flush |
| 1: Disrupted or not flush by >1 subchondral thickness | ||
| 7. Subchondral bone marrow signal intensity of graft relative to epiphyseal bone | 0: Normal | |
| 1: Abnormal (bone marrow edema pattern or hypointensity on all sequences) | ||
| 8. Osseous integration at host-graft junction | 0: Crossing trabeculae | |
| 1: Discernible cleft | ||
| 9. Presence of cystic changes of graft and host-graft junction | 0: Absent | |
| 1: Present | ||
| Ancillary features | 10. Opposing cartilage | 0: Normal |
| 1: Abnormal | ||
| 11. Meniscal tears | 0: Absent | |
| 1: Present | ||
| 12. Synovitis | 0: Absent | |
| 1: Present | ||
| 13. Fat pad scarring | 0: Absent | |
| 1: Present |
To evaluate interobserver reliability, images were independently scored by 2 fellowship-trained musculoskeletal radiologists and 2 fellowship-trained orthopaedic surgeons specializing in sports medicine. Prior to scoring, 4 hours of consensus training sessions were performed by the 2 orthopaedic surgeons to calibrate and standardize scoring on an independent data set. The 2 radiologists did not undergo a training session prior to scoring and their evaluation was entirely independent.
Clinical Evaluation
Clinical evaluation was performed postoperatively with the International Knee Documentation Committee (IKDC) subjective knee evaluation form,21 Knee injury and Osteoarthritis Outcome Score (KOOS) subcategories symptoms, pain, function in daily living, function in sport and recreation (Sports/Rec), and knee-related quality of life (QOL).
Statistical Analysis
Descriptive statistics were performed. Interobserver agreement was assessed using Cohen’s kappa coefficients for the OCAMRISS individual features and intraclass correlation coefficients for the OCAMRISS total scores. Cohen’s kappa coefficient and intraclass correlation coefficient vary from 0 (no agreement) to 1.0 (complete agreement). The intermediate values were interpreted as follows: 0 to 0.2, slight agreement; 0.21 to 0.4, fair agreement; 0.41 to 0.6, moderate agreement; 0.61 to 0.8, substantial agreement; 0.81 to 1.0, superior agreement. Spearman’s correlation coefficients were used to assess correlation between standardized clinical questionnaires (IKDC and KOOS) and the OCAMRISS total scores. For individual features of the OCAMRISS, Mann-Whitney U tests were performed to compare mean IKDC and KOOS scores (absence vs. presence of each feature). For the cartilage individual features, scores of 1 or 2 were collapsed together for analysis purposes.
Results
The mean time from OCA transplantation to MRI acquisition was 3 ± 2.3 years. The mean time from clinical outcome assessment to MRI acquisition was 0.4 ± 1.5 years. Interobserver agreement among the 4 examiners on individual features of the OCAMRISS was superior in 65% of comparisons (Table 4). Agreement among the readers was very strong for the cartilage, bone, ancillary, and total scores with 96% of comparisons having an intraclass correlation coefficient >0.80. Since the interobserver agreement between all examiners was considered superior for individual score features and for total scores, only the senior radiologist’s score was used for correlation with clinical outcome.
Table 4.
Descriptor, Coefficient Range, and Percentage of Comparisons for Each Descriptor.
| Descriptor | Coefficient (κ) Range | % of Comparisons |
|---|---|---|
| Superior | 0.81-1.0 | 65 |
| Substantial | 0.61-0.8 | 14 |
| Moderate | 0.41-0.6 | 18 |
| Fair | 0.21-0.4 | 3 |
The mean scores of IKDC and KOOS are reported in Table 5. The IKDC function scores were correlated with the OCAMRISS cartilage score (ρ = 0.53, P = 0.044) and total score (ρ = 0.67, P = 0.006). The KOOS sports/recreation subscale was correlated with the OCAMRISS ancillary score (ρ = 0.58, P = 0.049) and total score (ρ = 0.64, P = 0.024). Some of the clinical scores also differedamongspecific OCAMRISS individual features when comparing the presence or absence of a feature (Table 6). No significant correlation was observed with subchondral bone features of the OCAMRISS and any of the outcome scores.
Table 5.
Postoperative Outcomes After OCA Transplantation.
| Measure | Mean ± SD |
|---|---|
| IKDC Pain | 2.7 ± 2.5 |
| IKDC Function | 6.8 ± 1.8 |
| IKDC Total | 69.7 ± 19.6 |
| KOOS Symptoms | 75.6 ± 18.9 |
| KOOS Pain | 76.5 ± 18.9 |
| KOOS ADL | 84.1 ± 16.9 |
| KOOS Sports/Rec | 60.8 ± 22.7 |
| KOOS QOL | 45.7 ± 29.1 |
ADL = activities of daily living; IKDC = International Knee Documentation Committee subjective knee evaluation form; KOOS = Knee injury and Osteoarthritis Outcome Score; OCA = osteochondral allograft; QOL = knee-related quality of life; Sports/Rec = sports/recreation.
Table 6.
Positive Correlation of Clinical Outcomes With Osteochondral Allograft Magnetic Resonance Imaging Scoring System (OCAMRISS) Features.
| OCAMRISS Features | Clinical Outcomes | ρ | P Value |
|---|---|---|---|
| 1. Cartilage features total | |||
| IKDC Function | 0.53 | 0.044 | |
| 1.1 Cartilage fill | |||
| IKDC Function | 0.004 | ||
| IKDC Total | 0.05 | ||
| 1.2 Opposing cartilage | |||
| IKDC Function | 0.013 | ||
| KOOS Sports/Rec | (0.036) | ||
| 2. Ancillary features total | |||
| KOOS Sports/Rec | 0.58 | 0.049 | |
| 2.1 Meniscal tear | |||
| KOOS Symptoms | 0.03 | ||
| KOOS QOL | 0.03 | ||
| 3. Bone features | |||
| — | |||
| 4. OCAMRISS total score | |||
| IKDC Function | 0.67 | 0.006 | |
| KOOS Sports/Rec | 0.64 | 0.024 | |
IKDC = International Knee Documentation Committee subjective knee evaluation form; KOOS = Knee injury and Osteoarthritis Outcome Score; QOL = knee-related quality of life; Sports/Rec = sports and recreation.
Discussion
The present study showed that the OCAMRISS is a reliable scoring system to evaluate patients after OCA transplantation. Some subjective and objective outcomes, including the IKDC function, the KOOS sports/recreation, the KOOS symptoms, and the KOOS quality of life were correlated with some OCAMRISS features and/or total OCAMRISS score. No correlation was observed with subchondral bone features of the OCAMRISS and any of the outcome scores.
MRI is a noninvasive instrument useful to detect and monitor the cartilage status after several types of cartilage repair or reconstruction procedures. The International Cartilage Repair Society (ICRS) recommends appropriate sequences to conduct cartilage clinical trials as an important tool for postoperative assessment of cartilage status.22 New advancements in MRI techniques such as delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), T1 rho, T2-mapping, and diffusion-weighted imaging can show biomechanical details of cartilage, but studies performing correlation between clinical measures and radiological findings and measurements are lacking.16,23 Many of these new sequences are not widely available on clinical scanners. Similarly, UTE sequences, which allow for evaluation of the calcified cartilage layer, are not yet available as a product sequence on clinical MRI scanners and were excluded from the analysis in the current study.
The MOCART score was originally described for cartilage repair with cell-based transplantation. In the study that validated MOCART for the cartilage repair surgeries, Marlovits et al.12 in an observational study with 13 patients submitted to matrix-associated autologous chondrocyte transplantation showed that KOOS and visual analog scale were correlated with some MRI variables of the MOCART system such as “filling of the defect,” “structure of the repair tissue,” and “changes in the subchondral bone”. Later Blackman et al.15 and de Windt et al.16 could not prove the same with a systematic review of pooled and analyzed data from cartilage repair studies. No strong evidence was found to support MRI as a reliable method to predict clinical outcomes. Aldrian et al.24 also reported in a 10-year follow-up study after matrix-induced autologous chondrocyte transplantation that no correlation was found between clinical outcome and MRI, but a strong negative correlation was found between defect size and visual analog scale. This scoring system was not defined for osteochondral allograft transplantation. Some features, including “integration to the border zone” and “presence of adhesions” of the MOCART system may be more important for cell-based cartilage repair techniques than for osteochondral allograft transplantation.
The importance of the subchondral bone became evident for cartilage repair and reconstruction when the MOCART score was improved to the 3D MOCART score. The 3D MOCART introduced the “bone interface” to address integration of the donor and host as well as integration of a possible periosteal flap and “chondral osteophytes.”25 Chondral osteophytes or intralesional osteophytes, which are caused by the disruption of the subchondral plate and damage of the subchondral bone, are a possible complication after bone marrow stimulation techniques. Cell-based techniques such as autologous chondrocyte implantation showed 3 to 5 times greater failure after a bone marrow stimulating technique, highlighting the importance of the subchondral bone damage in cartilage repair survivorship.9,26 The subchondral bone findings and correlation with clinical outcomes remains controversial in the literature. For reparative procedures such as autologous chondrocyte implantation, edema of the subchondral bone seems to influence the clinical outcomes.12,27 Other authors show that the persistent edema of the subchondral bone 1 year of after an autologous chondrocyte implantation surgery may not have any significant clinical importance.28-30
In the case of osteochondral transfer such as osteochondral autograft transfer and osteochondral allograft transplantation, the subchondral bone is an important characteristic to be analyzed. The OCAMRISS score employed in our study is especially focused on the subchondral bone and has the advantage of including features of cartilage, ancillary features, and subchondral bone. Studies have shown that subchondral bone alterations are related to OCA transplantation survivorship. In a fresh osteochondral allograft retrieval study, Williams et al.3 analyzed 26 retrieval specimens and found some subchondral bone alterations such as osteonecrosis, creeping substitution, collapse of the bony architecture, thickened subchondral plate, and irregular morphologic characteristics. Although, in theory, subchondral bone integration may be part of a successful osteochondral allograft transplant, no strong correlation between subchondral bone damage and clinical outcome has been described in osteochondral allograft transplantation. Also in our study we could not find any correlation between subchondral bone damage and clinical outcome.
Our study is the first to use a comprehensive scoring system and to systematically correlate clinical outcomes with MRI features unique to OCA transplantation. Although our MRI study could not show correlation between subchondral bone features of the OCAMRISS and any of the clinical outcome scores, the subchondral bone remains a substantial part of the transplant and in theory failure of the bone is undesirable. The inability to correlate OCAMRISS bone parameters with outcome may be due to small sample size. However, OCAMRISS may provide a standardized method to communicate findings unique to OCA transplantation.
With advances in MRI technology, including the increased use of MRI systems at field strengths of 3T and higher, the possibility exists for higher resolution images with little or no increase in time, which may further increase the reliability of scoring. With the widespread deployment and subsequent validation of novel qualitative and quantitative MRI sequences, imaging may play a larger role in the postoperative evaluation of cartilage repair techniques. This includes UTE sequence allows qualitative and quantitative evaluation of the calcified layer of cartilage that plays an important role in the overall function of the osteochondral unit.31 In an exploratory subgroup analysis, preservation of the calcified cartilage layer was particularly useful in predicting the biomechanical properties of osteochondral allografts.18
Currently, there is no agreed indication for time interval for routine MRI after OCA transplantation. For cell-based repair techniques, Domayer et al.11 suggested MRI assessment at 3 months, 1 year, and 2 years. At 3 months, the goal is to check adherence of repair tissue and early complication; at 1 year to assess graft maturation; at 2 years to check late complications. For osteochondral repair techniques, authors recommend a MRI assessment at 1 and 2 years.32-34 We are unable to recommend a particular interval for postoperative MRI of OCA transplantation. This study did not evaluate longitudinal changes in MRI in individual patients. Currently, when MRI is performed within the first year after surgery, the surgeon and the radiologist should both be aware that subchondral bone marrow edema pattern may be a normal finding.33 Further prospective imaging studies are warranted to better characterize the MRI response to OCA transplantation.
The present study has several limitations inherent to a retrospective study such as variable inclusion and exclusion criteria. We understand that this study does not present the entire data set to support the relevance of bone features in the clinical outcome since we only have a few patients with subchondral bone alterations. Our study presented a time interval between the clinical outcome assessment and the MRI acquisition, which could have brought changes in the interval. The time interval from clinical outcome assessment to MRI acquisition may also have influenced the lack of clinical and radiological correlation for bone features. Our study should be considered a pilot study because of the small sample size with all types of clinical and imaging presentation, which may not be a representative sample. We did not employ a uniform high resolution technique and specific recommended sequences such as UTE. Despite this, some statistically significant correlations were found. Use of OCA transplantation and MRI after surgery will continue to increase and our results may encourage investigators to perform larger studies employing the OCAMRISS.22,32
The recently described OCAMRISS is a reproducible grading system for in vivo evaluation after osteochondral allograft transplantation. With slight modifications, orthopaedic surgeons can be educated to apply this scoring system. In the future, larger sample sizes and established osteochondral allograft transplantation protocols for postoperative assessment with MRI are needed.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: “Scripps Clinic Medical Group grant” was used as funding source for this study. William D. Bugbee, was consultant for “Joint Restoration Foundation.” Gokhan Meric was supported by The Scientific and Technological Research Council of Turkey (TUBITAK; Ref: B.14.2.TBT.0.06.01-219-6040). Guilherme C. Gracitelli was supported by Coordinating of Improvement of Higher Education Personnel–Capes/Brazil. Eric Y. Chang acknowledges grant support from the VA Clinical Science Research and Development Career Development Award (IK2CX000749).
Declaration of Conflicting Interests: One or more of the authors has declared the following potential conflict of interest or source of funding: W.D.B. is a consultant for and has received research support from the Joint Restoration Foundation, a nonprofit tissue bank that receives, processes, and distributes osteochondral allografts.
Ethical Approval: This study was approved by Scripps-Shiley Center of Orthopaedic Research and Education (SCORE) (San Diego, CA, USA) institutional review board.
References
- 1. Bugbee WD, Khanna G, Cavallo M, McCauley JC, Gortz S, Brage ME. Bipolar fresh osteochondral allografting of the tibiotalar joint. J Bone Joint Surg Am. 2013;95:426-432. [DOI] [PubMed] [Google Scholar]
- 2. Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res. 2010;468:1269-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams SK, Amiel D, Ball ST, Allen RT, Tontz WL, Jr, Emmerson BC, et al. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35:2022-32. [DOI] [PubMed] [Google Scholar]
- 4. Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907-14. [DOI] [PubMed] [Google Scholar]
- 5. Gross AE, Shasha N,d, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;(435):79-87. [DOI] [PubMed] [Google Scholar]
- 6. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22:121-33. [DOI] [PubMed] [Google Scholar]
- 7. Zak L, Krusche-Mandl I, Aldrian S, Trattnig S, Marlovits S. Clinical and MRI evaluation of medium- to long-term results after autologous osteochondral transplantation (OCT) in the knee joint. Knee Surg Sports Traumatol Arthrosc. 2014;22:1288-97. [DOI] [PubMed] [Google Scholar]
- 8. Zak L, Albrecht C, Wondrasch B, Widhalm H, Vekszler G, Trattnig S, et al. Results 2 years after matrix-associated autologous chondrocyte transplantation using the Novocart 3D scaffold: an analysis of clinical and radiological data. Am J Sports Med. 2014;42:1618-27. [DOI] [PubMed] [Google Scholar]
- 9. Nawaz SZ, Bentley G, Briggs TW, Carrington RW, Skinner JA, Gallagher KR, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am. 2014;96:824-30. [DOI] [PubMed] [Google Scholar]
- 10. Gomoll AH, Gillogly SD, Cole BJ, Farr J, Arnold R, Hussey K, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42:1074-81. [DOI] [PubMed] [Google Scholar]
- 11. Domayer S, Welsch G, Dorotka R, Mamisch TC, Marlovits S, Szomolanyi P, et al. MRI monitoring of cartilage repair in the knee: a review. Semin Musculoskelet Radiol. 2008;12:302-17. [DOI] [PubMed] [Google Scholar]
- 12. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16-23. [DOI] [PubMed] [Google Scholar]
- 13. Welsch GH, Zak L, Mamisch TC, Paul D, Lauer L, Mauerer A, et al. Advanced morphological 3D magnetic resonance observation of cartilage repair tissue (MOCART) scoring using a new isotropic 3D proton-density, turbo spin echo sequence with variable flip angle distribution (PD-SPACE) compared to an isotropic 3D steady-state free precession sequence (True-FISP) and standard 2D sequences. J Magn Reson Imaging. 2010;33:180-8. [DOI] [PubMed] [Google Scholar]
- 14. Trattnig S, Domayer S, Welsch GW, Mosher T, Eckstein F. MR imaging of cartilage and its repair in the knee—a review. Eur Radiol. 2009;19:1582-94. [DOI] [PubMed] [Google Scholar]
- 15. Blackman AJ, Smith MV, Flanigan DC, Matava MJ, Wright RW, Brophy RH. Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: a systematic review and meta-analysis. Am J Sports Med. 2013;41:1426-34. [DOI] [PubMed] [Google Scholar]
- 16. de Windt TS, Welsch GH, Brittberg M, Vonk LA, Marlovits S, Trattnig S, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41:1695-702. [DOI] [PubMed] [Google Scholar]
- 17. de Windt TS, Welsch GH, Brittberg M, Vonk L, Marlovits S, Trattnig S, et al. Correlation between magnetic resonance imaging and clinical outcomes after knee cartilage repair: letter to the editor. Am J Sports Med. 2013;41:NP48-50. [DOI] [PubMed] [Google Scholar]
- 18. Chang EY, Pallante-Kichura AL, Bae WC, Du J, Statum S, Wolfson T, et al. Development of a Comprehensive Osteochondral Allograft MRI Scoring System (OCAMRISS) with histopathologic, micro-computed tomography, and biomechanical validation. Cartilage. 2014;5:16-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22:121-33. [DOI] [PubMed] [Google Scholar]
- 20. Chang EY, Du J, Chung CB. UTE imaging in the musculoskeletal system. J Magn Reson Imaging. Epub 2014. July 16. 10.1002/jmri.24713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29:600-13. [DOI] [PubMed] [Google Scholar]
- 22. Mithoefer K, Saris DBF, Farr J, Kon E, Zaslav K, Cole BJ, et al. Guidelines for the design and conduct of clinical studies in knee articular cartilage repair: International Cartilage Repair Society recommendations based on current scientific evidence and standards of clinical care. Cartilage. 2011;2:100-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salzmann GM, Erdle B, Porichis S, Uhl M, Ghanem N, Schmal H, et al. Long-term T2 and qualitative MRI morphology after first-generation knee autologous chondrocyte implantation: cartilage ultrastructure is not correlated to clinical or qualitative MRI outcome. Am J Sports Med. 2014;42:1832-40. [DOI] [PubMed] [Google Scholar]
- 24. Aldrian S, Zak L, Wondrasch B, Albrecht C, Stelzeneder B, Binder H, et al. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: a prospective follow-up at a minimum of 10 years. Am J Sports Med. 2014;42:2680-8. [DOI] [PubMed] [Google Scholar]
- 25. Welsch GH, Zak L, Mamisch TC, Resinger C, Marlovits S, Trattnig S. Three-dimensional magnetic resonance observation of cartilage repair tissue (MOCART) score assessed with an isotropic three-dimensional true fast imaging with steady-state precession sequence at 3.0 Tesla. Invest Radiol. 2009;44:603-12. [DOI] [PubMed] [Google Scholar]
- 26. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37:902-8. [DOI] [PubMed] [Google Scholar]
- 27. Dhollander AA, Huysse WC, Verdonk PC, Verstraete KL, Verdonk R, Verbruggen G, et al. MRI evaluation of a new scaffold-based allogenic chondrocyte implantation for cartilage repair. Eur J Radiol. 2010;75:72-81. [DOI] [PubMed] [Google Scholar]
- 28. Gold GE, Bergman AG, Pauly JM, Lang P, Butts RK, Beaulieu CF, et al. Magnetic resonance imaging of knee cartilage repair. Top Magn Reson Imaging. 1998;9:377-92. [PubMed] [Google Scholar]
- 29. Recht M, Bobic V, Burstein D, Disler D, Gold G, Gray M, et al. Magnetic resonance imaging of articular cartilage. Clin Orthop Relat Res. 2001;(391 Suppl):S379-96. [DOI] [PubMed] [Google Scholar]
- 30. Ebert JR, Smith A, Fallon M, Wood DJ, Ackland TR. Degree of preoperative subchondral bone edema is not associated with pain and graft outcomes after matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2014;42:2689-98. [DOI] [PubMed] [Google Scholar]
- 31. Mow VC, Bachrach NM, Ateshian GA. The effects of a subchondral bone perforation on the load support mechanism within articular cartilage. Wear. 1994;175:167-75. [Google Scholar]
- 32. Brown DS, Durkan MG, Foss EW, Szumowski J, Crawford DC. Temporal in vivo assessment of fresh osteochondral allograft transplants to the distal aspect of the femur by dGEMRIC (Delayed Gadolinium-Enhanced MRI of Cartilage) and zonal T2 mapping MRI. J Bone Joint Surg Am. 2014;96:564-72. [DOI] [PubMed] [Google Scholar]
- 33. Chang G, Horng A, Glaser C. A practical guide to imaging of cartilage repair with emphasis on bone marrow changes. Semin Musculoskelet Radiol. 2011;15:221-37. [DOI] [PubMed] [Google Scholar]
- 34. Durkan MG, Szumowski J, Brown DS, Foss EW, Crawford DC. In vivo MRI of fresh stored osteochondral allograft transplantation with delayed gadolinium-enhanced MRI of cartilage: protocol considerations and recommendations. Magn Reson Med. 2013;69:1745-53. [DOI] [PubMed] [Google Scholar]


