Abstract
Background
Numerous treatment methods for osteochondral repair have been implemented, including auto- and allogeneic osteochondral transplantations, combined bone and chondrocyte transplantations, and synthetic implants, but no gold standard treatment has been established. We present preliminary data on a combined autologous bone and cartilage chips: autologous dual-tissue transplantation (ADTT); an easily applicable, low-cost treatment option for osteochondral repair. The aim of this study was to investigate the early biological and clinical outcome of ADTT.
Materials
Eight patients (age 32 ± 7.5 years) suffering from osteochondritis dissecans (OCD) in the knee were enrolled. The OCD lesion was debrided and the osteochondral defect was filled with autologous bone, to a level at the base of the adjacent cartilage. Cartilage biopsies from the intercondylar notch were chipped and embedded within fibrin glue in the defect. Evaluation was performed using magnetic resonance imaging, computed tomography, and clinical scores, preoperative and 1 year postoperative.
Results
Cartilage tissue repair evaluated using MOCART score improved from 22.5 to 52.5 (P < 0.01). Computed tomography imaging demonstrated very good subchondral bone healing with all 8 patients having a bone filling of >80%. We found improvements 1 year postoperative in the International Knee Documentation Committee score (from 35.9 to 68.1, P < 0.01), Tegner score (from 2.6 to 4.7, P < 0.05), and Knee injury and Osteoarthritis Outcome Score pain, symptoms, sport/recreation and quality of life (P < 0.05).
Conclusion
Treatment of OCD with ADTT resulted in very good subchondral bone restoration and good cartilage repair. Significant improvements in patient reported outcome was found at 1 year postoperative. This study suggests ADTT as a promising, low-cost, treatment option for osteochondral injuries.
Keywords: articular cartilage, tissue, bone, tissue, computed tomography, diagnostics, magnetic resonance imaging, diagnostics, cartilage transplantation, grafts
Introduction
Osteochondral lesions are caused by trauma, osteochondritis dissecans (OCD), or osteonecrosis. Current treatment options include minced bone and cartilage paste,1 mosaicplasty,2 allogeneic osteochondral transplantation,3-5 combined bone and chondrocyte transplantation, the so-called sandwich technique, and synthetic osteochondral implants.6-8 These treatment methods suffer from inconsistent clinical and biological outcome, high cost, and donor site morbidity. The limitations of the existing treatment methods call for new osteochondral treatment strategies to be developed and validated. A potential strategy is to use autologous or allogeneic cartilage chips. Chondrocytes from human cartilage chips embedded in fibrin glue has been shown to migrate from the cartilage chips and form extracellular matrix9,10 and in 1983, Albrecht et al.11 implanted minced autologous cartilage chips in osteochondral defects in rabbits and reported complete closure of the defects with hyaline cartilage. In recent years, clinical studies on cartilage chips have been emerging. Full-thickness chondral injuries have been treated successfully by Farr et al.12,13 and Buckwalter et al.14 using allogeneic particulated juvenile articular cartilage (PJAC), and by Cole et al.15,16 using autologous cartilage chips embedded in a biodegradable scaffold (Cartilage Autograt Implantation System, CAIS). These techniques are for full-thickness chondral defects, and until now, autologous cartilage chips have not been used to treat osteochondral defects clinically.
In this study, we present the preliminary findings on autologous dual-tissue transplantation (ADTT) for osteochondral repair. ADTT is a combination of fragmented autologous bone press-fitted into the bed of the defect, and autologous cartilage chips embedded in fibrin glue to cover the bone graft. The aim of the study was to evaluate the early biological repair potential of ADTT using magnetic resonance imaging (MRI), computed tomography (CT), and clinical outcome scores. We hypothesized that ADTT-treated patients would display improved clinical outcome and good biological healing 1 year postoperative.
Materials and Methods
Patients
Patients suffering from focal osteochondral defects in the knee were included in the study. Inclusion criteria were the following: symptomatic focal osteochondral lesions, age 18 to 55 years, patient agreement to follow rehabilitation protocol, and patient agreement to attend follow-up visits. Exclusion criteria were the following: advanced osteoarthritis defined as grade 2 or higher Tönnis score,17 untreated meniscal lesion, untreated ruptures of the cruciate ligaments, untreated ruptures of collateral ligaments, malalignment >5°, poor general health ASA (American Society of Anesthesiologists) 3 or higher, alcohol or drug abuse, systemic or intra-articular corticoid steroid treatment within the past 6 months, or diagnosed malignancy. The patient demographics are listed in Table 1.
Table 1.
Patient Demographics and Defect Details.
| Characteristics | Values |
|---|---|
| Age, years, mean ± SD | 32 ± 7 |
| Sex, male:female | 5:3 |
| Injury etiology | |
| Osteochondritis dissecans | 8 |
| Defect location | |
| Medial femoral condyle | 6 |
| Lateral femoral condyle | 1 |
| Trochlea | 1 |
| Defect size after debridement, cm2, [range] | 3.1 [1.5-4.7] |
| Defect depth after debridement, mm, [range] | 8.4 [6-12] |
Surgery
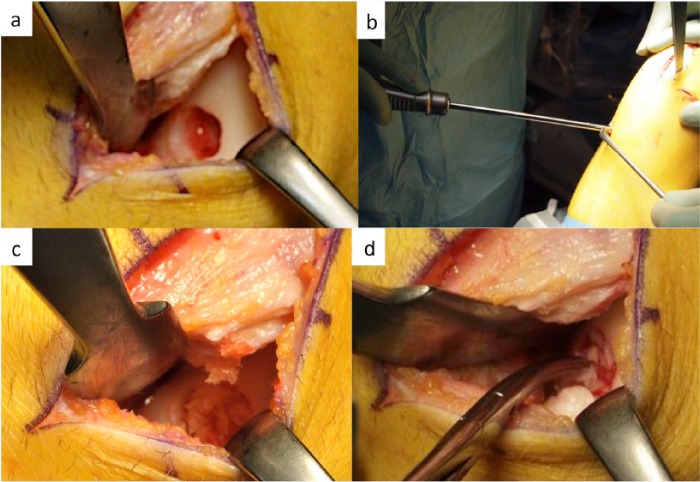
Prior to the open-surgery procedure, a knee arthroscopy was performed to identify the defect and additional joint pathology. Following the arthroscopy, the defect was exposed through a parapatellar arthrotomy. Using a hollow trephine, the defect was debrided to a point where the bottom of the defect consisted of healthy trabecular bone (clinical visual assessment), and the edges of the defect, of healthy articular cartilage. The defect size after debridement is described in Table 1. A biopsy of cancellous autologous bone was harvested through a drill hole in the proximal tibia and digitally broken down into fragments. The bone fragments were press-fitted into the bone part of the defect, to a level approximately 2 mm beneath the surface of the cartilage. Fibrin glue was applied to seal the bony part of the defect. For every square centimeter of defect being repaired, approximately 250 mg of hyaline cartilage was harvested. The biopsy was taken from the nonweightbearing femoral trochlea. Using surgical scissors, the cartilage was chipped to a size of approximately 0.25 to 0.5 mm2. The cartilage chips were dispersed throughout the defect, either touching or in very close proximity to each other (Within 0.5-1 mm). Fibrin glue was added and allowed to set for 5 minutes. To ensure proper placement of the cartilage chip–fibrin glue plug, the knee was continuously flexed and extended. The steps of the surgery are presented in Figure 1a-d.
Figure 1.
Autologous dual-tissue transplantation in 4 steps. (a) The defect has been debrided, so stable shoulders of healthy articular cartilage remain. The bottom of the defect consists of healthy subchondral bone. (b) A bone biopsy is taken from the proximal tibia. (c) The fragmented bone is press fitted into the defect. (d) The autologous cartilage chips, embedded in fibrin glue, are transplanted into the defect.
Postoperative Rehabilitation
All patients attended a stepwise activity progression program for the first 3 months postoperative. The patients were allowed 0° to 30° range of motion for the first 2 weeks in a hinged brace. From 2 weeks and onward, full range of motion was allowed. No weightbearing was allowed from 0 to 2 weeks. From 2 to 6 weeks, partial weightbearing was allowed. Impact activities were allowed after 6 months. Trained physiotherapists supervised the postoperative rehabilitation. Nonsteroidal anti-inflammatory drugs were prohibited for the initial 3 months.
Clinical Evaluation
The clinical outcome was evaluated using the International Knee Documentation Committee Subjective Knee Form (IKDC), the Knee injury and Osteoarthritis Outcome Score (KOOS), and the Tegner score.18-20 A qualified medical professional performed evaluation via telephone preoperative and 1 year postoperative.
Magnetic Resonance Imaging
For evaluation of osteochondral repair, patients were scanned prior to surgery and 1 year postoperative. The images were evaluated by an experienced blinded radiologist using the 3-dimensional (3D) MOCART (magnetic resonance observation of cartilage repair tissue) score.21 The images were evaluated according to defect fill, cartilage interface, bone interface, repair tissue surface, repair tissue structure, signal intensity, subchondral lamina, chondral osteophytes, bone marrow edema, subchondral bone, and joint effusion. The maximal achievable score was 100.
Magnetic resonance imaging on all patients was performed using an ONI Optima 430s 1.5T extremity scanner (GE Healthcare, Buckinghamshire, UK). Following sequences were acquired for evaluation: Axial STIR, coronal FSE T1, sagittal SmartFat-fat water separation technique (FSEfw) PD FS, sagittal water FSEfw PD FS, and sagittal 3D T2* mapping.
Computed Tomography
For subchondral bone evaluation, patients underwent CT scans 1 year postoperative. A Philips Brilliance 40 scanner (Eindhoven, the Netherlands) was used with a slice thickness of 0.625 mm. An experienced blinded observer evaluated the CT images using Osirix (Geneva, Switzerland, v. 5.7, 64-bit). Bone regeneration in the osteochondral defects was evaluated semiquantitatively according to the degree bone formation in the defect: ≤20%, 21% to 40%, 41% to 60%, 62% to 80% or >80%.
Statistical Analysis
The data from the MOCART, KOOS, IKDC, and Tegner scores were tested for normality using histograms and Q-Q plots, and for equality of variance using SD-tests. Paired Student t tests were performed on normally distributed data, while nonparametric data were analyzed using Wilcoxon rank sum test. Statistical analysis was performed using STATA/MP version 13.0 (StataCorp, College Station, TX, USA).
Results
No postoperative complications were seen in any patients. One patient was unavailable for clinical scoring
Clinical Scores
The individual clinical scores for each patient combined with the corresponding MRI and CT image is presented in Table 2. A statistical significant improvement from preoperative to 1 year postoperative was seen in the IKDC score, the KOOS pain, KOOS symptoms, KOOS sport/recreation, KOOS quality of life, and the Tegner score. KOOS activities of daily living did not improve significantly. The mean scores are listed in Table 3.
Table 2.
The Individual Clinical Scores and the Corresponding Figure Number.
| Score | Pt 1 |
Pt 2 |
Pt 3a |
Pt 4 |
Pt 5 |
Pt 6 |
Pt 7 |
Pt 8 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre OP | 1 year | Pre OP | 1 year | Pre OP | 1 year | Pre OP | 1 year | Pre OP | 1 year | Pre OP | 1 year | Pre OP | 1 year | Pre OP | 1 year | |
| IKDC | 57.5 | 71.3 | 37.9 | 86.2 | — | — | 36.8 | 36.8 | 10.3 | 60.9 | 10.3 | 71.3 | 37.5 | 75.9 | 60.9 | 74.4 |
| KOOS | ||||||||||||||||
| Pain | 83 | 97 | 56 | 97 | — | — | 58 | 58 | 25 | 72 | 31 | 83 | 75 | 92 | 81 | 92 |
| Symptoms | 82 | 86 | 54 | 86 | — | — | 64 | 58 | 32 | 79 | 57 | 93 | 61 | 89 | 68 | 93 |
| ADL | 81 | 82 | 81 | 96 | — | — | 24 | 76 | 24 | 76 | 26 | 84 | 93 | 100 | 94 | 99 |
| Sport/recreation | 40 | 60 | 30 | 95 | — | — | 0 | 25 | 0 | 25 | 5 | 35 | 5 | 65 | 40 | 65 |
| QOL | 31 | 69 | 19 | 75 | — | — | 44 | 64 | 6 | 56 | 6 | 63 | 19 | 50 | 44 | 63 |
| Tegner | 6 | 9 | 1 | 3 | — | — | 3 | 3 | 1 | 3 | 1 | 5 | 1 | 4 | 3 | 4 |
| Corresponding figure number | 1a | 1b, c | 1d | 1e, f | 1g | 1h, i | 1j | 1k, l | 2a | 2b, c | 2d | 2e, f | 2g | 2h, i | 2j | 2k, l |
| Previous surgery | None | None | Drilling | Trufit OC plug | None | None | None | MaioRegen OC scaffold | ||||||||
ADL = activities of daily living; IKDC = International Knee Documentation Committee; KOOS = Knee injury and Osteoarthritis Outcome Score; OC = osteochondral; PreOP = preoperative; Pt = patient; QOL = quality of life.
Patient number 3 was unavailable for clinical scoring.
Table 3.
The IKDC, KOOS, Tegner, and MOCART Scores.
| Score | Preoperative | SD | 1 year | SD | Difference | 95% CI | P |
|---|---|---|---|---|---|---|---|
| IKDC | 36.9 | 20 | 68.1 | 15.7 | 32.2 | 11.2-53.1 | <0.001 |
| KOOS | |||||||
| Pain | 58.3 | 23.4 | 84.5 | 14.5 | 26.2 | 3.5-48.8 | <0.05 |
| Symptoms | 63.3 | 17.3 | 83.3 | 12.1 | 20.1 | 2.6-37.5 | <0.05 |
| ADL | 74.8 | 24.0 | 85.4 | 14.0 | 10.6 | 12.2-33.5 | NS |
| Sport/recreation | 24.3 | 17.4 | 57.2 | 22.7 | 33.0 | 9.4-56.4 | <0.01 |
| QOL | 27.7 | 13.9 | 62.7 | 8.1 | 35.0 | 21.8-48.3 | <0.01 |
| Tegner | 2.6 | 1.8 | 4.7 | 2.2 | 2.1 | 0.9-3.4 | <0.01 |
| MOCART | 22.5 | 8.5 | 52.5 | 14.4 | 30 | 17.3-42.7 | <0.001 |
ADL = activities of daily living; IKDC = International Knee Documentation Committee; KOOS = Knee injury and Osteoarthritis Outcome Score; MOCART = magnetic resonance observation of cartilage repair tissue; NS = not significant; QOL = quality of life; SD = standard deviation.
Magnetic Resonance Imaging
The MOCART score significantly improved at 1 year in terms of defect fill (P < 0.01), cartilage interface (P < 0.01), repair tissue surface (P < 0.05), and repair tissue signal intensity (P < 0.01). No significant improvement was found in the categories; bone interface, repair tissue structure, subchondral lamina, chondral osteophytes, subchondral edema, subchondral bone, and effusion (P > 0.05). The total MOCART score improved from 22.5 to 52.5 at 1 year (P < 0.01) (Table 3). Preoperative and 1 year postoperative images of each patient can be found in Figures 2 and 3.
Figure 2.
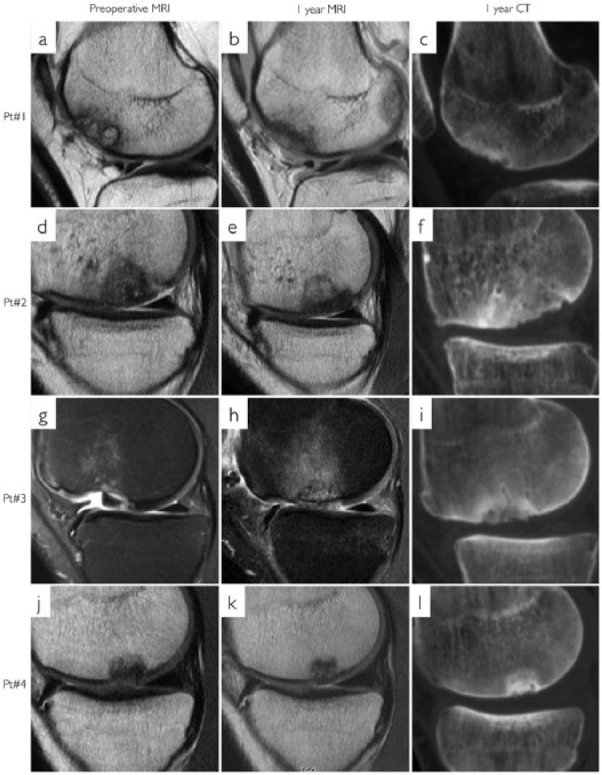
Magnetic resonance imaging (MRI) and computed tomography (CT) of patients 1 to 4. First column (a, d, g, and j) is preoperative MRI. Second column (b, e, h, and k) is 1 year MRI. Third column (c, f, i, and l) is 1 year CT. Each row represents 1 patient. (a) An International Cartilage Repair Society (ICRS) grade IV osteochondritis dissecans (OCD). (b, c) A good repair tissue integration and complete bone filling.
Figure 3.
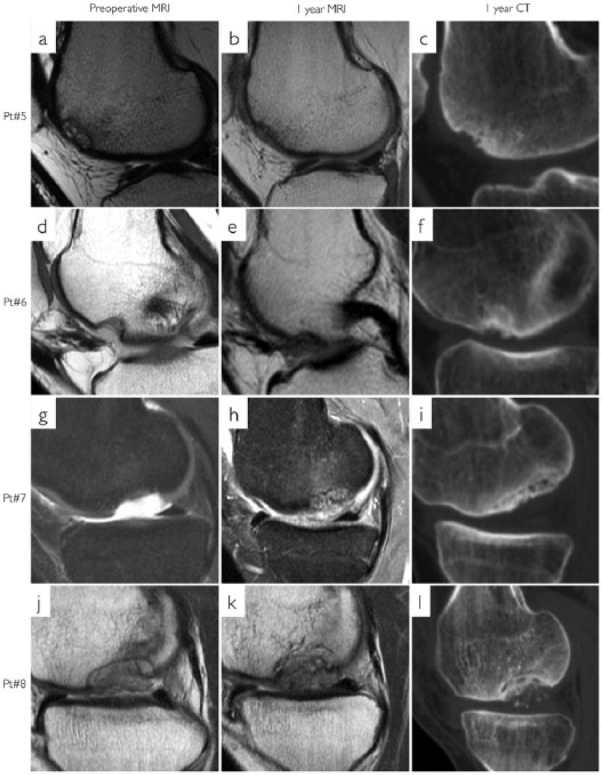
Magnetic resonance imaging (MRI) and computed tomography (CT) of patients 5 to 8. First column (a, d, g, and j) is preoperative MRI. Second column (b, e, h, and k) is 1 year MRI. Third column (c, f, i, and l) is 1 year CT. Each row represents 1 patient. (i, l) Incomplete subchondral bone integration. (l) Bone fragments in the chondral repair tissue.
Computed Tomography
All patients had >80% bone filling in the defects. The subchondral surface congruence of the repair tissue was very similar to the adjacent bone, but the surface seemed uneven in all 8 patients. Two patients had small bone fragments in the chondral repair tissue (Figs. 2l and 3l) and 2 patients had incomplete subchondral bone integration (Fig. 3i and l).
Discussion
This is the first clinical report on ADTT for OCD in the knee. The primary finding of this early report was a consistent, good biologic healing response of ADTT in osteochondral lesions. The biologic healing response was characterized by very good subchondral bone restoration and the cartilage repair was characterized by good cartilage interface integration, defect filling, surface structure and near normal tissue signal intensity.
The use of cartilage chips for hyaline cartilage repair was introduced in 1983.11 Chondrocytes from cartilage chips have been shown to migrate from the chips and form new extracellular matrix,9,10 and it has been shown that chondrocytes are not significantly damaged when cartilage is cut using sharp instruments.22 Since 1983, however, reports have been sparse and limited to in vitro and experimental studies, and studies on cartilage-bone paste.1,9,23-27 Recently, studies on cartilage chips have been emerging, perhaps due to the need for a universally available, low-cost treatment for injuries in this troublesome tissue.12,13,15,16 As in the present study PJAC (DeNovo NT Natural Tissue Graft, Zimmer Inc, Warsaw, IN, USA) and CAIS use cartilage chips. The main difference however is that ADTT treats osteochondral injuries rather than full-thickness chondral injuries. Furthermore, the PJAC treatment use 1 mm3 allogeneic cartilage chips embedded in fibrin glue, while CAIS use autologous chips of the same size, embedded in a biodegradable scaffold. Since data suggests that the increased surface achieved by reducing the size of the cartilage chips, increase the extracellular matrix production in vitro,28 we used chips sized 0.25 to 0.5 mm3. To avoid the need to purchase a commercially available scaffold or membrane, we used autologous bone graft and cartilage, both readily available in the patient, and sealed the defect with fibrin glue. Since the chondrocytes from cartilage chips expand and create extracellular matrix, it is possible to treat large lesions without increasing the donor site morbidity.
The subchondral unit, consisting of the articular cartilage and the subchondral bone, is important to restore in any attempt to repair an osteochondral defect. Signals from the subchondral bone have been shown to alter the differentiation potential of bone marrow stem cells and thereby induce phenotypic degenerative changes toward osteoarthritis.29 Furthermore, studies have indicated that osteoarthritis could be initiated through the activation of a secondary ossification center with thickening of the subchondral bone plate and thereby thinning of the articular cartilage.30
To ensure reestablishment of the subchondral unit, several different treatment methods have been implemented. The impact on clinical outcome of these treatment methods is overall relatively similar. Gel-type autologous chondrocyte implantation is a 2-step procedure using autologous bone graft, a fibrin scaffold, and cultured chondrocytes. Using gel-type autologous chondrocyte implantation on 9 patients suffering from osteochondral injuries on either the lateral or medial femoral condyles, Könst et al.31 found an increase in the IKDC score from 35 ± 16 preoperative to 57 ± 20 at 1 year (mean IKDC increase 22). In “mosaicplasty,” osteochondral allografts are transplanted from low weightbearing parts of the knee to the osteochondral defect. It provides good results in active younger patients with isolated osteochondral lesions; however, donor site morbidity is a concern with this technique, limiting treatment to smaller defects.32,33 Filardo et al.34 treated 31 patients, suffering from osteochondral injuries in the medial or lateral femoral condyle, with mosaicplasty, and found an IKDC increase from 40 ± 16 to 63 ± 18 at 1 year (mean IKDC increase 23). The MaioRegen scaffold is a layered biomimetic scaffold designed to be implanted into osteochondral defects and induce subchondral bone, and cartilage regeneration. It has shown promising clinical results in osteochondral defects of up to 3 to 8 cm2 on the femoral condyles, trochlea, and tibial plateau; however, the biological repair potential of the scaffold has recently been questioned.35 Authors have reported preoperative to 1 year IKDC increases from 36 ± 6 to 68 ± 13,36 45 ± 19 to 71 ± 18,37 and 48 ± 18 to 76 ± 1338 (mean IKDC increase 32, 26, and 28 respectively). In the present study, the ADTT treatment resulted in an IKDC increase from preoperative to 1 year of 36 ± 20 to 68 ± 15—a statistically and clinically significant increase of 32 points, which is similar to or better than previously published studies investigating methods for osteochondral repair.
Even though statistical significant clinical and radiological improvements were found in this study, 1 patient (patient 4) had a radiological improvement, but no clinical improvement, and 1 patient (patient 8) had a radiological deterioration, but a slight clinical improvement. Patient 4 had previously been treated with the Trufit osteochondral plug, and patient 8 had previously been treated with the MaioRegen osteochondral scaffold. Both scaffold treatments had failed and we speculate if failed treatment with osteochondral implants can impede future treatment attempts. An event observed in chondral defects after microfracture.39,40 Furthermore, a variable subchondral bone healing was found in patients 7 and 8. While a biological variation, even when using autologous bone grafts are expected, a possible explanation for the failure of patient 8 is the previous use of the MaioRegen scaffold, which has been shown to impede subchondral bone healing.35
This study is limited by the small sample size (n = 8), the short follow-up, and the lack of a control group. The risk of a type 1 error is present, but based on the small variance in data and the thorough clinical and radiological follow-up program showing improvements in subchondral bone and cartilage restoration, we conclude that these early results on ADTT are very encouraging. The fact that there is no need of a scaffold or cell culturing makes ADTT a promising, low-cost treatment method for osteochondral injuries.
Larger, controlled, long-term studies should be performed to validate this treatment principle.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was approved by the Central Denmark Region Committee on health research ethics, Journal number 1-10-72-26-14.
References
- 1. Stone KR, Walgenbach AW, Freyer A, Turek TJ, Speer DP. Articular cartilage paste grafting to full-thickness articular cartilage knee joint lesions: a 2- to 12-year follow-up. Arthroscopy. 2006;22(3):291-9. [DOI] [PubMed] [Google Scholar]
- 2. Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Jt Surg Br. 2012;94(4):504-9. [DOI] [PubMed] [Google Scholar]
- 3. Meyers MH, Akeson W, Convery FR. Resurfacing of the knee with fresh osteochondral allograft. J Bone Joint Surg Am. 1989;71(5):704-13. [PubMed] [Google Scholar]
- 4. Gross AE, Kim W, Las Heras F, Backstein D, Safir O, Pritzker KPH. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clin Orthop Relat Res. 2008;466:1863-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams RJ, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89:718-26. [DOI] [PubMed] [Google Scholar]
- 6. Kon E, Filardo G, Perdisa F, Di Martino A, Busacca M, Balboni F, et al. A one-step treatment for chondral and osteochondral knee defects: clinical results of a biomimetic scaffold implantation at 2 years of follow-up. J Mater Sci Mater Med. 2014;25(10):2437-44. [DOI] [PubMed] [Google Scholar]
- 7. Melton JTK, Wilson AJ, Chapman-Sheath P, Cossey AJ. TruFit CB bone plug: chondral repair, scaffold design, surgical technique and early experiences. Expert Rev Med Devices. 2010;7(3):333-41. [DOI] [PubMed] [Google Scholar]
- 8. Bekkers JE, Bartels LW, Vincken KL, Dhert WJ, Creemers LB, Saris DB. Articular cartilage evaluation after TruFit plug implantation analyzed by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC). Am J Sports Med. 2013;41(6):1290-5. [DOI] [PubMed] [Google Scholar]
- 9. Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24(6):1261-70. [DOI] [PubMed] [Google Scholar]
- 10. Sage A, Chang AA, Schumacher BL, Sah RL, Watson D. Cartilage outgrowth in fibrin scaffolds. Am J Rhinol Allergy. 2009;23:486-91. [DOI] [PubMed] [Google Scholar]
- 11. Albrecht FH. Closure of joint cartilage defects using cartilage fragments and fibrin glue [in German]. Fortschr Med. 1983;101(37):1650-2. [PubMed] [Google Scholar]
- 12. Farr J, Yao JQ. Chondral defect repair with particulated juvenile cartilage allograft. Cartilage. 2011;2(4):346-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42:1417-25. [DOI] [PubMed] [Google Scholar]
- 14. Buckwalter J, Bowman G, Albright J, Wolf B, Bollier M. Clinical outcomes of patellar chondral lesions treated with juvenile particulated cartilage allografts. Iowa Orthop J. 2014;34:44-9. [PMC free article] [PubMed] [Google Scholar]
- 15. Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39:1170-9. [DOI] [PubMed] [Google Scholar]
- 16. Farr J, Cole B, Sherman S, Karas V. Particulated articular cartilage: CAIS and DeNovo NT. J Knee Surg. 2012;25(1):23-9. [DOI] [PubMed] [Google Scholar]
- 17. Tönnis DD. Congenital dysplasia and dislocation of the hip in children and adults. Berlin: Springer; 1987. [Google Scholar]
- 18. Hefti E, Müller W, Jakob RP, Stäubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sport Traumatol Arthrosc. 1993;1:226-34. [DOI] [PubMed] [Google Scholar]
- 19. Bekkers JE, de Windt TS, Raijmakers NJ, Dhert WJ, Saris DB. Validation of the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the treatment of focal cartilage lesions. Osteoarthritis Cartilage. 2009;17(11):1434-9. [DOI] [PubMed] [Google Scholar]
- 20. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-9. [PubMed] [Google Scholar]
- 21. Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310-9. [DOI] [PubMed] [Google Scholar]
- 22. Redman SN, Dowthwaite GP, Thomson BM, Archer CW. The cellular responses of articular cartilage to sharp and blunt trauma. Osteoarthritis Cartilage. 2004;12:106-16. [DOI] [PubMed] [Google Scholar]
- 23. Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Rossi R, Piras L, et al. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2590-601. [DOI] [PubMed] [Google Scholar]
- 24. Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Von Degerfeld MM, Bignardi C, et al. Autologous cartilage fragments in a composite scaffold for one stage osteochondral repair in a goat model. Eur Cell Mater. 2013;26:15-32. [DOI] [PubMed] [Google Scholar]
- 25. Marmotti A, Bonasia DE, Bruzzone M, Rossi R, Castoldi F, Collo G, et al. Human cartilage fragments in a composite scaffold for single-stage cartilage repair: an in vitro study of the chondrocyte migration and the influence of TGF-β1 and G-CSF. Knee Surg Sport Traumatol Arthrosc. 2013;21:1819-33. [DOI] [PubMed] [Google Scholar]
- 26. Lind M, Larsen A. Equal cartilage repair response between autologous chondrocytes in a collagen scaffold and minced cartilage under a collagen scaffold: an in vivo study in goats. Connect Tissue Res. 2008;49(6):437-42. [DOI] [PubMed] [Google Scholar]
- 27. Frisbie DD, Lu Y, Kawcak CE, DiCarlo EF, Binette F, McIlwraith CW. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sport Med. 2009;37(Suppl 1):71S-80S. [DOI] [PubMed] [Google Scholar]
- 28. Bonasia D, Consentino A, Marmotti A, Spolaore S, Mattia S, Castoldi F, et al. Cartilage fragments for one stage cartilage repair: correlation between fragment size and extracellular matrix production in vitro. In: Poster session on Cartilage. Amsterdam: ESSKA Congress; 2014. Poster number P04-1476. [Google Scholar]
- 29. Leyh M, Seitz A, Dürselen L, Schaumburger J, Ignatius A, Grifka J, et al. Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow–derived mesenchymal stemcells and articular chondrocytes. Arthritis Res Ther. 2014;16:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burr DB, Radin EL. Microfractures and microcracks in subchondral bone: are they relevant to osteoarthrosis? Rheum Dis Clin North Am. 2003;29:675-85. [DOI] [PubMed] [Google Scholar]
- 31. Könst YE, Benink RJ, Veldstra R, van der Krieke TJ, Helder MN, van Royen BJ. Treatment of severe osteochondral defects of the knee by combined autologous bone grafting and autologous chondrocyte implantation using fibrin gel. Knee Surg Sport Traumatol Arthrosc. 2012;20(11):2263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(Suppl):25-32. [DOI] [PubMed] [Google Scholar]
- 33. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A(2):185-92. [DOI] [PubMed] [Google Scholar]
- 34. Filardo G, Kon E, Perdisa F, Balboni F, Marcacci M. Autologous osteochondral transplantation for the treatment of knee lesions: results and limitations at two years’ follow-up. Int Orthop. 2014;38(9):1905-12. [DOI] [PubMed] [Google Scholar]
- 35. Christensen BB, Foldager CB, Jensen J, Jensen NC, Lind M. Poor osteochondral repair by a biomimetic collagen scaffold: 1- to 3-year clinical and radiological follow-up. Knee Surg Sport Traumatol Arthrosc. Epub 2015. February 18. 10.1007/s00167-015-3538-3. [DOI] [PubMed] [Google Scholar]
- 36. Delcogliano M, de Caro F, Scaravella E, Ziveri G, De Biase CF, Marotta D, et al. Use of innovative biomimetic scaffold in the treatment for large osteochondral lesions of the knee. Knee Surg Sport Traumatol Arthrosc. 2014;22(6):1260-9. [DOI] [PubMed] [Google Scholar]
- 37. Berruto M, Delcogliano M, de Caro F, Carimati G, Uboldi F, Ferrua P, et al. Treatment of large knee osteochondral lesions with a biomimetic scaffold: results of a multicenter study of 49 patients at 2-year follow-up. Am J Sports Med. 2014;42(7):1607-17. [DOI] [PubMed] [Google Scholar]
- 38. Filardo G, Kon E, Di Martino A, Busacca M, Altadonna G, Marcacci M. Treatment of knee osteochondritis dissecans with a cell-free biomimetic osteochondral scaffold: clinical and imaging evaluation at 2-year follow-up. Am J Sports Med. 2013;41(8):1786-93. [DOI] [PubMed] [Google Scholar]
- 39. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-8. [DOI] [PubMed] [Google Scholar]
- 40. Pestka JM, Bode G, Salzmann G, Sudkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40:325-31. [DOI] [PubMed] [Google Scholar]