Abstract
Background
There is no data regarding the association between the platelet-to-lymphocyte ratio (PLR) and long-term mortality in patients with stable coronary artery disease (SCAD). The aim of this study is to evaluate the utility of the pre-procedural PLR for predicting long-term, all-cause mortality in patients with SCAD undergoing percutaneous coronary intervention (PCI) and stent implantation.
Methods
We analyzed a total of 2959 consecutive patients with SCAD who underwent PCI (balloon angioplasty followed by stent implantation or direct stenting) between July 2006 and December 2011 at our institution. The patients were stratified into tertiles according to their admission PLR. The association between the PLR value and the outcomes was assessed using Cox proportional regression analysis after adjusting for clinical angiographic and laboratory data.
Results
During median follow-up of 1124 days, mortality was highest in patients with PLR within the 3rd tertile as compared to the 2nd and the 1st tertile (11.0% vs 8.7% vs. 9.6%, respectively, p = 0.03). PLR remained associated with mortality in multivariable analysis including clinical variables, ejection fraction and angiographic parameters HR (per 10 units increase) = 1.02 [95%CI,1.01 ÷ 1.04, p = 0.006]. After adjustment for the eGFR and hemoglobin levels, PLR was however no longer significantly associated with mortality.
Conclusion
PLR has potential predictive value in patients with SCAD, which has not been reported previously, but statistical significance disappears after adjusting for estimated glomerular filtration rate (eGFR) and hemoglobin levels as a potential confounding variable.
Keywords: Platelet-to-lymphocyte ratio, Percutaneous coronary intervention, Mortality
Abbreviations
- HF
heart failure
- PLR
platelet/lymphocyte ratio
- NSTEMI
non-ST-elevation myocardial infarction
- STEMI
ST elevation myocardial infarction
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- SCAD
stable coronary artery disease
- CABG
coronary artery bypass grafting
- COPD
chronic obstructive pulmonary disease
- BMI
body mass index
- EF
ejection fraction
- Hb
hemoglobin
- eGFR
estimated glomerular filtration rate
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- CCS
Canadian Cardiovascular Society
- NYHA
New York Heart Association
Introduction
Numerous studies have shown the association between elevated platelet counts and cardiovascular mortality [1–3]. It has been also shown that the elevated level of neutrophils and relative lymphocytopenia are negative prognostic indexes of outcomes in patients with coronary artery disease and heart failure (HF) [4–9].
Platelet-to-lymphocyte ratio (PLR) which can be derived from the complete blood count is a novel index reflecting a systemic inflammatory burden that combines prognostic values of an individual’s platelet and lymphocyte count [4,9]. In patients with non-ST-elevation myocardial infarction (NSTEMI) and ST elevation myocardial infarction (STEMI), the PLR ratio is an independent predictor of mortality [10,11].
Moreover, in patients with myocardial infarction (MI) treated by primary PCI, PLR is an independent risk factor for more advanced coronary artery disease as reflected by higher Syntax scores and no-reflow phenomenon [12]. Data regarding PLR and its association with long-term prognosis in stable coronary artery disease (SCAD) patients are, however, lacking. We hypothesized that PLR is a potential marker of prognostic importance in patients with SCAD. The aim of our study was to establish the impact of the baseline PLR ratio on all-cause, long-term mortality in patients after PCI and stent implantation.
Methods
Data collection
For the purpose of this study, we examined a computer database to identify patients with SCAD referred to the Silesian Center for Heart Diseases in Poland who underwent coronary angiography and stent implantation between July 2006 and December 2011. In this database, information on coronary intervention, concomitant diseases, demographic data and laboratory parameters such as platelet and leukocyte counts are stored. The complete blood counts, which included total white blood cells, neutrophils, lymphocytes and platelets, were obtained using an automated blood counter Sysmex XS1000i and XE2100 (Sysmex Corporation, Kobe, Japan). Platelet-to-lymphocyte ratio was calculated as the ratio of the platelets to lymphocytes, obtained from the blood samples that were taken at the fasting state.
Patients undergoing hybrid revascularization, patients after orthotropic heart transplant, patients with known hematological diseases, patients on dialysis, or with other diseases limiting survival were excluded from the analysis. One patient died during the in-hospital period due to periprocedural complications. This patient was also excluded from this analysis.
The study was approved by the Local Ethics Committee at the District Chamber of Physicians.
Follow-up data
Information on survival was based on the National Health Fund insurance status, which can be electronically verified because the National Health Fund insurance policy is obligatory for all Polish citizens, and patients who were insured were marked as alive. We made an attempt to contact the relatives of uninsured patients and/or the relevant local registry office to obtain the date of death. Complete follow-up data (including eventual event and time of the event) were available for 2947 (99.6%) patients.
Statistical analysis
The normality of continuous variable distribution was tested with a Shapiro-Wilk test and deviation from the normal distribution was inspected by analysis of the normal probability plots (Quantile-Quantile plots). The continuous variables are not normally distributed, and are therefore presented as median and interquartile ranges. The dichotomous variables are presented as percentages. Patients were divided into sub-groups according to PLR tertiles. Group I included patients with PLR < 87 (n = 986); group II included patients with PLR value of ⩾87 and < 121 (n = 986); and group III included patients with PLR ⩾ 121 (n = 987).
To test for differences between the groups, the Kruskal–Wallis test and chi-square test were used for continuous variables, and dichotomous variables, respectively. The associations between groups and mortality were analyzed using the Kaplan-Meier method with log-rank testing. To assess the impact of the PLR on prognosis, a univariable and multivariable Cox regression analysis was performed. Variables used in the univariable Cox regression analysis included clinical, angiographic, and laboratory parameters. Sex, history of MI, previous PCI, previous coronary artery bypass grafting (CABG), previous stroke, hypercholesterolemia, hypertension, atrial fibrillation, diabetes, chronic obstructive pulmonary disease (COPD), family history of premature (<55 years of age) MI and sinus rhythm at admission were analyzed as dichotomous variables. Age, body mass index (BMI), ejection fraction (EF), hemoglobin (Hb), creatinine, sodium, and total cholesterol were analyzed as a continuous variable. Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [13] and was analyzed as a continuous variable, per 10 ml/min/1.73m2 increment. PLR was analyzed as a continuous variable per 10 units increase. Admission platelets and lymphocyte counts were excluded from the analysis as they were used to calculate PLR. The Canadian Cardiovascular Society (CCS) class was categorized as CCS class I and II vs. CCS class III and IV. Heart failure was categorized as “no heart failure” and “heart failure with symptoms in New York Heart Association Class (NYHA) I/II” and “heart failure with symptoms in NYHA class III/IV”. Smoking was categorized as “current smoker”, “former smoker” and “never smoked”. Type of stent implanted was categorized as “bare metal stent”, “drug eluting stent” or “both”. The number of stents implanted and number of PCI vessels were analyzed as ordinal categories. To minimize the impact of missing data on the Cox regression analysis, the multiple imputation method was used to impute missing data for the variables that were to be included in the Cox regression procedures. There were missing data regarding information on the BMI – 253 (8.6%), EF – 314 (10.6%), family history of premature coronary heart disease – 8 (0.3%). Hemodynamic data was available for all patients and was taken from angiography reports. Data on mortality was missing for 14 (0.5%) patients. Additionally for three (0.1%) patients, we were not able to establish the exact time of death.
To minimize the impact of the missing data on the multivariable Cox regression analysis, we used the missForest algorithm for mixed type data imputation [14]. All above-mentioned variables including the outcome variable were entered into the multiple imputation algorithm. We used the missForest R–package [15].
We performed Cox regression analysis on the imputed dataset. Variables that reached p-value lower than 0.2 in the univariable analysis were included in the multivariable model. We used backwards selection procedures to obtain variables significantly associated with long-term mortality in multivariable analysis. We created four models including PLR and clinical variables (model 1), clinical variables and ejection fractions (model 2), clinical variables, ejection fractions and angiographic parameters (number of stents implanted, number of PCI vessels, presence of multi-vessel coronary artery disease) (model 3), and additionally also hemoglobin and eGFR (model 4). We calculated Harrell’s concordance index (Harrell’s C-statistics) which describes discriminative ability of risk models obtained with the use of Cox-regression analysis. Harrell’s C-statistics range from 0.5 (no predictive ability) to 1.0 (perfect discrimination). The analyses were performed using Number Crunching Statistical Systems 8.0 (NCSS, Kaysville, UT, USA) and R software [16]. Figure was prepared using Graph Pad Prism Software (La Jolla, USA).
The creation of the database of patients with SCAD used in this study was supported by the National Science Center – Dec-2011/01/D/NZ5/04387.
Results
The study sample consisted of a cohort of 2959 patients with SCAD (2094 males), with a median age of 64.00 (IQR: 57 ÷ 71 years). The baseline demographic characteristics of patients stratified by the PLR tertiles are demonstrated in Table 1. The patients in the 3rd PLR tertile were significantly older, more often female and hypertensive whilst less often smokers and obese, as compared to patients in the 1st and 2nd PLR tertile. According to the admission laboratory work-up, patients in the 3rd PLR tertile had significantly lower red and white blood cell counts, sodium concentration, Hb levels and eGFR (Table 2). There were no differences between patients stratified by the PLR tertiles in the prescribed treatment at hospital discharge (Table 3).
Table 1.
Baseline clinical characteristics of analyzed cohort according to PLR values.
| Variable | 1st tertile, (n = 986) | 2nd tertile, (n = 986) | 3rd tertile, (n = 987) | P value | |
|---|---|---|---|---|---|
| Age (yrs) | 62.0 [56.0 ÷ 69.0] | 65.0 [57.0 ÷ 71.0] | 65.0 [58.0 ÷ 72.0] | <0.01 | |
| Men (%) | 746 (75.7%) | 693 (70.3%) | 655 (66.4%) | <0.01 | |
| Heart failure | NYHA class I/II | 107 (10.9%) | 93 (9.4%) | 101 (10.2%) | 0.70 |
| NYHA class III/IV | 33 (3.3%) | 42 (4.3%) | 40 (4.1%) | ||
| Atrial fibrillation | 104 (10.5%) | 106 (10.8%) | 110 (11.1%) | 0.91 | |
| Hypertension | 698 (70.8%) | 686 (69.6%) | 740 (75.0%) | 0.02 | |
| Previous MI | 578 (58.6%) | 562 (57.0%) | 589 (59.7%) | 0.48 | |
| Previous CABG | 136 (13.8%) | 134 (13.6%) | 108 (10.9%) | 0.11 | |
| Previous PCI | 460 (46.9%) | 485 (49.4%) | 504 (51.1%) | 0.17 | |
| Previous SCD | 28 (2.8%) | 31 (3.1%) | 26 (2.6%) | 0.79 | |
| PVD | 66 (6.7%) | 48 (4.9%) | 54 (5.5%) | 0.20 | |
| Prev. Stroke/TIA | 39 (4.0%) | 47 (4.8%) | 46 (4.7%) | 0.64 | |
| Diabetes mellitus | 366 (37.1%) | 344 (34.9%) | 365 (37.0%) | 0.51 | |
| Lipid abnormalities | 576 (58.4%) | 564 (57.2%) | 544 (55.1%) | 0.33 | |
| COPD | 68 (6.9%) | 71(7.2%) | 76 (7.7%) | 0.79 | |
| Obesity | 328 (36.5%) | 300 (33.3%) | 264 29.1 (%) | <0.01 | |
| Current smoker | 144 (14.6%) | 97 (9.9%) | 89 (9.0%) | <0.01 | |
| Previous smoker | 376 (38.2%) | 356 (36.2%) | 343 (34.8%) | ||
| FH of premature | 87 (8.8%) | 96 (9.8%) | 87 (8.8%) | 0.71 | |
| CCS class | I/II | 826 (83.8%) | 821 (83.3%) | 832 (84.3%) | 0.82 |
| III/IV | 160 (16.2%) | 165 (16.7%) | 155 (15.7%) | ||
| In sinus rhythm at admission | 64 (6.5%) | 54 (5.5%) | 59 (6.0%) | 0.63 | |
| Ejection fraction (%) | 48.0 [40.0 ÷ 53.5] | 48.0 [40.0 ÷ 55.0] | 50.0 [42.0 ÷ 55.0] | 0.08 | |
| BMS | 582 (59.0%) | 529 (53.7%) | 535 (54.2%) | 0.11 | |
| DES | 368 (37.3%) | 417 (42.3%) | 417 (42.2%) | ||
| BMS + DES | 36 (3.7%) | 40 (4.1%) | 35 (3.5%) | ||
| MVD | 194 (19.7%) | 189 (19.2%) | 196 (19.9%) | 0.92 | |
| No. of PCI vessels | 1 | 786 (79.7%) | 781 (79.2%) | 791 (80.1%) | 0.60 |
| No. of PCI vessels | 2 | 166 (16.8%) | 176 (17.8%) | 168 (17.0%) | |
| No. of PCI | 3 | 34 (3.0%) | 29 (2.7%) | 28 (2.3%) | |
| No. of stents | 1 | 715 (72.5%) | 678 (68.8%) | 679 (68.8%) | 0.62 |
| No. of stents | 2 | 212 (21.5%) | 246 (24.9%) | 241 (24.4%) | |
| No. of stents | 3 | 48 (4.9%) | 54 (5.5%) | 58 (5.9%) | |
| ⩾4 | 10 (0.7%) | 8 (0.7%) | 9 (0.7%) | ||
| BMI | 28.4 [26.0÷31.3] | 28.0 [25.3÷30.8] | 27.7 [25.2÷30.5] | <0.01 | |
Abbreviations: BMI - body mass index, BMS – bare metal stent, CABG – coronary artery bypass grafting, CCS – Canadian Cardiovascular Society, COPD - chronic obstructive pulmonary disease, DES – drug-eluting stent, FH – family history, MI – myocardial infarction, MVD – multivessel disease, NYHA – New York Heart Association, PCI – percutaneous coronary intervention, PVD – peripheral vascular disease, SCD - sudden cardiac death, TIA - transient ischemic attack.
Table 2.
Laboratory findings at hospital admission in the analyzed cohort according to PLR values.
| Variable | 1st tertile, N = 986 | 2nd tertile, N = 986 | 3rd tertile, N = 987 | P value |
|---|---|---|---|---|
| RBC (106/mm3) | 4.6 [4.3 ÷ 4.9] | 4.5 [4.2 ÷ 4.8] | 4.5 [4.1 ÷ 4.8] | <0.01 |
| Hb (mmol/l) | 8.8 [8.2 ÷ 9.3] | 8.7 [8.1 ÷ 9.2] | 8.4 [7.8 ÷ 9.0] | <0.01 |
| HCT (%) | 42.0 [39.0 ÷ 45.0] | 41.0 [39.0 ÷ 44.0] | 37.0 [40.0 ÷ 44.0] | <0.01 |
| WBC (103/mm3) | 7.6 [6.4 ÷ 8.9] | 6.8 [5.9 ÷ 8.1] | 6.8 [5.7 ÷ 8.1] | <0.01 |
| Neutrophils (103/mm3) | 4.0 [3.2 ÷ 5.0] | 4.0 [3.2 ÷ 5.0] | 4.3 [3.5 ÷ 5.5] | <0.01 |
| Lymphocyte (103/mm3) | 2.6 [2.2 ÷ 3.1] | 2.0 [1.7 ÷ 2.3] | 1.5 [1.2 ÷ 1.8] | <0.01 |
| PLT (103/mm3) | 176 [148 ÷ 206] | 204 [175 ÷ 236] | 239 [203 ÷ 283] | <0.01 |
| eGFR (ml/min/1.73 m2) | 85.5[71.0 ÷ 96.1] | 85.4 [69.4 ÷ 95.3] | 84.1 [67.6 ÷ 94.9] | <0.01 |
| Sodium (mmol/l) | 138.9 [137.1 ÷ 140.4] | 138.6 [137.0 ÷ 140.2] | 138.2 [136.6 ÷ 139.9] | <0.01 |
| Cholesterol (mmol/l) | 4.4 [3.7 ÷ 5.2] | 4.4 [3.7 ÷ 5.2] | 4.4 [3.6 ÷ 5.2] | 0.71 |
| PLR | 70.0 [59.7 ÷ 78.1] | 102.3 [94.3 ÷ 111.7] | 149.4 [132.5 ÷ 183.2] | <0.01 |
Abbreviations: eGFR – estimated glomerular filtration rate, Hb – hemoglobin, HCT – hematocrit, PLR – platelet/lymphocyte ratio, PLT – platelets, RBC – red blood cells, WBC – white blood cells.
Table 3.
Treatment at hospital discharge according to PLR values.
| Variable | 1st tertile, N = 986 | 2nd tertile, N = 986 | 3rd tertile, N = 987 | P value |
|---|---|---|---|---|
| Aspirin | 975 (98.9%) | 977 (99.1%) | 975 (98.8%) | 0.80 |
| Thienopyridines | 975 (98.9%) | 978 (99.2%) | 984 (99.7%) | 0.11 |
| ACE-I/ARB | 928 (94.1%) | 920 (93.3%) | 923 (93.5%) | 0.65 |
| Beta-blockers | 946 (95.9%) | 942 (95.5%) | 945 (95.7%) | 0.91 |
| Diuretics | 349 (35.4%) | 335 (34.0%) | 368 (37.3%) | 0.31 |
| Statin | 955 (96.9%) | 953 (96.7%) | 948 (96.0%) | 0.59 |
Abbreviations: ACE-I – angiotensin converting enzyme inhibitors, ARB – angiotensin receptor blockers.
PLR and Outcomes
Follow-up data (including eventual event and time of the event) were available for 2947 (99.6%) patients. The median follow-up was 1124 days (interquartile range 586 ÷ 1594 days). During the observation period, 287 deaths were reported. Mortality was highest in the 3rd tertile 108/983 (11.0%) as compared to the 1st 94/981 (9.6%) and 2nd tertile 85/981 (8.7%) (p = 0.03, log rank). Kaplan–Maier curves for survival are depicted in Fig. 1.
Figure 1.
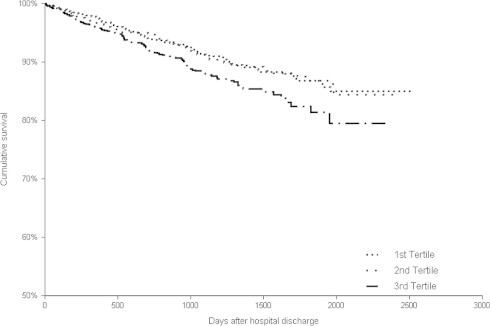
Kaplan–Meier survival plots for risk of death from all-cause, categorized according to the PLR ratio tertiles during follow-up.
Platelet-to-lymphocyte ratio was significantly associated with mortality in the univariable analysis (Fig. 2). It remained significantly associated with long-term mortality after consecutive adjustments for clinical variables (model 1), EF (model 2) and angiographic data (model 3). However, after adjustment for hemoglobin and eGFR, PLR was no longer significantly associated with the outcomes (model 4, Fig. 2).
Figure 2.
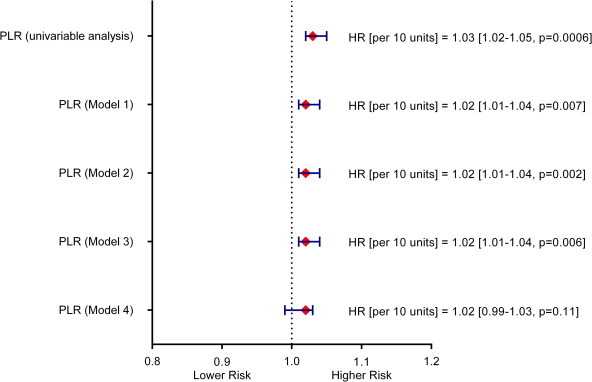
Risk associated with an increase in PLR in the setting of unadjusted analysis and after inclusion of clinical variables (model 1, Harrell’s C-statistics 0.742 [0.71 ÷ 0.77]), ejection fraction (model 2, Harrell’s C-statistics 0.745 [0.71 ÷ 0.78]), angiographic data (model 3, Harrell’s C-statistics 0.749 [0.72 ÷ 0.78]) and laboratory parameters (model 4, Harrell’s C-statistics – 0.762 [0.73 ÷ 0.79]).
Discussion
Platelet-to-lymphocyte ratio is a repeatable and easily obtainable blood index of the systemic inflammatory burden that combines both a prognostic value of the individual platelet and lymphocyte counts [4,9]. It has been found to be elevated in patients suffering from various oncologic disorders [17]. Platelet-to-lymphocyte ratio is a predictor of mortality in a cancer population [18–21] and a useful biomarker for predicting response to first-line chemotherapy [22].
Some studies have identified high platelet counts and worse outcomes after PCI [2,3], and the negative predictive role of lymphocyte counts in patients with coronary artery disease [7], unstable angina [6], and heart failure [4,5,8,9,23]. To our knowledge, no research has evaluated the significance of the PLR value in predicting long-term mortality in SCAD patients treated by elective PCI.
Gary et al. [24] reported that in patients with peripheral arterial occlusive disease, PLR ratio value of more than 150 can be used to discriminate patients at high risk for critical limb ischemia from those with a low risk. Additionally, it has been demonstrated that elevated platelet counts were independently associated with platelet resistance to aspirin [25], and increased PLR with a risk of thrombosis of prosthetic valves [26]. In our study, patients within the 3rd PLR tertile value were older, with a higher prevalence of female gender, a lower frequency of smoking, and lower body mass index compared to those within lower PLR tertiles. In addition, patients in the 3rd PLR tertile were more frequently hypertensive and had a higher heart rate at admission. The results of our study are in line with the findings of Gary at al, suggesting age influences on the PLR value [24].
Platelet-to-lymphocyte ratio was also found to be a marker of long-term mortality in patients with MI [10,12], and it was associated with more advanced coronary artery disease, in-hospital adverse events, and stent thrombosis in patients with STEMI treated with primary PCI [27,28].
In our study, we are able to demonstrate that a high PLR is a predictor of poor outcomes in patients after PCI and stent implantation after adjusting for clinical features, EF, and angiographic parameters (Fig. 2), but not after adjustment for hemoglobin and eGFR, which are well known predictors of long-term prognosis in that group of patients [29,30].
The mechanisms of the poor long-term outcomes of patients with elevated PLR discussed in the literature are multifactorial and not fully understood. It has long been recognized that low grade inflammation plays a key role in all aspects of atherosclerosis, including plaque formation, plaque rupture, and thrombosis. Prior studies reveal an association between major adverse cardiovascular outcomes and both higher platelet and low lymphocyte counts in patients with NSTEMI [10].
Abnormally low lymphocyte count was found to be a predictor of an overall mortality and MI in patients presenting to the emergency room with chest pain [31], and was associated with adverse clinical outcomes in patients with SCAD and NSTEMI [7,10]. On the contrary as platelets release over 300 proteins and molecules, including chemokines, and coagulation factors [32], higher platelet counts may reflect underlying inflammation and thrombocyte activation. In connection with its ability to ‘cross-talk’ with endothelial cells and leukocytes, platelets release substances that increase adhesion and transmigration of monocytes, therefore leading to a more aggressive course of atherosclerosis [33,34]. Indeed, platelet-neutrophil binding occurs after PCI [35]. However, inflammatory mediators and stress-induced steroid exposure can decrease the number of lymphocytes and hence modify the PLR [36]. At the end-stage renal disease, PLR is also positively correlated with the neutrophil-to-lymphocyte ratio, interleukin-6 and tumor necrosis factor-α, which are known mediators of inflammation [37]. Apart from its pro-inflammatory rating, PLR is also positively correlated with fibrinogen which leads to an increase in blood viscosity and therefore impairment of the tissue oxygen supply [24,38,39]. This is in line with both the study by Ayça et al, which proves that PLR is a predictor of no-reflow phenomenon in STEMI patients, and with our previous publication showing that baseline fibrinogen concentration in STEMI patients is an independent risk factor of the lack of myocardial reperfusion [27,40].
It has been also reported that an elevated platelet count is an independent predictor of aspirin resistance which may also affect outcomes in patients treated with stent implantation [25].
In summary, PLR is a surrogate marker of systemic inflammation and, as such, may provide an explanation for increased cardiovascular risk. Moreover, it could be assumed that high platelet concentrations and low lymphocyte counts may contribute to the progression of atherosclerosis, may accelerate restenosis and plaque instability, and hence be associated with worse prognosis in SCAD and patients with MI [10,11].
Strengths and limitations
This study has several limitations. It is a single-center retrospective study, and we used a single blood sample to calculate PLR. Nonetheless, the potential disadvantages of the retrospective analysis are diminished by the fact that patients’ data were inputted into an electronic database from report forms filled out by the attending physician upon the patient’s admission to our center. Strengths of this study include large cohort, detailed data on clinical, hemodynamic and laboratory parameters, and a long follow-up period with very few patients lost to follow-up.
Conclusion
Mortality in patients with SCAD undergoing elective stent implantation is higher in patients with the highest PLR values; and PLR has predictive value for all-cause long-term mortality in patients undergoing PCI and stent implantation, even after adjusting for the possible confounders, including clinical features, EF and angiographic data but not laboratory parameters. Future studies are warranted to clarify the prognostic value of PLR on cardiovascular event rates and all-cause mortality in SCAD patients.
Acknowledgements
The creation of the database of patients with SCAD used in this study was supported by the National Science Center – Dec-2011/01/D/NZ5/04387.
Disclosure: Authors have nothing to disclose with regard to commercial support.
References
- 1.Thaulow E., Erikssen J., Sandvik L., Stormorken H., Cohn P.F. Blood platelet count and function are related to total and cardiovascular death in apparently healthy men. Circulation. 1991;84(2):613–617. doi: 10.1161/01.cir.84.2.613. [DOI] [PubMed] [Google Scholar]
- 2.Iijima R., Ndrepepa G., Mehilli J., Bruskina O., Schulz S., Schömig A. Relationship between platelet count and 30-day clinical outcomes after percutaneous coronary interventions. Pooled analysis of four ISAR trials. Thromb Haemost. 2007;98(4):852–857. [PubMed] [Google Scholar]
- 3.Nikolsky E., Grines C.L., Cox D.A., Garcia E., Tcheng J.E., Sadeghi M. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial) Am J Cardiol. 2007;99(8):1055–1061. doi: 10.1016/j.amjcard.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 4.Ommen S.R., Hammill S.C., Gibbons R.J. The relative lymphocyte count predicts death in patients receiving implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2002;25(10):1424–1428. doi: 10.1046/j.1460-9592.2002.01424.x. [DOI] [PubMed] [Google Scholar]
- 5.Acanfora D., Gheorghiade M., Trojano L., Furgi G., Pasini E., Picone C. Relative lymphocyte count: a prognostic indicator of mortality in elderly patients with congestive heart failure. Am Heart J. 2001;142(1):167–173. doi: 10.1067/mhj.2001.115792. [DOI] [PubMed] [Google Scholar]
- 6.Zouridakis E.G., Garcia-Moll X., Kaski J.C. Usefulness of the blood lymphocyte count in predicting recurrent instability and death in patients with unstable angina pectoris. Am J Cardiol. 2000;86(4):449–451. doi: 10.1016/s0002-9149(00)00963-2. [DOI] [PubMed] [Google Scholar]
- 7.Ommen S.R., Gibbons R.J., Hodge D.O., Thomson S.P. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997;79(6):812–814. doi: 10.1016/s0002-9149(96)00878-8. [DOI] [PubMed] [Google Scholar]
- 8.Ommen S.R., Hodge D.O., Rodeheffer R.J., McGregor C.G., Thomson S.P., Gibbons R.J. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97(1):19–22. doi: 10.1161/01.cir.97.1.19. [DOI] [PubMed] [Google Scholar]
- 9.Arruda-Olson A.M., Reeder G.S., Bell M.R., Weston S.A., Roger V.L. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes. 2009;2(6):656–662. doi: 10.1161/CIRCOUTCOMES.108.831024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azab B., Shah N., Akerman M., McGinn J.T., Jr. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis. 2012;34(3):326–334. doi: 10.1007/s11239-012-0718-6. [DOI] [PubMed] [Google Scholar]
- 11.Ciçek G., Açıkgoz S.K., Bozbay M., Altay S., Uğur M., Uluganyan M. Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio Combination Can Predict Prognosis in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Angiology. 2014 doi: 10.1177/0003319714535970. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Yildiz A., Yuksel M., Oylumlu M., Polat N., Akyuz A., Acet H. The Utility of the Platelet-Lymphocyte Ratio for Predicting No Reflow in Patients With ST-Segment Elevation Myocardial Infarction. Clin Appl Thromb Hemost. 2014 doi: 10.1177/1076029613519851. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stekhoven D.J., Bühlmann P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–118. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 15.Stekhoven D.J. MissForest: Nonparametric missing value imputation using random forest, 1.4. http://cran.r-project.org/web/packages/mixOmics/index.html [accessed on 4 November 2014]
- 16.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria; 2013. http://www.R-project.org/.
- 17.Kwon H.C., Kim S.H., Oh S.Y., Lee S., Lee J.H., Choi H.J. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17(3):216–222. doi: 10.3109/1354750X.2012.656705. [DOI] [PubMed] [Google Scholar]
- 18.Proctor M.J., Morrison D.S., Talwar D., Balmer S.M., Fletcher C.D., O’Reilly D.S. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Lee S., Oh S.Y., Kim S.H., Lee J.H., Kim M.C., Kim K.H. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Açar G., Kalkan M.E., Avci A., Alizade E., Tabakci M.M., Toprak C. The Relation of Platelet-Lymphocyte Ratio and Coronary Collateral Circulation in Patients With Stable Angina Pectoris and Chronic Total Occlusion. Clin Appl Thromb. 2013 doi: 10.1177/1076029613508599. Oct 18. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Kalay N., Dogdu O., Koc F., Yarlioglues M., Ardic I., Akpek M. Hematologic parameters and angiographic progression of coronary atherosclerosis. Angiology. 2012;63(3):213–217. doi: 10.1177/0003319711412763. [DOI] [PubMed] [Google Scholar]
- 22.Liu H., Wu Y., Wang Z., Yao Y., Chen F., Zhang H. Pretreatment platelet-to-lymphocyte ratio (PLR) as a predictor of response to first-line platinum-based chemotherapy and prognosis for patients with non-small cell lung cancer. J Thorac Dis. 2013;5(6):783–789. doi: 10.3978/j.issn.2072-1439.2013.12.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudiger A., Burckhardt O.A., Harpes P., Müller S.A., Follath F. The relative lymphocyte count on hospital admission is a risk factor for long-term mortality in patients with acute heart failure. Am J Emerg Med. 2006;24(4):451–454. doi: 10.1016/j.ajem.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Gary T., Pichler M., Belaj K., Hafner F., Gerger A., Froehlich H. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS ONE. 2013;8(7):e67688. doi: 10.1371/journal.pone.0067688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lordkipanidzé M., Diodati J.G., Turgeon J., Schampaert E., Palisaitis D.A., Pharand C. Platelet count, not oxidative stress, may contribute to inadequate platelet inhibition by aspirin. Int J Cardiol. 2010;143(1):43–50. doi: 10.1016/j.ijcard.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 26.Gürsoy O.M., Karakoyun S., Kalçik M., Gökdeniz T., Yesin M., Gündüz S. Usefulness of novel hematologic inflammatory parameters to predict prosthetic mitral valve thrombosis. Am J Cardiol. 2014;113(5):860–864. doi: 10.1016/j.amjcard.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Ayça B., Akin F., Celik O., Yüksel Y., Oztürk D., Tekiner F. Platelet to lymphocyte ratio as a prognostic marker in primary percutaneous coronary intervention. Platelets. 2014:1–7. doi: 10.3109/09537104.2014.968117. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Oylumlu M., Yıldız A., Yüksel M., Korkmaz A., Aydın M., Bilik M.Z. Usefulness of Platelet-Lymphocyte Ratio to Predict Stent Thrombosis in Patients with ST Elevation Myocardial Infarction. Kosuyolu Kalp Derg. 2014;17(2):81–85. [Google Scholar]
- 29.Kitai Y., Ozasa N., Morimoto T., Bao B., Furukawa Y., Nakagawa Y. Prognostic implications of anemia with or without chronic kidney disease in patients undergoing elective percutaneous coronary intervention. Int J Cardiol. 2013;168(6):5221–5228. doi: 10.1016/j.ijcard.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 30.Osadnik T., Wasilewski J., Lekston A., Strzelczyk J., Kurek A., Gutowski A.R. Comparison of modification of diet in renal disease and chronic kidney disease epidemiology collaboration formulas in predicting long-term outcomes in patients undergoing stent implantation due to stable coronary artery disease. Clin Res Cardiol. 2014;103(7):569–576. doi: 10.1007/s00392-014-0687-1. [DOI] [PubMed] [Google Scholar]
- 31.Evans M.A., Hodge D.O., Smars P.A., Thomson S.P., Gibbons R.J. The prognostic value of the leukocyte differential in the evaluation of patients presenting with chest pain. Circulation. 1995;92(Suppl. I):664. [Abstract] [Google Scholar]
- 32.Smyth S.S., McEver R.P., Weyrich A.S., Morrell C.N., Hoffman M.R., Arepally G.M. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7(11):1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 33.Stokes K.Y., Granger D.N. Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol. 2012;590(Pt 5):1023–1034. doi: 10.1113/jphysiol.2011.225417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gawaz M., Langer H., May A.E. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115(12):3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi S., Watanabe N., Nakazawa K., Suzuki J., Tsushima K., Tamatani T. Roles of P-selectin in inflammation, neointimal formation, and vascular remodeling in balloon-injured rat carotid arteries. Circulation. 2000;102(14):1710–1717. doi: 10.1161/01.cir.102.14.1710. [DOI] [PubMed] [Google Scholar]
- 36.Park C.S., Ihm S.H., Yoo K.D., Kim D.B., Lee J.M., Kim H.Y. Relation between C-reactive protein, homocysteine levels, fibrinogen, and lipoprotein levels and leukocyte and platelet counts, and 10-year risk for cardiovascular disease among healthy adults in the USA. Am J Cardiol. 2010;105(9):1284–1288. doi: 10.1016/j.amjcard.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 37.Turkmen K., Erdur F.M., Ozcicek F., Ozcicek A., Akbas E.M., Ozbicer A. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial Int. 2013;17(3):391–396. doi: 10.1111/hdi.12040. [DOI] [PubMed] [Google Scholar]
- 38.Narvaez I., Sagastagoitia J.D., Vacas M., Saez Y., Lafita M., Monica S. Prevalence and biologic profile of aspirin resistance in patients with angiographically proven coronary artery disease. Thromb Res. 2007;120(5):671–677. doi: 10.1016/j.thromres.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 39.Prajapati J.H., Sahoo S., Nikam T., Shah K.H., Maheriya B., Parmar M. Association of high density lipoprotein with platelet to lymphocyte and neutrophil to lymphocyte ratios in coronary artery disease patients. J Lipids. 2014;2014:686791. doi: 10.1155/2014/686791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wasilewski J., Osadnik T., Poloński L. High baseline fibrinogen concentration as a risk factor of no tissue reperfusion in ST-segment elevation acute myocardial infarction treated with successful primary percutaneous coronary intervention. Kardiol Pol. 2006;64(9):967–972. discussion 973–4. [PubMed] [Google Scholar]


