Abstract
Objective
Circulating fibroblast growth factor 21 (FGF21) is an important auto- and endocrine player with beneficial metabolic effects on obesity and diabetes. In humans, thermogenic brown adipose tissue (BAT) was recently suggested as a source of FGF21 secretion during cold exposure. Here, we aim to clarify the role of UCP1 and ambient temperature in the regulation of FGF21 in mice.
Methods
Wildtype (WT) and UCP1-knockout (UCP1 KO) mice, the latter being devoid of BAT-derived non-shivering thermogenesis, were exposed to different housing temperatures. Plasma metabolites and FGF21 levels were determined, gene expression was analyzed by qPCR, and tissue histology was performed with adipose tissue.
Results
At thermoneutrality, FGF21 gene expression and serum levels were not different between WT and UCP1 KO mice. Cold exposure led to highly increased FGF21 serum levels in UCP1 KO mice, which were reflected in increased FGF21 gene expression in adipose tissues but not in liver and skeletal muscle. Ex vivo secretion assays revealed FGF21 release only from BAT, progressively increasing with decreasing ambient temperatures. In association with increased FGF21 serum levels in the UCP1 KO mouse, typical FGF21-related serum metabolites and inguinal white adipose tissue morphology and thermogenic gene expression were altered.
Conclusions
Here we show that the genetic ablation of UCP1 increases FGF21 gene expression in adipose tissue. The removal of adaptive nonshivering thermogenesis renders BAT a significant source of endogenous FGF21 under thermal stress. Thus, the thermogenic competence of BAT is not a requirement for FGF21 secretion. Notably, high endogenous FGF21 levels in UCP1-deficient models and subjects may confound pharmacological FGF21 treatments.
Keywords: FGF21, UCP1, Thermogenesis, BAT, iWAT, Obesity
Highlights
-
•
Genetic ablation of UCP1 increases FGF21 gene expression in adipose tissue.
-
•
The removal of adaptive nonshivering thermogenesis renders BAT a significant source of endogenous FGF21 under thermal stress.
-
•
The thermogenic competence of BAT is not a requirement for FGF21 secretion.
1. Introduction
Brown adipose tissue (BAT), the main site of non-shivering thermoregulation (NST), defends body temperature in small mammals and human infants [1]. In adult humans, the presence of active BAT inversely correlates with body mass index [2–5], suggesting that BAT is a natural defense mechanism against obesity. Thermogenic functionality of BAT is regulated at multiple levels, but the mitochondrial uncoupling protein 1 (UCP1) is crucial. UCP1 converts nutrient energy directly to heat by uncoupling substrate oxidation from ATP synthesis [6]. UCP1 KO mice are cold sensitive but can survive after stepwise acclimatization to the cold [7]. Canonical activation of BAT requires sympathetic noradrenaline release, but the search for peripheral hormones that increase UCP1 gene expression revealed several serum proteins, including fibroblast growth factor 21 (FGF21). FGF21 is a pleiotropic regulator of glucose homeostasis, lipid and energy metabolism [8–10], that is mainly released from the liver. Recently, other tissues such as the stressed muscle and adipose tissue were identified as sources of FGF21 [11,12]. In white adipose tissue (WAT), FGF21 appears to act solely in an autocrine or paracrine manner [8,13]. In BAT of rodents, FGF21 expression is induced by cold exposure and beta3-adrenergic stimulation [14–16]. So far, only one study showed secretion of FGF21 from BAT, suggesting an endocrine role of activated BAT [16].
Increased systemic FGF21 serum levels, either endogenously induced or exogenously administered, lead to the appearance of brown fat like-structures in WAT depots by the recruitment of beige fat cells – generally termed the “browning” of WAT [8,11,15,17]. The beige adipocytes possess thermogenic potential through expression of functional UCP1 [18] but whether they contribute to systemic adaptive thermogenesis is under debate [19]. In adult humans, the major proportion of UCP1-positive cells is classified as beige adipocytes [20,21] while the minor proportion is classical neonatal BAT that is found in the neck region [22]. Cold exposure of humans increased thermogenic gene expression including UCP1 and circulating FGF21, suggesting augmentation of BAT thermogenesis in concert with FGF21 [23]. Furthermore, the human data suggested that FGF21 secretion during cold exposure may require functionally active BAT [24]. Given the effects of exogenous FGF21 on “browning”, it is possible that elevated serum FGF21 levels increase thermogenic gene programming in beige adipose tissue of humans. Accumulating evidence from rodents and man suggests that the BAT-FGF21 axis plays a key role in the regulation of energy expenditure and thermogenesis.
In the UCP1 KO mouse model, which lacks the ability to recruit adrenergic thermogenesis in BAT [25,26], we discovered that circulating FGF21 levels are highly elevated in response to cold exposure and specifically released from non-functional BAT. The release of full-length FGF21 suggests endocrine crosstalk with other tissues, presumably altering serum metabolites and inducing thermogenic programs in WAT.
2. Material and methods
2.1. Animals
The experiments were performed in homozygous WT and UCP1 KO littermates (genetic background C57BL/6J – originally from Jackson Laboratory - Strain Name: B6.129-Ucp1tm1Kz/J). Mice were bred, born and weaned at 30 °C. They were housed in groups with ad libitum access to food and water and a 12:12-h dark–light cycle (lights on: 7:00 CET). At the age of 10–12 weeks, mice were single housed and randomly assigned to warm (30 °C) or cold (2–3 wks at 18 °C followed by 4 wks at 5 °C) acclimation. At 16–18 weeks of age, mice were euthanized 3–4 h after lights went on, and serum and tissue samples were collected. Another cohort of mice was acclimated to either 23 °C or 18 °C for 2 wks. The animal welfare authorities approved animal maintenance and experimental procedures.
2.2. Gene expression analysis
RNA was extracted from BAT, liver and inguinal white adipose tissue (iWAT) using Qiazol according to the manufacturer's instructions (Qiazol Lysis Reagent, Qiagen). Synthesis of cDNA and DNAse treatment were performed from 1 μg of total RNA using the QuantiTect Reverse Transcription Kit (Qiagen). Quantitative real-time PCR (qRT-PCR) was performed on the ViiA™ 7 Real-Time PCR System (Applied Biosystems). The PCR mix contained (5 μl) SybrGreen Master Mix, (Applied Biosystems), a cDNA amount corresponding to 5 ng of RNA used for cDNA synthesis and gene specific primer pairs. Gene expression was calculated as ddCT, using HPRT or B2 microglobulin (B2M) for normalization and relative to the WT 30 °C, which was normalized to a value of 1. The oligonucleotide primer sequences are available on request.
2.3. Serum analysis
For all serum analyses, commercially available assay kits were used according to the manufacturer's recommendations. Serum triglycerides (Triglyceride Colorimetric Assay Kit – Cayman Chemical), glycerol (Glycerol Colorimetric Assay Kit, Cayman) and intact/active FGF21 (Intact FGF-21 ELISA Kit, Eagle Biosciences) were measured undiluted. Sera for FGF21 (Mouse/Rat FGF-21 Quantikine ELISA Kit - R&D Systems) and NEFA (NEFA-HR2 Wako Chemicals) detection were diluted 1:2. Serum samples were stored at −80 °C until use.
2.4. Ex vivo analysis of FGF21 secretion in BAT and WAT
BAT and iWAT tissues were collected and washed several times with phosphate-buffered saline (PBS, Life technologies). Tissues were cut in pieces (10–15 mg), washed again 3x in PBS and incubated in Dulbecco's modified Eagle's medium (DMEM/F-12, Life technologies) containing 1% essential fatty acid-free bovine serum albumin (Sigma) for 4 h at 37 °C in a humidified incubator containing 5% CO2. Thereafter, the supernatants were removed and analyzed for FGF21 (R&D Systems). BAT and iWAT tissue pieces were washed in PBS, weighted, snap frozen in liquid nitrogen and stored at −80 °C for protein detection.
2.5. Ex vivo analysis of FGF21 secretion in soleus and EDL muscle
Ex vivo analysis of FGF21 secreted from EDL and soleus muscles was measured as described before [11].
2.6. Histology
Fat tissue specimens were fixed in 4% paraformaldehyde (Roth chemicals) for 24 h and embedded in low melting paraffin (Paraplast Plus®, Sigma Aldrich) for histological examination. Four μm-thick sections were cut using a rotary microtome (HSM55, Microm). Sections were mounted on superfrost glass slides (Menzel glass), dehydrated in increasing ethanol series and stained with hematoxylin and eosin (H&E) (Merck). Bright field images were obtained with the Keyence Microscope BZ-9000.
2.7. Statistics
Statistical analyses were performed using Stat Graph Prism (6.0) (Graphpad). All data are reported as mean ± SEM. After testing for normal distribution of the data and equal variances within the data sets, a Student's t-test (unpaired, two-tailed) was used to determine differences between the genotypes under different temperatures, whereby asterisks indicate the degree of statistical significance which was assumed P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001). Statistical analyses testing differences in tissue-specific levels and genotype differences were performed by one-way ANOVA followed by Bonferroni's multiple comparison adjustment. Statistical significance was assumed at P < 0.05. Statistical differences between groups are indicated by superscript letters, whereby different letters indicate significant different at P < 0.05.
3. Results
3.1. Chronic cold exposure induces serum levels of intact FGF21 in UCP1 KO mice
UCP1 KO mice kept at chronic cold (5 °C) showed significantly increased FGF21 serum levels as compared to WT mice, whereas no genotype difference was detectable under thermoneutral conditions (Figure 1A). To investigate whether the blood serum FGF21 levels can potentially mediate endocrine crosstalk between tissues, we assessed full-length FGF21 protein levels using a Sandwich ELISA assay, detecting the full-length protein by binding to the N-terminal and the C-terminal of FGF21. We found highly increased intact FGF21 in the blood serum of cold exposed UCP1 KO mice (Figure 1B).
Figure 1.
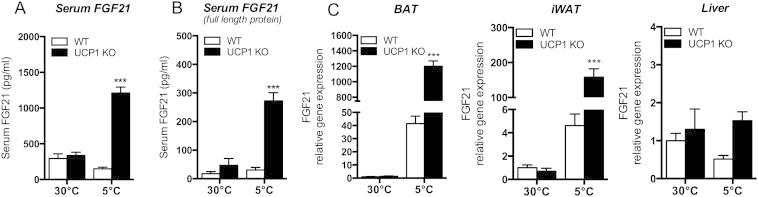
Cold induced increase of FGF21 serum levels and gene expression in UCP1 KO mice compared to WT littermates. Mice were maintained at 30 °C or exposed to 5 °C for 3 weeks (upon acclimation to 18 °C for 2 weeks). (A) Serum levels of FGF21 and (B) active FGF21 of WT or UCP1 KO mice. (C) quantitative PCR (qPCR) analysis of FGF21 in brown adipose tissue (BAT), inguinal white adipose tissue (iWAT) and liver. ***P < 0.001, significant differences between the genotypes. Data are means ± SEM (n = 6–8/group).
3.2. FGF21 gene expression is elevated in BAT and iWAT of UCP1 KO mice
We aimed to identify tissues that contribute to increased FGF21 serum levels of UCP1 KO mice under cold conditions and determined FGF21 mRNA levels in multiple tissues. FGF21 mRNA was highly induced in BAT and iWAT, but not in the liver, of cold-acclimated UCP1 KO mice (Figure 1C). At thermoneutrality, FGF21 mRNA levels in BAT and iWAT were low in both genotypes. The cold-induced increase of FGF21 gene expression in functional BAT and iWAT was expected in WT mice, given the concurrent literature [14–16]. However, the genetic ablation of UCP1 resulted in a dramatic increase of FGF21 mRNA levels in BAT and iWAT but not in the liver (Figure 1C), suggesting that BAT or iWAT is a relevant source of endocrine FGF21. To comparatively estimate the relative contribution of the tissues to increasing FGF21 serum levels, we also determined FGF21 mRNA levels only considering ct-values without normalizing to tissue-specific housekeeping genes in liver, skeletal muscle, heart, white and brown adipose tissue (Fig. S1), thus allowing for multiple tissue comparison. Among those tissues, we found the highest FGF21 mRNA concentrations in BAT and iWAT of cold acclimated UCP1 KO mice. As expected from the literature, liver shows the highest gene expression under thermoneutral conditions while FGF21 mRNA was nearly not expressed in muscle and heart.
3.3. FGF21 serum levels in UCP1 KO mice respond to mild temperatures below thermoneutrality
We asked whether high FGF21 serum levels are a result of prolonged cold exposure and adaptive non-shivering thermogenesis or whether temperatures below thermoneutrality directly control them. In order to distinguish between these two scenarios we performed mild cold challenges of WT and UCP1 KO mice raised and bred at 30 °C. Exposure to room temperature (23 °C, 11 days) significantly increased serum FGF21 levels in UCP1 KO mice, while no change was observed in WT mice. Acclimatization to 18 °C, considered the critical temperature for survival of UCP1 KO mice prior to cold exposure [7], further elevated FGF21 serum levels in UCP1 KO mice to the magnitude observed in response to prolonged cold of 5 °C (Figure 2A). The results demonstrate ambient temperature-dependent increase of FGF21 serum levels in UCP1 KO mice.
Figure 2.
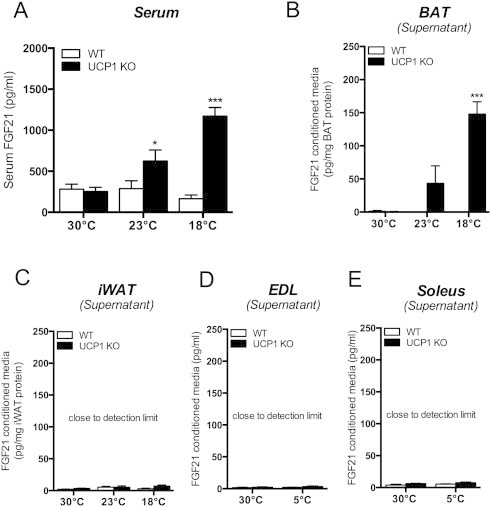
Mild ambient temperatures below thermoneutrality induce FGF21 serum levels and secretion from BAT of UCP1 KO mice. Mice were maintained at 30 °C or exposed to mild cold (23 °C or 18 °C) (A) Serum levels of FGF21 and ex vivo secretion of FGF21 from (B) BAT, (C) iWAT, EDL (extensior digitorum longus) (EDL) and (F) soleus muscle of WT and UCP1 KO mice after 4 h incubation. *P < 0.05; ***P < 0.001, significant differences between the genotypes. Data are means ± SEM (n = 4–6/group).
3.4. BAT is the source of cold-induced circulating FGF21 in UCP1 KO mice
To explore the source of increased FGF21 serum levels in UCP1 KO mice, we analyzed secretion of FGF21 from excised BAT, iWAT tissue samples and skeletal muscle (extensor digitorum longus (EDL) and soleus muscle). The ex vivo secretion analyses confirmed BAT but not iWAT or muscle of UCP1 KO mice as the source of circulating FGF21 by showing significant release of FGF21 in an acclimatization temperature-dependent manner (Figure 2B–E). In contrast, levels of secreted FGF21 from WT mouse BAT samples were close to the detection limit (Figure 2B). The liver is a known source of FGF21; however, we found no evidence for differential up-regulation of liver FGF21 mRNA in response to cold and due to genotype (Figure 1C). The secretion assays of this soft tissue are prone to error, due to unregulated protein leaking and therefore, were not performed in this study. Thus, although there is no evidence for regulation of liver FGF21, we cannot fully exclude the contribution of the liver to circulating FGF21 levels.
3.5. Cold-induction changes serum metabolites, iWAT morphology and gene expression
The typical effects of FGF21 action, such as decreased serum triglycerides, free fatty acids and glycerol levels (Figure 3A–C), were observed in UCP1 KO mice kept at chronic cold (5 °C). Further indication for enhanced endogenous FGF21 signaling is supported by increased FGF21-cofactor beta-klotho (Klb) in iWAT (Figure 3D; BAT/liver: Fig. S2). As expected, the iWAT of UCP1 KO mice displayed enhanced multilocularity, thermogenic and lipolytic gene expression (Figure 3E–G). The direct link between increased FGF21 of cold-exposed UCP1 KO mice and iWAT remodeling requires further experimentation. Interestingly, the remodeling of iWAT in UCP1 KO mice appears associated with metabolic futile cycles but not mitochondrial thermogenesis. Mitochondrial oxidative capacity (measured as COX activity) and the protein content of respiratory chain complexes in iWAT were not changed between genotypes (Fig. S3). Searching for alternative metabolic (thermogenic) pathways revealed evidence for increased futile cycling of lipids in cold exposed UCP1 KO mice, reflected in trends toward higher lipogenic (ACC, FASN) (Fig. S4) and significantly increased lipolytic (ATGL) gene expression (Fig. 3G). The futile cycling of triglyceride hydrolysis and re-synthesis is promoted by glycerol-kinase in WAT [27]. Cold-induction of glycerolkinase (Gyk) and adipocyte glycerol transporter (aquaporin 7; Aqp7) were more pronounced in iWAT of UCP1 KO compared to WT mice (Fig. S4). Altogether, UCP1-independent browning of iWAT appears to be associated with futile cycling of triglycerides and thus, higher ATP turnover (heat) of beige adipocytes.
Figure 3.
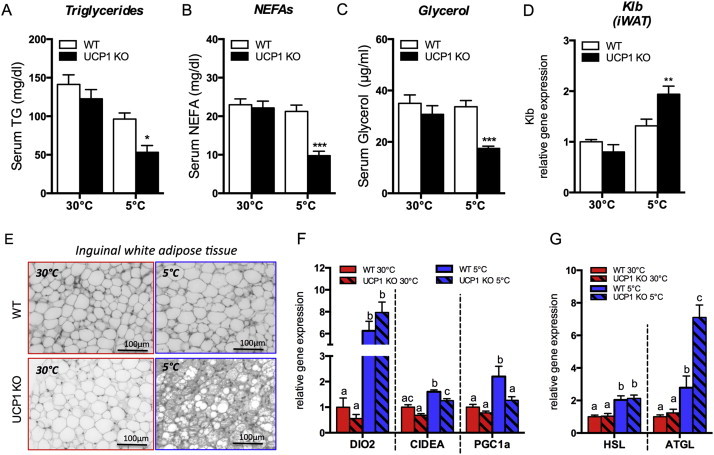
Serum levels of metabolites and iWAT morphology and gene expression of UCP1 KO mice and WT littermates. Mice were maintained at 30 °C or exposed to 5 °C for 3 weeks (upon acclimation to 18 °C for 2 weeks). Serum levels of (A) Triglycerides, (B) NEFAs, (C) Glycerol; iWAT Klb gene expression (D), morphology (E), thermogenic (F) and lipolytic (G) gene expression. *P < 0.05, **P < 0.01, and ***P < 0.0001, significant differences between the genotypes or statistical differences of the genotypes are indicated by superscript letters, whereby means annotated with different letters are significantly different. Statistical significance was assumed at P < 0.05. Data are means ± SEM (n = 6–8/group).
4. Discussion and conclusion
Obesity and diabetes research is geared toward the increase of UCP1 in brown and beige adipocytes to combust surplus energy. Similarly, FGF21 has been suggested as a therapeutic target to lower body weight by increasing energy expenditure. As both, BAT and FGF21, are associated in cold exposed humans, a positive functional relationship of between FGF21 and thermogenic BAT has been suggested [23]. Data on cold exposed humans suggested the augmentation of BAT thermogenesis in concert with FGF21 [23] and the requirement of functionally active BAT for FGF21 secretion [24].
The idea that FGF21 is released as an adipokine from thermogenically-competent BAT remained controversial [14–16], and was demonstrated by a single study in mice [16]. Here, not only do we confirm the release of FGF21 from BAT but also we demonstrate that classical UCP1-mediated BAT thermogenesis in mice is not required for cold-induced secretion of full-length FGF21 protein. Unexpectedly, the lack of UCP1 potentiated FGF21 expression in BAT and iWAT, rendering BAT the major source of circulating FGF21 serum levels at temperatures below thermoneutrality. The control for increased FGF21 release is presumably extrinsic (e.g. sympathetic nervous over-activation) as primary brown adipocytes of WT and UCP1 KO mice show no cell-autonomous differences in agonist-mediated FGF21 induction (CL316,243) (Fig. S5). Increased FGF21 plasma levels have pleiotropic metabolic effects but several studies established ‘browning’ of iWAT as a typical FGF21 target. Morphological remodeling such as multilocularity in iWAT [28–30], the induction of the β-Klotho/FGFR receptor complex, thermogenic and lipid metabolism gene programs (except UCP1) support the effects of increased FGF21 levels in coordinating adaptive responses in the absence of UCP1. In the absence of UCP1, thermogenesis may be supported by increased ATP turnover which can be enhanced by ATP consuming futile cycles. Whether these effects are solely mediated by FGF21 and assist to rescue metabolic homeostasis during cold exposure by mobilization of energy storage and futile metabolic cycles has to be determined in future studies, possibly utilizing UCP1-FGF21 double knockout mice.
Conflict of interests
The authors declare no competing financial interests.
Author contributions
SK and MK performed all experiments except histology, which was performed by LB and FN. SK, MK, and MJ analyzed the data. MJ and SK drafted, wrote and edited the manuscript, SK, RO, CWM, AK, and MJ conceptualized and designed the study.
Funding
This work was supported by the German Center for Diabetes Research (DZD).
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 2.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B. Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsushita M., Yoneshiro T., Aita S., Kameya T., Sugie H., Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. International Journal of Obesity. 2014;38:812–817. doi: 10.1038/ijo.2013.206. [DOI] [PubMed] [Google Scholar]
- 4.Ouellet V., Routhier-Labadie A., Bellemare W., Lakhal-Chaieb L., Turcotte E., Carpentier A.C. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of F-18-FDG-detected BAT in humans. Journal of Clinical Endocrinology and Metabolism. 2011;96:192–199. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- 5.Pfannenberg C., Werner M.K., Ripkens S., Stef I., Deckert A., Schmadl M. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–1793. doi: 10.2337/db10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholls D.G., Locke R.M. Thermogenic mechanisms in brown fat. Physiological Reviews. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Golozoubova V., Hohtola E., Matthias A., Jacobsson A., Cannon B., Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- 8.Coskun T., Bina H.A., Schneider M.A., Dunbar J.D., Hu C.C., Chen Y. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 9.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharitonenkov A., Adams A.C. Inventing new medicines: the FGF21 story. Molecular Metabolism. 2014;3:221–229. doi: 10.1016/j.molmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keipert S., Ost M., Johann K., Imber F., Jastroch M., van Schothorst E.M. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. American Journal of Physiology, Endocrinology and Metabolism. 2014;306:E469–E482. doi: 10.1152/ajpendo.00330.2013. [DOI] [PubMed] [Google Scholar]
- 12.Tyynismaa H., Carroll C.J., Raimundo N., Ahola-Erkkila S., Wenz T., Ruhanen H. Mitochondrial myopathy induces a starvation-like response. Human Molecular Genetics. 2010;19:3948–3958. doi: 10.1093/hmg/ddq310. [DOI] [PubMed] [Google Scholar]
- 13.Adams A.C., Coskun T., Cheng C.C., O.F L.S., Dubois S.L., Kharitonenkov A. Fibroblast growth factor 21 is not required for the antidiabetic actions of the thiazoladinediones. Molecular Metabolism. 2013;2:205–214. doi: 10.1016/j.molmet.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chartoumpekis D.V., Habeos I.G., Ziros P.G., Psyrogiannis A.I., Kyriazopoulou V.E., Papavassiliou A.G. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Molecular Medicine. 2011;17:736–740. doi: 10.2119/molmed.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F. FGF21 regulates PGC-1 alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & Development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hondares E., Iglesias R., Giralt A., Gonzalez F.J., Giralt M., Mampel T. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. Journal of Biological Chemistry. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kharitonenkov A., Wroblewski V.J., Koester A., Chen Y.F., Clutinger C.K., Tigno X.T. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 18.Shabalina I.G., Petrovic N., de Jong J.M., Kalinovich A.V., Cannon B., Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Reports. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 19.Keipert S., Jastroch M. Brite/beige fat and UCP1-is it thermogenesis? Biochimica et Biophysica Acta. 2014;1837:1075–1082. doi: 10.1016/j.bbabio.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Sharp L.Z., Shinoda K., Ohno H., Scheel D.W., Tomoda E., Ruiz L. Human BAT possesses molecular signatures that resemble beige/brite cells. PLOS One. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cypess A.M., White A.P., Vernochet C., Schulz T.J., Xue R., Sass C.A. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature Medicine. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee P., Linderman J.D., Smith S., Brychta R.J., Wang J., Idelson C. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metabolism. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virtanen K.A. BAT thermogenesis: linking shivering to exercise. Cell Metabolism. 2014;19:352–354. doi: 10.1016/j.cmet.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Enerback S., Jacobsson A., Simpson E.M., Guerra C., Yamashita H., Harper M.E. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 26.Golozoubova V., Cannon B., Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. American Journal of Physiology, Endocrinology and Metabolism. 2006;291:E350–E357. doi: 10.1152/ajpendo.00387.2005. [DOI] [PubMed] [Google Scholar]
- 27.Rosell M., Kaforou M., Frontini A., Okolo A., Chan Y.W., Nikolopoulou E. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. American Journal of Physiology, Endocrinology and Metabolism. 2014;306:E945–E964. doi: 10.1152/ajpendo.00473.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granneman J.G., Burnazi M., Zhu Z., Schwamb L.A. White adipose tissue contributes to UCP1-independent thermogenesis. American Journal of Physiology, Endocrinology and Metabolism. 2003;285:E1230–E1236. doi: 10.1152/ajpendo.00197.2003. [DOI] [PubMed] [Google Scholar]
- 29.Meyer C.W., Willershauser M., Jastroch M., Rourke B.C., Fromme T., Oelkrug R. Adaptive thermogenesis and thermal conductance in wild-type and UCP1-KO mice. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2010;299:R1396–R1406. doi: 10.1152/ajpregu.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ukropec J., Anunciado R.P., Ravussin Y., Hulver M.W., Kozak L.P. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1(-/-) mice. Journal of Biological Chemistry. 2006;281:31894–31908. doi: 10.1074/jbc.M606114200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


