Abstract
Although early myocardial reperfusion via primary percutaneous coronary intervention (PCI) allows the preservation of left ventricular function and improves outcome, the acute restoration of blood flow may contribute to the pathophysiology of infarction, a complex phenomenon called reperfusion injury. First described in animal models of coronary obstruction, mechanical post-conditioning, a sequence of repetitive interruption of coronary blood flow applied immediately after reopening of the occluded vessel, was able to reduce the infarct size. However, evidence of its real benefit remains controversial. This review describes the mechanisms of post-conditioning action and the different protocols employed focusing on its impact on primary PCI outcome.
Keywords: Post-conditioning, Balloon inflation, Primary PCI, Reperfusion injury
Abbreviations
- CK
creatine-kinase
- eNO
endogenous nitric-oxide
- LVEF
left ventricular ejection fraction
- MACE
major adverse cardiac events
- mKATP
mitochondrial potassium ATP
- mPTP
mitochondrial permeability transition pore
- MRI
magnetic resonance imaging
- PCI
percutaneous coronary intervention
- ROS
reactive oxygen species
- STEMI
ST-segment elevation myocardial infarction
- Tn
troponin
Introduction
For patients with ST-segment elevation myocardial infarction (STEMI), “time is myocardium”. Infarct size can be limited by early myocardial reperfusion via primary percutaneous coronary intervention (PCI), thus allowing the preservation of left ventricular function and improving clinical outcome [1,2]. However, the acute restoration of blood flow may contribute to the pathophysiology of infarction, a complex phenomenon called reperfusion injury [3,4]. Indeed, lethal reperfusion injury accounts for up to 50% of the final size of a myocardial infarct [4]. In 2003, Zhao et al. [5] were the first to describe a phenomenon known as “post-conditioning” in which a sequence of repetitive interruption of coronary blood flow was applied immediately after reopening of the occluded vessel. This adjunct treatment attenuated reperfusion injury, reduced infarct size and preserved vascular endothelial function comparable to ischemic preconditioning in a canine model of coronary obstruction [5]. Since the first report in a human heart by Staat et al. [6] in 2005, the interest in post-conditioning has increased. Although various parameters have been employed to assess its benefits, the real impact of post-conditioning in PCI remains controversial. The current review describes the mechanisms of post-conditioning action and the different protocols employed, focusing on its real impact on primary PCI outcome.
Mechanisms of post-conditioning action
In experimental models, the effect of post-conditioning on decreasing final infarct size is mediated through different pathways [5–8]. The molecular basis of post-conditioning action can be subdivided into the following three headings: triggers, mediators and end-effectors [7,8] (Fig. 1). Several activators of the signaling cascades (triggers) have been identified: adenosine, opioids, bradykinin, erythropoietin, endogenous nitric-oxide (eNO), acetylcholine, pro-inflammatory cytokines (particularly TNF-a and IL-6) and reactive oxygen species (ROS) [7]. The trigger phase is characterized by extracellular receptor/ligand interactions with autacoid, endocrine or paracrine signaling molecules [8]. The mediators of post-conditioning action can be subdivided into two pathways: the first is represented by reperfusion injury salvage kinase pathway, which includes phosphoinositide-3-kinases and extracellular regulated kinase-mitogen activated protein kinase, while the second consists of the reduction of intracellular calcium overload [7,8]. The mitochondrial permeability transition pore (mPTP) and mitochondrial potassium ATP (mKATP) channel [7] are currently considered as the most important end-effectors. The opening of mPTP, which is a voltage-dependent pore localized in the inner mitochondrial membrane, has been reported to occur within the first minutes of reperfusion. Such a phenomenon allows the transfer of small molecules into the matrix by osmosis, which is responsible for the swelling and rupturing of outer mitochondrial membrane with subsequent accumulation of calcium and other oxidants, eventually leading to alkalization of the intracellular matrix, and thus to apoptosis [7,8]. In the rabbit heart model, Argaud et al. [9] demonstrated that the magnitude of the protective effect of post-conditioning was similar to that obtained with NIM811, which specifically inhibited mPTP opening at the time of reperfusion. TRO40303 also inhibits mPTP opening, and has been shown to reduce infarct size in animal models of myocardial infarction [10,11]. However, in the MITOCARE study randomizing patients with STEMI requiring primary PCI to TRO40303 (n = 83) or placebo (n = 80) prior to balloon inflation, no significant differences were observed between groups regarding infarct size assessed by necrosis biomarkers and cardiac magnetic resonance imaging (MRI) [12].
Figure 1.
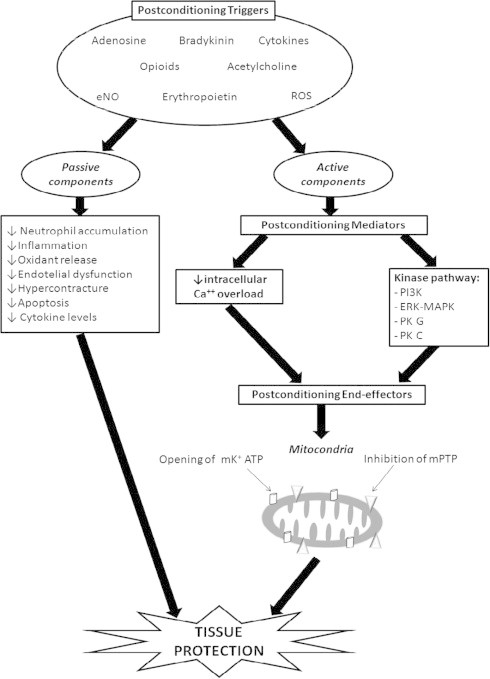
Molecular basis of post-conditioning action. The mechanisms of action of post-conditioning can be subdivided into three headings: triggers, mediators, and end-effectors. Several triggers have been recognized such as adenosine, opioids, bradykinin, erythropoietin, endogenous nitric-oxide, acetylcholine, pro-inflammatory cytokines and ROS. The mediators of post-conditioning action can be subdivided into two pathways: reperfusion injury salvage kinase pathway (PI3K, ERK-MAPK, PK G, PK C) and reduction of intracellular calcium overload. The mitochondria is the key target of post-conditioning action in order to avoid apoptosis due to ischemia/reperfusion injury, and the end-effectors are mPTP and mKATP. On the other hand, post-conditioning may also have a passive effect on improving endothelial dysfunction and decreasing oxidant release, hypercontracture, cytokine levels, inflammation and apoptosis. Abbreviations: Ca = calcium; ERK-MAPK = extracellular regulated kinase-mitogen activated protein kinase; eNO = endogenous nitric oxide; mKATP = mitochondrial potassium ATP; mPTP = mitochondrial permeability transition pore; PI3K = phosphoinositide-3-kinases; PK = protein kinase; ROS = reactive oxygen species.
On the other hand, the use of glibenclamide and 5-OH decanoate, which are inhibitors of mKATP channel, has been shown to reverse the protective effects of post-conditioning [13]. It has also been reported that intermittent targeting of the mKATP channel in the early minutes of reperfusion triggers post-conditioning protection through reactive oxygen species generation [14].
Otherwise, post conditioning may also have a passive impact on endothelial dysfunction, oxidant release, hypercontracture, cytokine levels, inflammation and apoptosis [7,8].
The majority of cellular signaling elements involved in such a process might be affected by confounders, co-morbidities or co-medication, as these conditions are associated with fundamental molecular alterations, potentially explaining the variability of responses to post-conditioning between individuals [3].
Mechanical post-conditioning protocols and modalities
Accumulating evidence has suggested that several technical issues may improve cardioprotection by post-conditioning, such as the balloon position, the conditioning delay to first inflation, and even the stenting technique. Thuny et al. [15] suggested that the post-conditioning protocol should be performed upstream of the site of the culprit lesion in order to reduce microembolisms. On the other hand, the delay to first inflation has been recognized as an important determinant of reduction in infarct size [16]. In fact, delay prolongation from 10 to 30 to 60 s [17,18] or 10 min [19] has been shown to result in cardioprotection failure in animal models.
In currently available trials, the post-conditioning protocols consisted of two to four cycles of ischemia and reperfusion (produced by inflations/deflations of angioplasty balloon) after direct stenting [20–22]. Taking into account an average of three cycles plus one balloon inflation for direct stenting, the cut-off of four inflations would mimic post-conditioning in a real-life practice. After PCI of the presumed culprit lesion, the stent balloon is commonly used to perform alternating inflations and deflations. In the majority of studies, post-conditioning was performed by four 30–60-s cycles of low pressure balloon inflations (4–6 atm) at the site of previous occlusion, each separated by 30–60 s of reflow [20–23]. Table 1 summarizes the various post-conditioning protocols employed in different studies.
Table 1.
Protocols employed in different trials on post-conditioning in PCI.
| Study | Year | Protocol of POC | N POC/controls |
|---|---|---|---|
| Staat et al. [6] | 2005 | 60 s × 4 | 14/16 |
| Ma et al. [34] | 2006 | 30 s × 3 | 47/47 |
| Yang et al. [26] | 2007 | 30 s × 3 | 23/18 |
| Thibault et al. [25] | 2008 | 60 s × 4 | 17/21 |
| Sorensson et al. [27] | 2010 | 60 s × 4 | 38/38 |
| Freixa et al. [28] | 2012 | 60 s × 4 | 39/40 |
| Tarantini et al. [29] | 2012 | 60 s × 4 | 39/39 |
| Zhao et al. [38] | 2012 | 60 s × 4 | 32/30 |
| Hahn et al. [20] | 2013 | 60 s × 4 | 350/350 |
| Dwyer et al. [41] | 2013 | 30 s × 4 | 50/52 |
| Limalanathan et al. [23] | 2014 | 60 s × 4 | 136/136 |
Abbreviations: PCI = percutaneous coronary intervention and POC = post-conditioning.
Post-conditioning effects
Post-conditioning and injury biomarkers
Serum creatine-kinase (CK) release was the most widely used study endpoint, and peak CK values strongly correlate with infarct size and predict cardiac outcomes in STEMI patients treated with primary PCI [24].
Yetgin et al. [21] found a lower CK peak in post-conditioning group compared with controls, corresponding to 21% reduction of enzymatic infarct size. This finding was similar to those reported by previous randomized studies, in which post-conditioning resulted in CK release reduction ranging from 27% to 40% [6,15,25,26]. However, Hahn et al. [20] failed to demonstrate any advantage of post-conditioning in terms of reperfusion markers as assessed by complete ST-segment resolution (⩾70%), 30 min post-PCI and post-procedural TIMI flow or myocardial blush grade in a large randomized trial involving 700 STEMI patients.
Other results from recent studies assessed with troponin I (TnI) or TnT [27–30], were also controversial with earlier observations. When TnT or TnI were used as endpoints, there was no significant difference between post-conditioning group and controls (p = 0.74) [30]. In a multi-center randomized controlled study, Roubille et al. [31] failed to show any significant decrease in CK and TnI release, even after adjustment for the size of the area at risk. More recently, Limalanathan et al. [23] showed no significant difference in TnT peak between controls and post-conditioning group (p = 0.63).
On the other hand, some factors may have an influence on the impact of post-conditioning on injury markers. According to Yetgin et al. [21], the decrease in CK peak was more pronounced in women, patients without diabetes or hypercholesterolemia, patients presenting within 3–6 h or with delay of first balloon re-occlusion ⩽ 1 min. In a meta-analysis of 13 studies, Wang et al. [30] found that myocardial injury biomarkers were significantly reduced in cases of non-use of Gp IIb/IIIa inhibitors. A possible explanation is that Gp IIb/IIIa inhibitors and post-conditioning procedure may act via similar pathways, thus achieving balance on protection, particularly against no-reflow phenomenon.
Post-conditioning and no-reflow
Despite the continuous improvement in PCI equipment and techniques, 60–70% of patients with optimal angiographic reperfusion still display microvascular obstruction (the so-called no-reflow phenomenon) detected by cardiac MRI studies after reperfusion [32]. After prolonged ischemia reperfusion, endothelium destruction or swelling, vasospasm, plugging of leukocytes or red blood cells and micro-thrombi may lead to the microcirculation perfusion deficit. Myocardial edema and hemorrhage may contribute by extrinsic compression causing microvascular obstruction. Using contrast-enhanced cardiac-MRI within 3 days after reperfusion, Mewton et al. [32] showed that post-conditioning was associated with smaller, early and late microvascular obstruction size (p = 0.01). Furthermore, such a significant reduction was persistent after adjustment for thrombus aspiration [32].
Dong and colleagues [33] assessed the impact of post-conditioning on various reflow determinants. Compared with controls, patients who underwent post-conditioning showed better rates of ST-segment resolution (93.8% versus 73.3%, p = 0.029), final TIMI grade-3 flow (81.3% versus 56.7%, p = 0.036), and final myocardial blush grade 3 (23% versus 14%, p = 0.043).
Although the number of patients in these two studies was relatively limited (50 and 62 respectively), post-conditioning could improve myocardial reperfusion in patients with STEMI patients undergoing PCI by reducing no-reflow [32,33].
In order to evaluate the impact of post-conditioning on coronary blood flow velocity, Ma et al. [34] reported that patients with post-conditioning had much faster corrected TIMI frame count 8 weeks after the primary PCI. Moreover, the peak of malondialdehyde, an oxidative agent which is actively implicated in coronary endothelial cytotoxicity, was significantly lower after post-conditioning compared with controls [34]. This latter finding revealed the probable impact of post-conditioning in improving cardiac vascular endothelial function after myocardial infarction.
Conversely, Hahn et al. [20] did not find significant differences neither in 30 min ST-segment resolution (p = 0.79) nor in blush flow grade 30 days later (p = 0.20).
Post-conditioning and left ventricular function
Comparing 32 patients who underwent post-conditioning to 30 controls treated by PCI for acute myocardial infarction, Dong et al. [33] found a better echocardiographic left ventricular ejection fraction (LVEF) in a post-conditioned group (55.1 ± 9.8% versus 42.9 ± 10.7%, p = 0.042), 7 days after the procedure. Two meta-analyses [30,35] reported the same findings with an improvement of LVEF after post-conditioning of 4.2% and 3.55%, respectively. However, it is usually recognized that only an increase of LVEF > 5% is considered significant improvement in patients with abnormal LVEF.
In canine models, Zhao et al. [5] reported that ischemic post-conditioning did not improve myocardial segment contractile function in the first 3 h after reperfusion. Moreover, Vinten-Johansen et al. [36] and Couvreur et al. [37] revealed that ischemic post-conditioning was not able to protect against myocardial stunning in dogs and rabbits. Such findings may explain the fact that at short-term follow up, Zhao et al. [38] found similar contractile function in post-conditioned and control groups. However, at 6-month follow-up, the authors demonstrated an increased LVEF and reduced wall motion score index in the post-conditioned group compared with controls [38]. Thibault et al. [25] also observed a persistent infarct size reduction at 6 months assessed by myocardial scintigraphy, and an improved recovery of myocardial contractile function at 1-year control echocardiography. Conversely, Wang et al. [30] found LVEF reduction during medium and long-term follow-up, which could be due to left ventricular remodeling.
Furthermore, studies using cardiac MRI have emerged revealing modest reductions [15,39–41], no impact [30,22], and perhaps even potential increase in infarct size without improvement [28,29]. By studying myocardial salvage after 3 months as judged by delayed enhancement cardiac MRI, Lønborg et al. [41] found a 19% relative reduction of infarct size in the post-conditioning group (51 ± 16% of total area at risk versus 63 ± 17%, p < 0.01), corresponding to a 31% increase in salvage ratio. Conversely, Dwyer et al. [22] showed that post-conditioning neither significantly increases myocardial salvage (p = 0.08) nor reduces infarct size (p = 0.18) assessed by cardiac MRI in patients with STEMI undergoing primary PCI. The same finding was reported by Roubille et al. [31] Moreover, Freixa et al. [28] found that post-conditioning was associated with lower myocardial salvage (4.1 ± 7.2 versus 9.1 ± 5.8% in controls; p = 0.004) and lower myocardial salvage index (18.9 ± 27.4 versus 30.9 ± 20.5% in controls; p = 0.038) with no significant differences in infarct size; and LVEF was found among the groups at 1 week and 6 months. More recently, in the POSTEMI trial, 272 patients were randomized to post-conditioning group (n = 136) and control group (n = 136); primary endpoint was infarct size measured by cardiac MRI [23]. After 4 months, no difference was observed between control group and post-conditioning group in percentage of left ventricular mass measured by cardiac MRI (14.4% versus 13.5%, respectively; p = 0.18) and LVEF after 4 months (55% versus 56.5%, respectively; p = 0.19) [23].
Post-conditioning and clinical outcome
Data regarding mid-term clinical follow-up are limited. In a cohort of 225 patients, Deftereos et al. [42] showed a lower 30-day rate of death or re-hospitalization for any cause in post-conditioned group compared with the control group (12.4% versus 22.3%; p = 0.05). Conversely, in a multicenter randomized trial including 700 patients, Hahn et al. [20] assessed clinical outcomes at 1 month from procedures. No differences were observed between post-conditioned patients and controls regarding death (3.7% versus 2.7%; p = 0.53) and major adverse cardiac events (MACE) (4.3% versus 3.7%; p = 0.70). At 4 months, Limalanathan et al. [23] reported no significant difference between post-conditioned patients and controls regarding re-hospitalization for acute coronary syndromes or heart failure.
In another report, Tarantini et al. [29] showed even higher rates of major adverse events at 6-month follow-up in post-conditioned group compared with control group (16.7% versus 2.6%; p = 0.08).
In a meta-analysis of 15 randomized trials including 1545 patients with a mean follow-up of 4.7 months, Khalili et al. [43] did not note any impact of mechanical post-conditioning on mortality (OR = 1.52; 95% CI 0.77–2.99; p = 0.23), recurrent myocardial infarction (OR = 3.04; 95% CI 0.74–12.54; p = 0.12), stent thrombosis (OR = 1.24, 95% CI 0.51–3.04; p = 0.83), or the composite MACE outcome (OR = 1.53; 95% CI 0.89–2.63; p = 0.13).
Pharmacological post-conditioning alternative
As an alternative to intra-coronary balloon inflations, several pharmacological agents designed to prevent lethal myocardial reperfusion injury by targeting its components have been evaluated in STEMI patients undergoing primary PCI. The most investigated of these pharmacological adjuncts were natriuretic peptide [44], cyclosporine A [45], and adenosine [46]. Kitakaze et al. [44] randomly assigned 277 patients to receive intravenous atrial natriuretic peptide for 3 days and 292 patients to receive the same dose of placebo. Patients with acute myocardial infarction and who were given atrial natriuretic peptide had lower infarct size of 14.7% (95% CI 3.0–24.9%), and better LVEF at 6–12 months (ratio 1.05, 95% CI 1.01–1.10, p = 0.024) [44]. Piot et al. [45] randomly assigned 58 patients receiving either an intravenous bolus of cyclosporine A or normal saline (control group) immediately before undergoing primary PCI. The release of CK was significantly reduced in the cyclosporine group as compared with the control group (p = 0.04), while the release of troponin I was not significantly reduced (p = 0.15) [45]. On the fifth day, the absolute mass of the area of hyper-enhancement on MRI was significantly reduced in the cyclosporine group as compared with the control group, with a median of 37 g (interquartile range, 21–51) versus 46 g (interquartile range, 20–65; p = 0.04). No adverse effects of cyclosporine administration were detected [45]. More recently, in placebo-controlled, randomized multicenter trial including 240 STEMI patients, Nicolli et al. [46] showed that the use of adenosine results not only in significant improvement of microvascular obstruction assessed by ST-segment resolution but also in MACE occurrence at 30 days. Although these are promising results, further studies are required to confirm the efficacy of these therapeutic agents and to determine whether they can improve clinical outcomes, especially if combined with intra-coronary balloon inflations.
Remote ischemic conditioning alternative
Remote ischemic conditioning is based on the fact that transient non-injurious ischemia of one organ or tissue can protect a distant organ or tissue from ischemic injury. Gho et al. [47] reported that brief ischemia of the kidney or small intestine was able to protect the myocardium against prolonged ischemia. Birnbaum et al. [48] demonstrated that rabbit hindlimb ischemia protected the heart against later ischemia. In 2005, Kerendi et al. [49] moved the remote ischemic stimulus in time, from before ischemia to later during the period of organ ischemia, and showed that renal ischemia was cardioprotective.
To understand the mechanisms involved, three theories have been advanced: (1) humoral factors acting via the systemic circulation, particularly eNO, ROS, adenosine, kininogens and opioids; (2) neurogenic transmission with involvement of muscle afferents and the autonomic nervous system; and (3) effects on immune cells with reduction of neutrophil activation and adherence to endothelium, and inflammatory gene expression [50]. The final common pathway of protection in the target organ involves activation of the reperfusion injury salvage kinases or survival-activating factor enhancement pathways that ultimately converge on the mitochondria to open mKATP channels, thereby preventing the opening of the mPTP [7,8].
In a meta-analysis of 23 randomized clinical trials of remote conditioning, most involving patients undergoing cardiac surgery, limb conditioning did not reduce mortality or major adverse cardiovascular events compared with no conditioning but did reduce the incidence of myocardial infarction and troponin release [51]. Bøtker and colleagues [52] reported the use of blood pressure cuff inflation/deflation for four cycles of 5-min occlusion in patients with STEMI before primary PCI. The intervention group (n = 73) had higher mean and median salvage indices at 30 days, estimated by gated single photon emission computed tomography, than the control group (n = 69). The benefit was greatest in the subset of patients with coronary vessel occlusion on admission angiography. More recently, Crimi et al. [53] showed that in patients with anterior STEMI, remote ischemic conditioning of the lower limb at the time of primary PCI reduced enzymatic infarct size and was also associated with ST-segment resolution and improvement of T2-weighted edema volume in cardiac MRI.
Conclusion
Although the majority of the studies demonstrate that post-conditioning might reduce injury biomarkers, the other benefits of post-conditioning remain controversial, and data on long-term outcomes are limited. Several confounders such as co-morbidities, co-medication and the difference in post-conditioning protocols might be responsible for the huge disparity observed between the various studies. Moreover, the limited number of randomized trials, including large cohorts, and the significant variations encountered in clinical events when the STEMI model is employed as a post conditioning model make it difficult to confirm the impact of post-conditioning. Further studies are therefore needed to better identify the best protocol to adopt, which patient might gain more benefit from such a procedure, and the overall possible benefits of associating post-conditioning with pharmacological and remote ischemic conditioning.
Disclosures: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Prati F., Petronio S., Van Boven A.J., Tendera M., De Luca L., de Belder M.A. Evaluation of infarct-related coronary artery patency and microcirculatory function after facilitated percutaneous primary coronary angioplasty: the FINESSE-ANGIO (facilitated intervention with enhanced reperfusion speed to stop events-angiographic) study. JACC Cardiovasc Interv. 2010;3(12):1284–1291. doi: 10.1016/j.jcin.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 2.van Domburg R.T., Sonnenschein K., Nieuwlaat R., Kamp O., Storm C.J., Bax J.J. Sustained benefit 20 years after reperfusion therapy in acute myocardial infarction. J Am Coll Cardiol. 2005;46(1):15–20. doi: 10.1016/j.jacc.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 3.Ovize M., Baxter G.F., Di Lisa F., Ferdinandy P., Garcia-Dorado D., Hausenloy D.J. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the working group of cellular biology of the heart of the European society of cardiology. Cardiovasc Res. 2010;87(3):406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 4.Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Z.Q., Corvera J.S., Halkos M.E., Kerendi F., Wang N.P., Guyton R.A. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 6.Staat P., Rioufol G., Piot C., Cottin Y., Cung T.T., L’Huillier I. Postconditioning the human heart. Circulation. 2005;112(14):2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 7.Kaur S., Jaggi A.S., Singh N. Molecular aspects of ischaemic postconditioning. Fundam Clin Pharmacol. 2009;23(5):521–536. doi: 10.1111/j.1472-8206.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 8.Bell R.M., Yellon D.M. Conditioning the whole heart-not just the cardiomyocyte. J Mol Cell Cardiol. 2012;53(1):24–32. doi: 10.1016/j.yjmcc.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Argaud L., Gateau-Roesch O., Raisky O., Loufouat J., Robert D., Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111(2):194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 10.Javadov S., Karmazyn M., Escobales N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J Pharmacol Exp Ther. 2009;330(3):670–678. doi: 10.1124/jpet.109.153213. [DOI] [PubMed] [Google Scholar]
- 11.Heusch G., Boengler K., Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol. 2010;105(2):151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 12.Atar D., Arheden H., Berdeaux A., Bonnet J.L., Carlsson M., Clemmensen P. Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: MITOCARE study results. Eur Heart J. 2014;Sep 1 doi: 10.1093/eurheartj/ehu331. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Penna C., Rastaldo R., Mancardi D., Raimondo S., Cappello S., Gattullo D. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res Cardiol. 2006;101(2):180–189. doi: 10.1007/s00395-006-0584-5. [DOI] [PubMed] [Google Scholar]
- 14.Penna C., Mancardi D., Rastaldo R., Losano G., Pagliaro P. Intermittent activation of bradykinin B2 receptors and mitochondrial KATP channels trigger cardiac postconditioning through redox signaling. Cardiovasc Res. 2007;75(1):168–177. doi: 10.1016/j.cardiores.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Thuny F., Lairez O., Roubille F., Mewton N., Rioufol G., Sportouch C. Post-conditioning reduces infarct size and edema in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59(24):2175–2181. doi: 10.1016/j.jacc.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y., Xu S. Post-conditioning the human heart: technical concerns beyond the protocol algorithm. J Am Coll Cardiol. 2013;62(13):1216–1217. doi: 10.1016/j.jacc.2013.05.083. [DOI] [PubMed] [Google Scholar]
- 17.Philipp S., Downey J.M., Cohen M.V. Postconditioning must be initiated in less than 1 minute following reperfusion and is dependent on adenosine receptors and PI3-kinase. Circulation. 2004;110 III–168. [Google Scholar]
- 18.Kin H., Zhao Z.Q., Sun H.Y., Wang N.P., Corvera J.S., Halkos M.E. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62(1):74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Yang X.M., Proctor J.B., Cui L., Krieg T., Downey J.M., Cohen M.V. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44(5):1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 20.Hahn J.Y., Song Y.B., Kim E.K., Yu C.W., Bae J.W., Chung W.Y. Ischemic postconditioning during primary percutaneous coronary intervention: the effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation. 2013;128(17):1889–1896. doi: 10.1161/CIRCULATIONAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- 21.Yetgin T., Manintveld O.C., Duncker D.J., van der Giessen W.J. Postconditioning against ischaemia-reperfusion injury: ready for wide application in patients? Neth Heart J. 2010;18(7–9):389–392. doi: 10.1007/BF03091803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dwyer N.B., Mikami Y., Hilland D., Aljizeeri A., Friedrich M.G., Traboulsi M. No cardioprotective benefit of ischemic postconditioning in patients with ST-segment elevation myocardial infarction. J Interv Cardiol. 2013;26(5):482–490. doi: 10.1111/joic.12064. [DOI] [PubMed] [Google Scholar]
- 23.Limalanathan S., Andersen G.Ø., Kløw N.E., Abdelnoor M., Hoffmann P., Eritsland J. Effect of ischemic postconditioning on infarct size in patients with ST-elevation myocardial infarction treated by primary PCI results of the POSTEMI (POstconditioning in ST-elevation myocardial infarction) randomized trial. J Am Heart Assoc. 2014;3(2):e000679. doi: 10.1161/JAHA.113.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chia S., Senatore F., Raffel O.C., Lee H., Wackers F.J., Jang I.K. Utility of cardiac biomarkers in predicting infarct size, left ventricular function, and clinical outcome after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2008;1(4):415–423. doi: 10.1016/j.jcin.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Thibault H., Piot C., Staat P., Bontemps L., Sportouch C., Rioufol G. Long-term benefit of postconditioning. Circulation. 2008;117(8):1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 26.Yang X.C., Liu Y., Wang L.F., Cui L., Wang T., Ge Y.G. Reduction in myocardial infarct size by postconditioning in patients after percutaneous coronary intervention. J Invasive Cardiol. 2007;19(10):424–430. [PubMed] [Google Scholar]
- 27.Sörensson P., Saleh N., Bouvier F., Böhm F., Settergren M., Caidahl K. Effect of postconditioning on infarct size in patients with ST elevation myocardial infarction. Heart. 2010;96(21):1710–1715. doi: 10.1136/hrt.2010.199430. [DOI] [PubMed] [Google Scholar]
- 28.Freixa X., Bellera N., Ortiz-Pérez J.T., Jiménez M., Paré C., Bosch X. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2012;33(1):103–112. doi: 10.1093/eurheartj/ehr297. [DOI] [PubMed] [Google Scholar]
- 29.Tarantini G., Favaretto E., Marra M.P., Frigo A.C., Napodano M., Cacciavillani L. Postconditioning during coronary angioplasty in acute myocardial infarction: the POST-AMI trial. Int J Cardiol. 2012;162(1):33–38. doi: 10.1016/j.ijcard.2012.03.136. [DOI] [PubMed] [Google Scholar]
- 30.Wang L., Wang J., Xu H., Li B. Postconditioning in patients treated with primary percutaneous coronary intervention: an updated meta-analysis. Catheter Cardiovasc Interv. 2013;82(5):E662–E671. doi: 10.1002/ccd.25095. [DOI] [PubMed] [Google Scholar]
- 31.Roubille F., Mewton N., Elbaz M., Roth O., Prunier F., Cung T.T. No post-conditioning in the human heart with thrombolysis in myocardial infarction flow 2–3 on admission. Eur Heart J. 2014;35(25):1675–1682. doi: 10.1093/eurheartj/ehu054. [DOI] [PubMed] [Google Scholar]
- 32.Mewton N., Thibault H., Roubille F., Lairez O., Rioufol G., Sportouch C. Postconditioning attenuates no-reflow in STEMI patients. Basic Res Cardiol. 2013;108(6):383. doi: 10.1007/s00395-013-0383-8. [DOI] [PubMed] [Google Scholar]
- 33.Dong M., Mu N., Guo F., Zhang C., Ren F., Li J. The beneficial effects of postconditioning on no-reflow phenomenon after percutaneous coronary intervention in patients with ST-elevation acute myocardial infarction. J Thromb Thrombolysis. 2014;38(2):208–214. doi: 10.1007/s11239-013-1010-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Ma X.J., Zhang X.H., Li C.M., Luo M. Effect of postconditioning on coronary blood flow velocity and endothelial function in patients with acute myocardial infarction. Scand Cardiovasc J. 2006;40(6):327–333. doi: 10.1080/14017430601047864. [DOI] [PubMed] [Google Scholar]
- 35.Hansen P.R., Thibault H., Abdulla J. Postconditioning during primary percutaneous coronary intervention: a review and meta-analysis. Int J Cardiol. 2010;144(1):22–25. doi: 10.1016/j.ijcard.2009.03.118. [DOI] [PubMed] [Google Scholar]
- 36.Vinten-Johansen J., Zhao Z.Q., Jiang R., Zatta A.J., Dobson G.P. Preconditioning and postconditioning: innate cardioprotection from ischemia-reperfusion injury. J Appl Physiol. 2007;103(4):1441–1448. doi: 10.1152/japplphysiol.00642.2007. [DOI] [PubMed] [Google Scholar]
- 37.Couvreur N., Lucats L., Tissier R., Bize A., Berdeaux A., Ghaleh B. Differential effects of postconditioning on myocardial stunning and infarction: a study in conscious dogs and anesthetized rabbits. Am J Physiol Heart Circ Physiol. 2006;291(3):H1345–H1350. doi: 10.1152/ajpheart.00124.2006. [DOI] [PubMed] [Google Scholar]
- 38.Zhao C.M., Yang X.J., Yang J.H., Cheng X.J., Zhao X., Zhou B.Y. Effect of ischaemic postconditioning on recovery of left ventricular contractile function after acute myocardial infarction. J Int Med Res. 2012;40(3):1082–1088. doi: 10.1177/147323001204000327. [DOI] [PubMed] [Google Scholar]
- 39.Nijveldt R., Beek A.M., Hirsch A., Stoel M.G., Hofman M.B., Umans V.A. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol. 2008;52(3):181–189. doi: 10.1016/j.jacc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Grybauskas P. Role of tissue factor in atherothrombosis. Medicina (Kaunas) 2003;39(12):1165–1170. [Article in Lithuanian] [PubMed] [Google Scholar]
- 41.Lønborg J., Kelbaek H., Vejlstrup N., Jørgensen E., Helqvist S., Saunamäki K. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv. 2010;3(1):34–41. doi: 10.1161/CIRCINTERVENTIONS.109.905521. [DOI] [PubMed] [Google Scholar]
- 42.Deftereos S., Giannopoulos G., Tzalamouras V., Raisakis K., Kossyvakis C., Panagopoulou V. Renoprotective effect of remote ischemic post-conditioning by intermittent balloon inflations in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2013;61(19):1949–1955. doi: 10.1016/j.jacc.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Khalili H., Patel V.G., Mayo H.G., de Lemos J.A., Brilakis E.S., Banerjee S. Surrogate and clinical outcomes following ischemic postconditioning during primary percutaneous coronary intervention of ST-segment elevation myocardial infarction: a meta-analysis of 15 randomized trials. Catheter Cardiovasc Interv. 2014;84(6):978–986. doi: 10.1002/ccd.25581. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Kitakaze M., Asakura M., Kim J., Shintani Y., Asanuma H., Hamasaki T. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370(9597):1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 45.Piot C., Croisille P., Staat P., Thibault H., Rioufol G., Mewton N. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359(5):473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 46.Niccoli G., Rigattieri S., De Vita M.R., Valgimigli M., Corvo P., Fabbiocchi F. Open-label, randomized, placebo-controlled evaluation of intracoronary adenosine or nitroprusside after thrombus aspiration during primary percutaneous coronary intervention for the prevention of microvascular obstruction in acute myocardial infarction: the REOPEN-AMI study (intracoronary nitroprusside versus adenosine in acute myocardial infarction) JACC Cardiovasc Interv. 2013;6(6):580–589. doi: 10.1016/j.jcin.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Gho B.C., Schoemaker R.G., van den Doel M.A., Duncker D.J., Verdouw P.D. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94(9):2193–2200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 48.Birnbaum Y., Hale S.L., Kloner R.A. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96(5):1641–1646. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 49.Kerendi F., Kin H., Halkos M.E., Jiang R., Zatta A.J., Zhao Z.Q. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100(5):404–412. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- 50.Hess D.C., Hoda M.N., Bhatia K. Remote limb preconditioning [corrected] and postconditioning: will it translate into a promising treatment for acute stroke? Stroke. 2013;44(4):1191–1197. doi: 10.1161/STROKEAHA.112.678482. [DOI] [PubMed] [Google Scholar]
- 51.Brevoord D., Kranke P., Kuijpers M., Weber N., Hollmann M., Preckel B. Remote ischemic conditioning to protect against ischemia-reperfusion injury: a systematic review and meta-analysis. PLoS ONE. 2012;7(7):e42179. doi: 10.1371/journal.pone.0042179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bøtker H.E., Kharbanda R., Schmidt M.R., Bøttcher M., Kaltoft A.K., Terkelsen C.J. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375(9716):727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 53.Crimi G., Pica S., Raineri C., Bramucci E., De Ferrari G.M., Klersy C. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACC Cardiovasc Interv. 2013;6(10):1055–1063. doi: 10.1016/j.jcin.2013.05.011. [DOI] [PubMed] [Google Scholar]


