Abstract
Acute pancreatitis (AP) is an acute inflammatory disease of the exocrine pancreas. In spite of the pivotal role of the endocrine pancreas in glucose metabolism, the impact of impaired glucose tolerance on AP has not been fully elucidated. A meta-analysis of seven observational studies showed that type 2 diabetes mellitus (DM) was associated with an increased risk of AP. The increased risk of AP shown in the meta-analysis was independent of hyperlipidemia, alcohol use and gallstones. Anti-diabetic drugs including incretins might increase the risk of AP, but no intervention trials have confirmed this. Although a controversial finding, DM seems to be associated with severe attacks and organ failure in AP. We analyzed the results of a nationwide epidemiological survey of AP in Japan. We studied the impact of pre-existing DM on the clinical course of AP in 1954 cases for which information on DM status was available at the onset of AP. The prevalence of DM in AP patients (12.8%) was higher than that in the general population in Japan (10.5%). AP patients with DM had higher morbidity of cardiovascular and renal failure than those without DM. About 35% of the idiopathic AP patients with DM had renal failure. The mortality of AP patients with DM (4.0%) was higher than that of AP patients without DM (1.7%). If stratified by etiology, idiopathic, but not alcoholic or biliary, AP patients with DM were predisposed to increased mortality (9.7%). In conclusion, impaired glucose tolerance might have an impact on the development and clinical outcome of AP. However, the impact might depend on the cause of hyperglycemia, the condition of DM including severity, duration and treatment, and the characteristics of the AP patients including age, etiology and comorbidity.
Keywords: Alcohol, Diabetes mellitus, Epidemiology, Gallstone, Mortality, Organ failure
Core tip: We analyzed the results of a nationwide epidemiological survey of acute pancreatitis (AP) in Japan to clarify the impact of pre-existing diabetes mellitus (DM) on the clinical course of AP. AP patients with DM had higher mortality and morbidity of cardiovascular and renal failure than those without DM. About 35% of the idiopathic AP patients with DM had renal failure. If stratified by etiology, idiopathic, but not alcoholic or biliary, AP patients with DM had higher mortality than those without DM. Impaired glucose tolerance might have an impact on the development and clinical outcome of AP.
INTRODUCTION
Acute pancreatitis (AP) is an acute inflammatory disease of the pancreas characterized by a sudden onset of upper abdominal pain, nausea, emesis, and an increase of pancreatic digestive enzymes in the serum and urine[1,2]. Most patients with AP have a mild disease that only affects the pancreas and resolves spontaneously. However, 10%-20% of the patients develop necrosis of the pancreas and multiple organ failure, which may eventually lead to death[1,2]. In Japan, the overall mortality of AP was found to be 2.6% and in severe AP, 10.1%[3]. Various factors such as gallstones, alcohol, comorbidity and age affect the development and the clinical condition of AP[1-4]. Diabetes mellitus (DM), a metabolic disease with a globally increasing prevalence, has an impact on various diseases including cardiovascular diseases[5], cancer[6] and sepsis[7]. In spite of the pivotal role of the endocrine pancreas in glucose metabolism, the impact of impaired glucose tolerance on AP remains to be fully elucidated. In this editorial, we review the association of DM with AP. In addition, we analyzed the results of a nationwide survey of AP in Japan to clarify the susceptibility of diabetic patients to AP and the impact of impaired glucose tolerance on the clinical outcome of AP.
PRE-EXISTING DM AND RISK OF DEVELOPING AP
Epidemiological evidence suggests that individuals with type 2 DM have an increased risk of AP. Noel et al[8] evaluated the risk of AP in patients with type 2 DM in a United States insurance claims database and found that the risk of AP was 2.83 times higher in the overall diabetic cohort than in the non-diabetic cohort, and five times higher in the youngest diabetic cohort (under the age of 45) than in the age-matched non-diabetic cohort. In the United Kingdom, patients with type 2 DM had a 1.49-fold higher risk of AP than those without diabetes[9]. In Taiwan, Lai et al[10] reported that patients with type 2 DM had 1.95-fold greater incidence of AP compared with non-diabetics (27.7 vs 14.2 per 10000 person-years). In agreement with the result of Noel et al[8], the age-specific risk was highest in type 2 DM subjects aged 20-39 years, followed by aged 40-64 years, and aged 65 years and older, compared with the non-diabetic age-matched group[10]. A meta-analysis of seven observational studies including these three studies[8-14] showed that type 2 DM was associated with an increased risk of AP [relative risk = 1.92; 95% confidence interval (CI): 1.50-2.47][15].
Although DM may play a causative role in AP, the underlying mechanisms remain largely unknown. Solanki et al[16] conducted a systematic search of the scientific literature for the years 1965-2011 exploring the mechanisms of pathogenesis of AP in patients with DM. They found that no clinical studies could be identified which directly provide pathogenetic mechanisms of DM in the causation of AP. The available data on DM and its associated metabolic changes and therapy indicate that hyperglycemia coupled with the factors influencing insulin resistance (tumor necrosis-α, nuclear factor-κB, and amylin) cause an increase in the generation of reactive oxygen species in pancreatic acinar cells[16]. On the other hand, the susceptibility of DM to AP might result from confounding factors because the risk factors for AP such as alcohol consumption, gallstones, obesity, hypertriglyceridemia and chronic pancreatitis are also complications and risk factors for DM[17-19]. A history of DM might represent both an underlying pathogenetic mechanism and a potential confounding factor for the development of AP. However, the increased risk of AP shown in the meta-analysis was independent of hyperlipidemia, alcohol use and gallstones[15].
ANTI-DIABETIC DRUGS AND RISK OF AP
It has been of debate whether anti-diabetic drugs increase the risk of AP. Among the biguanides, both phenformin and metformin, the latter in patients with renal insufficiency, have been reported in case reports as a potential cause of AP[20,21]. Sulphonylureas have also been found in cohort studies to increase the AP risk. Gonzalez-Perez[14] reported that past use of sulphonylureas was associated with an increased risk of AP compared with that of non-users (OR = 2.58, 95%CI: 1.34-4.96). Conversely, Lai et al[10] reported that patients taking anti-diabetic drugs had a reduced risk of AP. The hazard ratios (HRs) of developing AP ranged from 0.44 to 0.63 (all P < 0.05) for subjects taking α-glucosidase inhibitors, metformin, sulfonylureas, or thiazolidinediones, after controlling for sex, age and co-morbidities. The beneficial effect of insulin was likely, but not statistically significant (HR = 0.81, 95%CI: 0.64-1.02). Interestingly, the beneficial effects were increased as the number of drugs taken increased. From an adjusted HR of 0.7 for patients who had used two medications, the adjusted HR was reduced to 0.31 (95%CI: 0.18-0.56) in patients taking five anti-diabetic drugs. There was a significant dose-response effect of the duration of taking anti-diabetic drugs on reducing the risk of AP[10]. The HR decreased to 0.14 among patients taking metformin, and to 0.13 among those taking sulfonylureas, when the treatment was longer than 3 years. The beneficial effect was also observed among those using insulin for more than a year.
Several case reports and the United States Food and Drug Administration pharmacovigilance database indicate an association between AP and incretin-based agents: dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 receptor agonists (reviewed in[22]). In the drug development program and clinical trials of both incretin classes, the incidence of AP was similar to placebo-treated cohorts, and a meta-analysis confirmed this result[23]. In addition, none of the intervention trials investigating these compounds, including two large randomized controlled trials with cardiovascular endpoints, confirmed the increased risk of AP with incretin use. In the EXAMINE trial, the incidence of AP was 0.4% in the alogliptin group and 0.3% in the placebo group[24]. In the SAVOR-TIMI53 trial, cases of definite AP were confirmed in 17 (0.2%) vs 9 (0.1%) (HR = 1.88, 95%CI: 0.86-4.41, P = 0.17) and definite plus possible pancreatitis in 22 vs 16 (HR = 1.36, 95%CI: 0.72-2.64, P = 0.42) in the saxagliptin and placebo arms, respectively[25]. No differences in time to event onset, concomitant risk factors for pancreatitis, investigator-reported causality from study medication or disease severity, and outcome were found between treatment arms. Further understanding of the association between anti-diabetic drugs and AP may provide clues to developing preventive strategies in patients with DM.
IMPACT OF IMPAIRED GLUCOSE TOLERANCE ON THE CLINICAL COURSE OF AP
Some studies have shown that AP patients with diabetes had a lower risk of hospital mortality, but with conflicting results. The University HealthSystem Consortium database and the prospective Mayo Clinic database showed that diabetic patients discharged with a diagnosis of AP experienced a significantly decreased mortality (Table 1)[26]. A retrospective cohort study in Taiwan also showed that AP patients with DM had a lower risk of hospital mortality (adjusted OR = 0.77, 95%CI: 0.65-0.91)[27]. It is not clear why diabetes may have such an intriguingly protective effect on the mortality in AP patients. A survival advantage of DM has been reported in patients with other critical illnesses[28] such as pneumonia, congestive heart failure[26], and sepsis[29]. The proposed biological mechanisms that favor diabetes during critical illnesses include the anti-inflammatory effect of some antidiabetic agents such as insulin[30] and thiazolidinediones[31], and the preventive effects against acute lung injury[29,32]. Furthermore, nonbiological mechanisms such as an improvement of lifestyle before the onset of AP and vigilant treatments after admission for patients with DM could explain, at least in part, the finding of decreased mortality in patients with DM.
Table 1.
Mortality of acute pancreatitis in patients with pre-existing diabetes mellitus and those without diabetes mellitus
| Ref. | Country | Diagnosis of DM | Prevalence of DM | Mortality of AP with DM | Mortality of AP without DM | Mortality of AP with DM compared to AP without DM |
| Graham et al[26], (2010) | United States | Two patients databases | Undescribed | Undescribed | Undescribed | Lower |
| Shen et al[27], (2012) | Taiwan | The Taiwan National Insurance Research Databese | Case-control study | 3.5% | 4.1% | Lower |
| Zhao et al[33], (2012) | China | History or HbA1c | 12.6% (40/318) | 15.0% | 1.1% | Higher |
| Kikuta et al, (present study) | Japan | Questionnaire | 12.8% (250/1954) | 4.0% | 1.7% | Higher |
AP: Acute pancreatitis; DM: Diabetes mellitus.
In contrast, several studies have shown that AP patients with DM might have more severe courses and poor outcomes. Frey et al[4] reported that DM increased the risk of multi-organ failure (≥ 2 systems) (OR = 1.6, 95%CI: 1.4-1.8). AP patients with a previous history of DM or with glycosylated hemoglobin A1c higher than 6.5% had a higher mortality than those without DM[33]. Shen et al[27] retrospectively compared 18990 first-attack AP patients with DM to 37980 matched control subjects from Taiwan’s National Health Insurance Research Database between 2000 and 2009. They showed that AP patients with DM had a higher risk of severe attack than their non-diabetic counterparts (adjusted OR = 1.21, 95%CI: 1.16-1.26). When severity criteria, defined by the modified Atlanta classification, were individually analyzed, diabetic AP patients had a 58% higher risk of intensive care unit admittance and more life-supporting treatments, especially hemodialysis and mechanical ventilation, than non-diabetic AP patients[27]. Diabetic AP patients had a 30% higher risk of local complications than AP patients without diabetes. Zhao et al[33] reported that AP patients with DM had a longer length of hospitalization than those without DM (18.3 ± 4.6 d vs 13.2 ± 5.1 d).
TRANSIENT HYPERGLYCEMIA IN THE EARLY COURSE OF AP
Hyperglycemia is the common early feature of AP and is used in prognostic models[34,35]. The association between hyperglycemia and poor clinical outcomes in critically ill patients has been demonstrated in a number of observational studies[28,36], but the adverse effect of hyperglycemia may be complicated by pre-existing diabetes. Egi et al[28] showed that hyperglycemia while staying in an intensive care unit was strongly associated with the outcome in patients without pre-existing DM but showed no significant association with the outcome in patients with pre-existing DM. This finding suggests that acute and chronic impaired glucose tolerance may be distinct pathophysiological entities and may have different clinical consequences. Hence, hyperglycemia in the early phase of AP seems to be complicated and may arise from mechanisms such as uncontrolled pre-existing DM, damage to the endocrine pancreas due to severe attack of AP, and metabolic stress associated with critical illness[37].
THE IMPACT OF DM ON CLINICAL COURSE OF AP BASED ON THE NATIONWIDE SURVEY IN JAPAN
As described above, the impact of impaired glucose tolerance in AP seems to be complicated. To clarify this issue, we examined the impact of DM on the clinical course of AP using the results of the nationwide epidemiological survey of AP in Japan.
Methods
A nationwide epidemiological survey was conducted by the Research Committee of Intractable Diseases of the Pancreas, targeting AP patients treated in 2007 in Japan. The details of the survey were published elsewhere[38]. Briefly, a first survey was performed by sending a questionnaire to randomly selected 3027 departments to determine the number of hospitalized patients with AP during 2007. A second survey was conducted by sending a questionnaire to 693 departments in which hospitalized patients with AP were treated based on the first questionnaire. This study was approved by the Ethics Committee of Tohoku University School of Medicine (article No. 2008-327, 2014-220). We studied the impact of DM on the clinical outcome of AP using 1954 cases for which information on DM status was available at the onset of AP. DM was diagnosed by respondents to the questionnaire, based on clinical information such as history, hemoglobin A1c and blood sugar level. The clinico-epidemiological information on individual patients including patient profile, etiology, symptoms, blood biochemical findings, computed tomography (CT) findings, and prognosis was collected from medical records of patients. The characteristics of the study population are shown in Table 2.
Table 2.
Characteristics of the study population n (%)
| Total AP patients | AP patients with DM | AP patients without DM | P value | |
| Patients, n | 1954 | 250 | 1704 | |
| Sex | ||||
| Male | 1289 (66.0) | 185 (74.0) | 1104 (64.8) | 0.004 |
| Female | 665 (34.0) | 65 (26.0) | 600 (35.2) | |
| Age, yr, mean ± SD | 59.3 ± 18.0 | 60.3 ± 16.6 | 59.1 ± 18.2 | 0.319 |
| Etiology | ||||
| Alcoholic | 621 (31.8) | 97 (38.8) | 524 (30.8) | 0.107 |
| Biliary | 482 (24.7) | 52 (20.8) | 430 (25.2) | |
| Idiopathic | 319 (16.3) | 34 (13.6) | 285 (16.7) | |
| Miscellaneous | 482 (24.7) | 60 (24.0) | 422 (24.8) | |
| Undescribed | 50 (2.6) | 7 (2.8) | 43 (2.5) | |
| BMI, mean ± SD | 22.5 ± 4.0 | 23.5 ± 4.2 | 22.3 ± 4.0 | < 0.001 |
| Triglyceride, mg/dL, | ||||
| median (1st, 3rd quartile) | 97 (64, 170.5) | 137 (79.5, 526) | 94 (63, 157.75) | 0.004 |
AP: Acute pancreatitis; DM: Diabetes mellitus.
Respiratory failure was defined as necessity of ventilatory support. Cardiovascular failure was defined as systolic blood pressure ≤ 80 mmHg or symptoms of shock. Renal failure was defined as blood urea nitrogen ≥ 40 mg/dL or serum creatinine ≥ 2.0 mg/dL. Three organ failures were evaluated daily until 72 h after AP onset.
CT findings within 48 h of onset were graded based on the severity scoring system of AP proposed by the Japanese Ministry of Health, Labour and Welfare[39]. Extrapancreatic inflammation was graded into four categories: none, anterior pararenal space, root of mesocolon, and beyond lower pole of kidney. The pancreas was conveniently divided into three segments (head, body, and tail), and hypoenhanced lesion of the pancreas by contrast-enhanced CT was also graded into four categories: none, localized in each segment, extends to 2 segments, and occupies 2 whole segments or more.
The number and percentage of patients were calculated and compared between groups by the chi-square test or a two-tailed Fisher’s exact test (when at least one expected cell value in 2 × 2 table was less than 5). Risk factors for mortality of AP were assessed using logistic regression analysis. Stepwise logistic regression analysis with a variable entry criterion of P < 0.10, and a variable retention criterion of P < 0.05 was used to identify multivariable risk factors. Logistic regression results were summarized as the OR, 95%CI for OR, and P value. Data were analyzed using JMP7 software (SAS Institute, Inc., Cary, NC). All statistical tests were 2-sided and P < 0.05 was used to indicate statistical significance.
Prevalence of DM at the onset of AP
The prevalence of DM in AP patients (12.8%) was significantly higher than that in the general population in Japan (10.5%) (Table 3). If stratified by etiology, patients with alcoholic AP (15.6%) had a significantly higher prevalence of DM than the Japanese general population. The prevalence of DM in patients with biliary or idiopathic AP was not different from that in the Japanese general population. Of note, the prevalence of DM in AP patients under 60 years old was significantly higher than in the Japanese general population of the same generation.
Table 3.
Prevalence of diabetes mellitus in patients with acute pancreatitis stratified by age
| Age | General population (MHLW 2007) | All AP patients | Alcoholic AP | Biliary AP | Idiopathic AP |
| Total | 10.5% | 12.8%b | 15.6%d | 10.8% | 10.7% |
| (420/4003) | (250/1954) | (97/621) | (52/482) | (34/319) | |
| < 19 | Not available | 5.3% | 0% | No patients | 16.7% |
| (1/19) | (0/1) | (1/6) | |||
| 20-29 | 0.5% | 9.3%d | 11.9%d | 0% | 3.4% |
| (1/204) | (9/97) | (5/42) | (0/4) | (1/29) | |
| 30-39 | 1.4% | 12.0%d | 13.0%d | 0% | 13.8%c |
| (8/589) | (25/208) | (15/115) | (0/18) | (4/29) | |
| 40-49 | 4.7% | 12.8%d | 12.8%d | 14.3%a | 16.7%a |
| (26/557) | (34/265) | (21/164) | (4/28) | (4/24) | |
| 50-59 | 8.0% | 12.2%a | 13.9%a | 7.8% | 15.6% |
| (57/711) | (41/336) | (21/151) | (5/64) | (5/32) | |
| 60-69 | 17.7% | 14.8% | 22.6% | 13.0% | 13.5% |
| (165/934) | (50/338) | (19/84) | (13/100) | (7/52) | |
| > 70 | 16.2% | 13.3% | 29.1%a | 11.2% | 8.5%a |
| (163/1008) | (87/652) | (16/55) | (29/259) | (12/141) | |
| Uncertain age | No patients | 7.7% | 0% | 11.1% | 0% |
| (3/39) | (0/9) | (1/9) | (0/6) |
The prevalence of DM in the Japanese general population in 2007 was cited from a report by the Ministry of Health, Labour and Welfare (http://www.mhlw.go.jp/bunya/kenkou/eiyou09/dl/01-kekka-01.pdf).
P < 0.05,
P < 0.01,
P < 0.005,
P < 0.001 vs the prevalence of DM in Japanese general population. AP: Acute pancreatitis; DM: Diabetes mellitus.
Organ failure
The morbidity of organ failures diagnosed within 72 h of AP onset is summarized in Figure 1. In AP patients without pre-existing DM, the morbidity of cardiovascular, respiratory and renal failures was around 1%, 2% and 5%, respectively. AP patients with DM had higher morbidity of cardiovascular and renal failure than those without DM, although the morbidity of respiratory failure was not different between the patients with DM and those without DM. Interestingly, the morbidity of organ failure varied among etiologies in DM patients, but not in non-DM patients. About 35% of the idiopathic AP patients with DM had renal failure.
Figure 1.
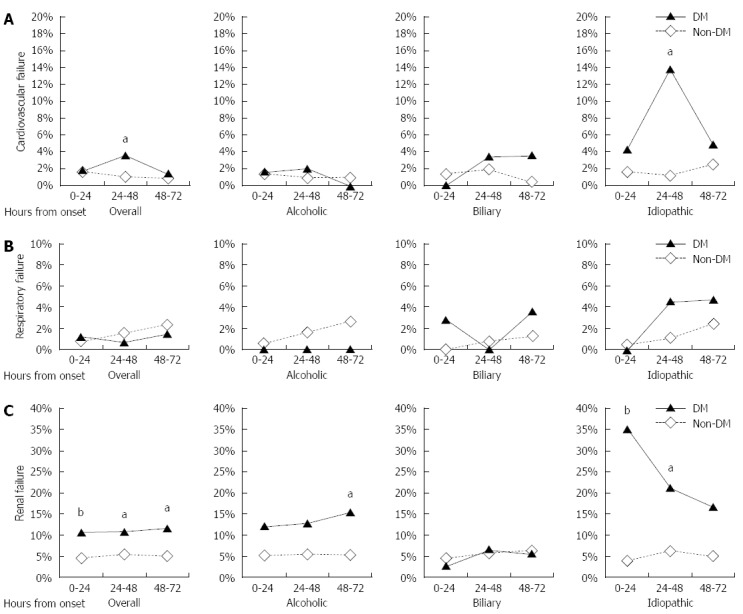
Morbidity of organ failure. The morbidity of cardiovascular failure (A), respiratory failure (B) and renal failure (C) was compared between the AP patients with or without DM (aP < 0.05, bP < 0.01 vs non-DM; Fisher’s exact test). AP: Acute pancreatitis; DM: Diabetes mellitus.
CT findings
CT findings within 48 h of AP onset are shown in Figure 2. CT findings did not differ between the patients with DM and those without DM. Even if stratified by etiology, pre-existing DM did not have an impact on CT findings (data not shown).
Figure 2.
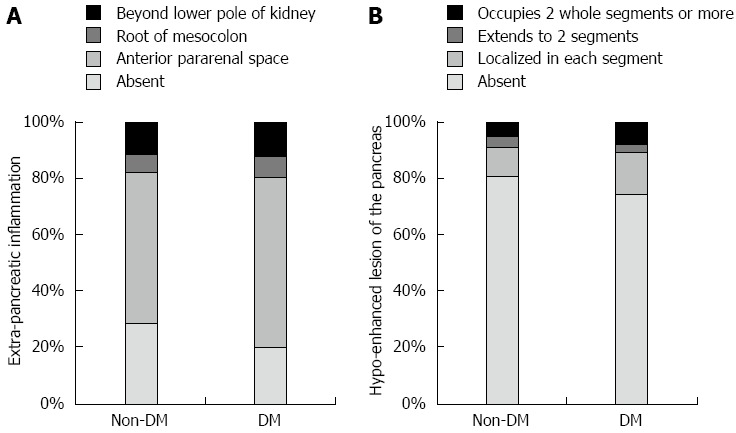
Comparison of the computed tomography findings between the acute pancreatitis patients with or without diabetes mellitus. A: Extra-pancreatic inflammation; B: Hypo-enhanced lesion of the pancreas. There were no significant differences in the distribution of the extra-pancreatic inflammation and hypo-enhanced lesions between non-DM and DM patients (χ2 test). DM: Diabetes mellitus.
Mortality
Mortality was assessed excluding the patients whose outcome was unspecified and who died due to other causes than AP. The mortality of AP patients with DM (4.0%) was higher than that of AP patients without DM (1.7%) (Tables 1 and 4). In particular, AP patients with DM aged 70 or older manifested an increased mortality (9.5%). If stratified by etiology, idiopathic AP patients with DM were predisposed to increased mortality (9.7%). On the other hand, DM did not affect the mortality of alcoholic AP patients. In addition, sex, body mass index (BMI) and triglyceride level, in which there was a significant difference between the patients with DM and those without DM (Table 2), did not affect the mortality of the study population. The mortality of male and female AP patients was 1.8% (22/1197) and 2.3% (14/616) (P = 0.59), and that of AP patients with BMI ≥ 30 and < 30 was 0% (0/62) and 1.8% (25/1390) (P = 0.62), respectively. Stepwise logistic regression revealed that age and DM were independently associated with mortality of AP (Table 5).
Table 4.
Mortality of the acute pancreatitis patients stratified by age, etiology and diabetes mellitus
| AP patients with DM | AP patients without DM | P value | |
| Total AP patients | 4.0% (9/226) | 1.7% (27/1587) | 0.036 |
| < 19 | 0% (0/1) | 0% (0/17) | > 0.999 |
| 20-29 | 0% (0/8) | 0% (0/82) | > 0.999 |
| 30-39 | 0% (0/25) | 1.2% (2/173) | > 0.999 |
| 40-49 | 0% (0/34) | 0.5% (1/217) | > 0.999 |
| 50-59 | 0% (0/41) | 1.1% (3/277) | > 0.999 |
| 60-69 | 4.9% (2/41) | 1.8% (5/271) | 0.231 |
| > 70 | 9.5% (7/74) | 3.1% (16/516) | 0.017 |
| Uncertain age | 0% (0/2) | 0% (0/34) | > 0.999 |
| Alcoholic AP | 1.1% (1/92) | 1.2% (6/489) | > 0.999 |
| < 19 | No patients | 0% (0/1) | Not determined |
| 20-29 | 0% (0/4) | 0% (0/34) | > 0.999 |
| 30-39 | 0% (0/15) | 1.1% (1/93) | > 0.999 |
| 40-49 | 0% (0/21) | 0.7% (1/134) | > 0.999 |
| 50-59 | 0% (0/21) | 0.8% (1/120) | > 0.999 |
| 60-69 | 0% (0/16) | 4.9% (3/61) | > 0.999 |
| > 70 | 6.7% (1/15) | 0% (0/38) | 0.283 |
| Uncertain age | No patients | 0% (0/8) | Not determined |
| Biliary AP | 2.3% (1/44) | 1.0% (4/409) | 0.402 |
| < 19 | No patients | No patients | Not determined |
| 20-29 | No patients | 0% (0/4) | Not determined |
| 30-39 | No patients | 0% (0/17) | Not determined |
| 40-49 | 0% (0/4) | 0% (0/23) | > 0.999 |
| 50-59 | 0% (0/5) | 1.8% (1/57) | > 0.999 |
| 60-69 | 0% (0/11) | 0% (0/86) | > 0.999 |
| > 70 | 4.3% (1/23) | 1.4% (3/214) | 0.337 |
| Uncertain age | 0% (0/1) | 0% (0/8) | > 0.999 |
| Idiopathic AP | 9.7% (3/31) | 2.7% (7/261) | 0.078 |
| < 19 | 0% (0/1) | 0% (0/4) | > 0.999 |
| 20-29 | 0% (0/1) | 0% (0/26) | > 0.999 |
| 30-39 | 0% (0/4) | 0% (0/24) | > 0.999 |
| 40-49 | 0% (0/4) | 0% (0/20) | > 0.999 |
| 50-59 | 0% (0/5) | 0% (0/27) | > 0.999 |
| 60-69 | 16.7% (1/6) | 2.6% (1/39) | 0.252 |
| > 70 | 20.0% (2/10) | 5.2% (6/115) | 0.125 |
| Uncertain age | No patients | 0% (0/6) | Not determined |
AP: Acute pancreatitis; DM: Diabetes mellitus.
Table 5.
Multivariate analyses of risk factors of mortality
| Variable |
Univariate |
Multivariate |
||||
| OR | 95%CI | P value | Adjusted OR | 95%CI | P value | |
| Age (yr) | 1.05 | 1.02-1.07 | < 0.001 | 1.05 | 1.02-1.07 | < 0.001 |
| DM (present/absent) | 2.40 | 1.05-4.97 | 0.038 | 2.50 | 1.09-5.24 | 0.032 |
| BMI (kg/m2) | 0.98 | 0.88-1.08 | 0.672 | |||
| TG (mg/dL) | 1.00 | 0.98-1.00 | 0.712 | |||
BMI: Body mass index; CI: Confidence interval; OR: Odds ratio; TG: Triglyceride.
Of note, the impact of DM on the mortality of AP varies according to the etiology. Alcoholic AP with DM patients might have a concomitant disorder of the exocrine pancreas, which might result in lower mortality than idiopathic AP with DM. The impact of DM on the mortality of AP might depend on the characteristics of patients, which might partially account for the discrepancies in the impact of DM among previous reports.
It was of interest to see whether the type of anti-diabetic medicine, number of drugs and poor glycemic control status are associated with the course of AP. However, because such items were not included in the questionnaire of this nationwide survey, we could not analyze the data from these points of view. Further studies are required to clarify the effects of these factors on AP.
CONCLUSION
In conclusion, patients with DM appear to have an increased risk of AP. The impact of impaired glucose tolerance on AP may depend on the cause of hyperglycemia, the condition of DM including severity, duration and treatment, and the characteristics of the AP patients including age, etiology and comorbidity. Further elucidation of the mechanisms linking impaired glucose tolerance and AP would facilitate better understanding of the pathogenesis of AP and development of preventive strategies.
Footnotes
Supported by Grants from the Smoking Research Foundation (to Masamune A); the Research Committee on Intractable Pancreatic Diseases, the Ministry of Health, Labour and Welfare of Japan (Principal investigators: Shimosegawa T and Takeyama Yoshifumi).
Conflict-of-interest: The authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 27, 2014
First decision: February 10, 2015
Article in press: April 28, 2015
P- Reviewer: Brisinda G, Talukdar R S- Editor: Yu J L- Editor: Logan S E- Editor: Wang CH
References
- 1.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 2.Wu BU, Banks PA. Clinical management of patients with acute pancreatitis. Gastroenterology. 2013;144:1272–1281. doi: 10.1053/j.gastro.2013.01.075. [DOI] [PubMed] [Google Scholar]
- 3.Hamada S, Masamune A, Kikuta K, Hirota M, Tsuji I, Shimosegawa T. Nationwide epidemiological survey of acute pancreatitis in Japan. Pancreas. 2014;43:1244–1248. doi: 10.1097/MPA.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 4.Frey C, Zhou H, Harvey D, White RH. Co-morbidity is a strong predictor of early death and multi-organ system failure among patients with acute pancreatitis. J Gastrointest Surg. 2007;11:733–742. doi: 10.1007/s11605-007-0164-5. [DOI] [PubMed] [Google Scholar]
- 5.Stolar M. Addressing cardiovascular risk in patients with type 2 diabetes: focus on primary care. Am J Med Sci. 2011;341:132–140. doi: 10.1097/MAJ.0b013e3181ec04e9. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 7.Koh GC, Peacock SJ, van der Poll T, Wiersinga WJ. The impact of diabetes on the pathogenesis of sepsis. Eur J Clin Microbiol Infect Dis. 2012;31:379–388. doi: 10.1007/s10096-011-1337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32:834–838. doi: 10.2337/dc08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girman CJ, Kou TD, Cai B, Alexander CM, O’Neill EA, Williams-Herman DE, Katz L. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab. 2010;12:766–771. doi: 10.1111/j.1463-1326.2010.01231.x. [DOI] [PubMed] [Google Scholar]
- 10.Lai SW, Muo CH, Liao KF, Sung FC, Chen PC. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am J Gastroenterol. 2011;106:1697–1704. doi: 10.1038/ajg.2011.155. [DOI] [PubMed] [Google Scholar]
- 11.Blomgren KB, Sundström A, Steineck G, Wiholm BE. Obesity and treatment of diabetes with glyburide may both be risk factors for acute pancreatitis. Diabetes Care. 2002;25:298–302. doi: 10.2337/diacare.25.2.298. [DOI] [PubMed] [Google Scholar]
- 12.Eland IA, Sundström A, Velo GP, Andersen M, Sturkenboom MC, Langman MJ, Stricker BH, Wiholm B. Antihypertensive medication and the risk of acute pancreatitis: the European case-control study on drug-induced acute pancreatitis (EDIP) Scand J Gastroenterol. 2006;41:1484–1490. doi: 10.1080/00365520600761676. [DOI] [PubMed] [Google Scholar]
- 13.Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care. 2010;33:2349–2354. doi: 10.2337/dc10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Perez A, Schlienger RG, Rodríguez LA. Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes Care. 2010;33:2580–2585. doi: 10.2337/dc10-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue Y, Sheng Y, Dai H, Cao H, Liu Z, Li Z. Risk of development of acute pancreatitis with pre-existing diabetes: a meta-analysis. Eur J Gastroenterol Hepatol. 2012;24:1092–1098. doi: 10.1097/MEG.0b013e328355a487. [DOI] [PubMed] [Google Scholar]
- 16.Solanki NS, Barreto SG, Saccone GT. Acute pancreatitis due to diabetes: the role of hyperglycaemia and insulin resistance. Pancreatology. 2012;12:234–239. doi: 10.1016/j.pan.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 18.Lomberk G. Diabetes. Pancreatology. 2009;9:200–202. doi: 10.1159/000187722. [DOI] [PubMed] [Google Scholar]
- 19.Chen LY, Qiao QH, Zhang SC, Chen YH, Chao GQ, Fang LZ. Metabolic syndrome and gallstone disease. World J Gastroenterol. 2012;18:4215–4220. doi: 10.3748/wjg.v18.i31.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;39:709–716. doi: 10.1097/01.mcg.0000173929.60115.b4. [DOI] [PubMed] [Google Scholar]
- 21.Mallick S. Metformin induced acute pancreatitis precipitated by renal failure. Postgrad Med J. 2004;80:239–240. doi: 10.1136/pgmj.2003.011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giorda CB, Nada E, Tartaglino B, Marafetti L, Gnavi R. A systematic review of acute pancreatitis as an adverse event of type 2 diabetes drugs: from hard facts to a balanced position. Diabetes Obes Metab. 2014;16:1041–1047. doi: 10.1111/dom.12297. [DOI] [PubMed] [Google Scholar]
- 23.Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract. 2012;98:271–284. doi: 10.1016/j.diabres.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 24.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 25.Raz I, Bhatt DL, Hirshberg B, Mosenzon O, Scirica BM, Umez-Eronini A, Im K, Stahre C, Buskila A, Iqbal N, et al. Incidence of pancreatitis and pancreatic cancer in a randomized controlled multicenter trial (SAVOR-TIMI 53) of the dipeptidyl peptidase-4 inhibitor saxagliptin. Diabetes Care. 2014;37:2435–2441. doi: 10.2337/dc13-2546. [DOI] [PubMed] [Google Scholar]
- 26.Graham BB, Keniston A, Gajic O, Trillo Alvarez CA, Medvedev S, Douglas IS. Diabetes mellitus does not adversely affect outcomes from a critical illness. Crit Care Med. 2010;38:16–24. doi: 10.1097/CCM.0b013e3181b9eaa5. [DOI] [PubMed] [Google Scholar]
- 27.Shen HN, Lu CL, Li CY. Effect of diabetes on severity and hospital mortality in patients with acute pancreatitis: a national population-based study. Diabetes Care. 2012;35:1061–1066. doi: 10.2337/dc11-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, Bailey M. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36:2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 29.Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care. 2009;13:R18. doi: 10.1186/cc7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Q, Li J, Gao F. New insights into insulin: The anti-inflammatory effect and its clinical relevance. World J Diabetes. 2014;5:89–96. doi: 10.4239/wjd.v5.i2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consoli A, Devangelio E. Thiazolidinediones and inflammation. Lupus. 2005;14:794–797. doi: 10.1191/0961203305lu2223oa. [DOI] [PubMed] [Google Scholar]
- 32.Moss M, Guidot DM, Steinberg KP, Duhon GF, Treece P, Wolken R, Hudson LD, Parsons PE. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28:2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Zhao X, Chang Mei H, Chen L, Jiang L, He M, Chen J, Hu Z, Ye H, Hu H, Zhou L, et al. An increased level of haemoglobin A1C predicts a poorer clinical outcome in patients with acute pancreatitis. Clin Endocrinol (Oxf) 2012;77:241–245. doi: 10.1111/j.1365-2265.2011.04252.x. [DOI] [PubMed] [Google Scholar]
- 34.Ranson JH. Etiological and prognostic factors in human acute pancreatitis: a review. Am J Gastroenterol. 1982;77:633–638. [PubMed] [Google Scholar]
- 35.Mentula P, Kylänpää ML, Kemppainen E, Jansson SE, Sarna S, Puolakkainen P, Haapiainen R, Repo H. Early prediction of organ failure by combined markers in patients with acute pancreatitis. Br J Surg. 2005;92:68–75. doi: 10.1002/bjs.4786. [DOI] [PubMed] [Google Scholar]
- 36.Whitcomb BW, Pradhan EK, Pittas AG, Roghmann MC, Perencevich EN. Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations. Crit Care Med. 2005;33:2772–2777. doi: 10.1097/01.ccm.0000189741.44071.25. [DOI] [PubMed] [Google Scholar]
- 37.Xiu F, Stanojcic M, Diao L, Jeschke MG. Stress hyperglycemia, insulin treatment, and innate immune cells. Int J Endocrinol. 2014;2014:486403. doi: 10.1155/2014/486403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satoh K, Shimosegawa T, Masamune A, Hirota M, Kikuta K, Kihara Y, Kuriyama S, Tsuji I, Satoh A, Hamada S. Nationwide epidemiological survey of acute pancreatitis in Japan. Pancreas. 2011;40:503–507. doi: 10.1097/MPA.0b013e318214812b. [DOI] [PubMed] [Google Scholar]
- 39.Takeda K, Yokoe M, Takada T, Kataoka K, Yoshida M, Gabata T, Hirota M, Mayumi T, Kadoya M, Yamanouchi E, et al. Assessment of severity of acute pancreatitis according to new prognostic factors and CT grading. J Hepatobiliary Pancreat Sci. 2010;17:37–44. doi: 10.1007/s00534-009-0213-4. [DOI] [PubMed] [Google Scholar]


