Abstract
AIM: To evaluate the prognostic factors in patients with spontaneously ruptured hepatocellular carcinoma (HCC).
METHODS: Seventy-nine patients experiencing spontaneous rupture of HCC between April 2004 and August 2014 were enrolled in this study. The clinical features, treatment modalities and outcomes were reviewed. The statistical methods used in this work included univariate analysis, Kaplan-Meier survival analysis with log-rank tests, and multivariate analysis using a Cox regression hazard model.
RESULTS: Of the 79 patients with HCC rupture, 17 (21.5%) underwent surgery, 32 (40.5%) underwent transarterial embolization (TAE), and 30 (38%) received conservative treatment. The median survival time was 125 d, and the mortality rate at 30 d was 27.8%. Multivariate analysis revealed that lesion length (HR = 1.46, P < 0.001), lesion number (HR = 1.37, P = 0.042), treatment before tumor rupture (HR = 4.36, P = 0.019), alanine transaminase levels (HR = 1.0, P = 0.011), bicarbonate levels (HR = 1.18, P < 0.001), age (HR = 0.96, P = 0.026), anti-tumor therapy during the follow-up period (HR = 0.21, P = 0.008), and albumin levels (HR = 0.89, P = 0.010) were independent prognostic factors of survival after HCC rupture. The Barcelona-Clinic Liver Cancer (BCLC) stage was also an important prognostic factor; the median survival times for BCLC stages A, B and C were 251, 175 and 40 d, respectively (P < 0.001).
CONCLUSION: Anti-tumor therapy during the follow-up period, without a history of anti-tumor therapy prior to HCC rupture, small tumor length and number, and early BCLC stage are the most crucial predictors associated with satisfactory overall survival. Other factors play only a small role in overall survival.
Keywords: Hepatocellular carcinoma, Rupture, Prognosis, Surgery, Transarterial embolization
Core tip: The most important predictors of survival in patients with spontaneous rupture of hepatocellular carcinoma (HCC) are continuous anti-tumor therapies during the follow-up period, tumor length and number, Barcelona-Clinic Liver Cancer stage, and a history of anti-tumor therapies prior to HCC rupture. Therefore, the present study may provide useful information for doctors making judgments on the prognosis of spontaneously ruptured HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a common tumor and is a major health problem worldwide[1]. One of the life-threatening complications of HCC is spontaneous tumor rupture with catastrophic intraperitoneal hemorrhage. Given the advances in treatment and increased early detection of HCC, the incidence of spontaneous HCC rupture has decreased to approximately 3%-15%, but mortality rates remain high (range: 25%-75%) during the acute phase[2-4]. If left untreated, the median survival is only 1.2 to 4 mo, and the prognosis is very poor[5].
To improve our understanding of HCC rupture, a number of studies have been conducted to identify possible predictive factors[4,6,7]. Age, tumor characteristics, tumor stage, hemoglobin levels, hepatic function, renal function, and treatment modality at the time of tumor rupture have been considered possible predictors for spontaneous rupture of HCC. These factors also play important roles in deciding the treatment modality at the time of tumor rupture. From our perspective, treatment before HCC rupture and therapies during follow-up are also important factors; however, to the best of our knowledge, no research has been conducted to evaluate the roles of these variables on survival after HCC rupture.
Therefore, we conducted this retrospective study to evaluate all possible risk factors in patients with HCC rupture and to identify independent predictors of survival.
MATERIALS AND METHODS
Between April 2004 and August 2014, seventy-nine patients (67 males, 12 females; age range: 25-77 years) were initially diagnosed with spontaneous HCC rupture at our hospital. The diagnostic criteria of HCC followed the guidelines issued by the American Association for the Study of Liver Diseases. HCC rupture was diagnosed as abrupt abdominal pain, disruption of the peritumoral liver capsule with enhanced fluid collection in the perihepatic area adjacent to HCC by contrast enhanced computed tomography or ultrasound, or when an abdominal paracentesis showed bloody ascites.
The following patient information at the time of HCC rupture was reviewed: age, tumor characteristics on imaging, HCC stage, laboratory data, Child-Pugh scores, and treatment modality. Other information including whether the patients received anti-tumor therapies before tumor rupture or during the follow-up period, was also recorded. The anti-tumor therapies included chemotherapy, sorafenib, transarterial embolization (TAE), and surgery. Tumor characteristics included the location of the ruptured tumor, the size of the tumor in its greatest dimension, the number of intrahepatic nodules, and the presence of liver cirrhosis. If the number of intrahepatic nodules was greater than five, it was regarded as five. HCC staging was performed using the Barcelona Clinic Liver Cancer (BCLC) staging system. The collected laboratory data included white blood cell count (WBC), hemoglobin (HB), platelet count (PLT), international normalized ratio (INR), activated partial thromboplastin time (APTT), alanine transaminase (ALT), albumin (ALB), total bilirubin (TBil), bicarbonate (HCO3-) and creatinine (Crea).
At the time of HCC rupture, the patients immediately received careful conservative treatment, including intensive care, anti-shock measures, and assessment of the patient’s condition. All treatment suggestions were made according to the clinical judgment of the doctors and the performance of the patients was explained to the patients and their family members. After definite treatment decisions were made by the patients and their family members, written informed consent, including the risks and benefits of the treatment modality, was obtained. Of the 79 ruptured HCC cases enrolled, 17 (21.5%) underwent surgery, 32 (40.5%) underwent TAE, and the remaining 30 cases (38%) underwent conservative treatment.
The surgical contraindications included uncontrolled hemodynamic status, poor liver function, severe coagulopathy, hepatic encephalopathy, and poor performance status. Segmentectomy (n = 11, 64.7%) and perihepatic packing (n = 6, 35.3%) were performed depending on the circumstances. The TAE group was contraindicated due to severe poor liver function, severe coagulopathy, hepatic encephalopathy, and tumor thrombus in the main portal vein. Embolization of the feeding artery was performed after selective angiography, with lipiodol or PVA particles. In the conservative treatment group, the patients received intensive care, anti-shock measures, blood replacement, and correction of coagulopathy. Follow-up was performed every 1 to 3 mo, and contrast-enhanced CT and alpha-fetoprotein levels were evaluated to determine further therapy for these patients.
Statistical analysis
The patients’ characteristics were analyzed to determine whether the prognostic factors influenced survival. Continuous variables were expressed as the mean ± SD, and categorical variables were expressed as a number. The survival rate was analyzed using Kaplan-Meier method, and the differences were compared using the log-rank test. If factors were found to be significant in univariate analysis, then multivariate analysis was performed using a Cox regression hazard model to identify the independent factors. To identify an effective value of the ruptured tumor size to predict 30-d mortality, receiver operating characteristic (ROC) curve analysis was conducted to obtain the cut-off value, sensitivity and specificity. Two-tailed P-values of < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS software (version 16.0; Chicago, IL, United States).
RESULTS
Seventy-nine patients with a mean age of 55.8 years and HCC rupture were enrolled in our study. According to imaging results, the mean number of lesions was 2.7 ± 1.9, and the lesion length in the greatest dimension was 7.7 ± 3.5 cm (range: 1.5-14.8 cm). The ruptured site was located in the right lobe more often than in the left lobe (57% vs 43%). Forty-nine patients were diagnosed with liver cirrhosis (62%). Before treatment, 10 (12.7%), 47 (59.5%), and 22 (27.8%) patients were classified with BCLC A, B, or C stage HCC, respectively. Twenty-two patients were classified as Child-Pugh class A (27.8%), thirty-seven were classified as Child-Pugh class B (46.9%), and twenty were classified as Child-Pugh class C (25.3%). The median survival time was 125 d, and the mean survival time was 210.6 d (range: 0-1523 d). The 30-d mortality rate was 27.8% (22 patients). Fifty-seven patients had hepatitis B virus (72.2%), and two patients had hepatitis C virus (2.5%). Twenty-six patients received anti-tumor therapies prior to HCC rupture (32.9%), and nineteen patients received anti-tumor therapies during the follow-up period (24.1%).
Univariate analysis revealed that age, lesion length, lesion number, cirrhosis, BCLC stage, treatment before HCC rupture, treatment during follow-up, WBC level, HB level, PLT level, INR level, APTT level, ALT level, ALB level, TBil level, HCO3- level, Crea level, and Child-Pugh score were associated with overall survival rates in patients with HCC rupture (Table 1). Multivariate analysis revealed that lesion length (HR = 1.46, P < 0.001), lesion number (HR = 1.37, P = 0.042), treatment before tumor rupture (HR = 4.36, P = 0.019), ALT level (HR = 1.00, P = 0.011) and HCO3- level (HR = 1.18, P < 0.001) were positively associated with poor survival in patients with HCC rupture. Age (HR = 0.96, P = 0.026), treatment during the follow-up period (HR = 0.21, P = 0.008), and ALB level (HR = 0.89, P = 0.010) were inversely associated with poor survival (Table 2).
Table 1.
Univariate analysis of risk factors related to spontaneous rupture of hepatocellular carcinoma
| Variables | Patients (n = 79) | P value |
| Age (yr) | 55.8 ± 12.6 | < 0.001 |
| Lesion length (cm) | 7.7 ± 3.5 | < 0.001 |
| Number (1/2-3/≥ 5) | 39/10/30 | < 0.001 |
| Location (left lobe/right lobe) | 34/45 | 0.934 |
| Cirrhosis (absence/presence) | 30/49 | < 0.001 |
| Virus (None/HBV/HCV) | 20/57/2 | 0.150 |
| BCLC stage (A/B/C) | 10/47/22 | < 0.001 |
| Treatment before rupture (absence/presence) | 53/26 | < 0.001 |
| Treatment at rupture (conservative/TAE/surgery) | 30/32/17 | 0.086 |
| Treatment in the follow-up period (absence/presence) | 60/19 | < 0.001 |
| WBC, × 109/L | 12.4 ± 5.4 | < 0.001 |
| HB, g/L | 106.2 ± 25.6 | < 0.001 |
| PLT, × 1010/L | 171 ± 84.4 | < 0.001 |
| INR | 1.3 ± 0.3 | < 0.001 |
| APTT, S | 38.4 ± 7.2 | < 0.001 |
| ALT, U/L | 127 ± 186.9 | < 0.001 |
| ALB, g/L | 30.8 ± 7.3 | < 0.001 |
| TBil, umol/L | 28.3 ± 20.9 | < 0.001 |
| HCO3-, mmol/L | 23.3 ± 5.6 | < 0.001 |
| Crea, umol/L | 95.7 ± 50.1 | < 0.001 |
| Child-Pugh | 7.9 ± 1.8 | < 0.001 |
HBV: Hepatitis B virus; HCV: Hepatitis C virus; BCLC: Barcelona Clinic Liver Cancer; TAE: Transarterial embolization; WBC: White blood cell count; HB: Hemoglobin; PLT: Platelet count; INR: International normalized ratio; APTT: Activated partial thromboplastin time; ALT: Alanine transaminase; ALB: Albumin; TBil: Total bilirubin; HCO3-: Bicarbonate; Crea: Creatinine.
Table 2.
Multivariate analysis of risk factors related to survival in patients with hepatocellular carcinoma rupture
| Variables | HR | 95%CI | P value |
| Age (yr) | 0.96 | 0.93-1.00 | 0.026 |
| Lesion length (cm) | 1.46 | 1.23-1.73 | < 0.001 |
| Number | 1.37 | 1.01-1.85 | 0.042 |
| Cirrhosis (presence) | 2.47 | 0.77-7.96 | 0.131 |
| BCLC stage A (control) | - | - | - |
| BCLC stage B | 0.10 | 0.02-0.41 | 0.002 |
| BCLC stage C | 0.19 | 0.03-1.01 | 0.052 |
| Treatment before rupture (presence) | 4.36 | 1.27-14.99 | 0.019 |
| Treatment in follow-up period (presence) | 0.21 | 0.07-0.67 | 0.008 |
| WBC, × 109/L | 1.04 | 0.96-1.13 | 0.375 |
| HB, g/L | 0.99 | 0.97-1.00 | 0.314 |
| PLT, × 1010/L | 1.00 | 0.99-1.00 | 0.143 |
| INR | 0.39 | 0.04-3.60 | 0.406 |
| APTT, S | 1.07 | 0.99-1.15 | 0.103 |
| ALT, U/L | 1.00 | 1.00-1.01 | 0.011 |
| ALB, g/L | 0.89 | 0.81-0.97 | 0.010 |
| TBil, umol/L | 1.00 | 0.97-1.02 | 0.735 |
| HCO3-, mmol/L | 1.18 | 1.08-1.28 | < 0.001 |
| Crea, umol/L | 1.01 | 1.00-1.02 | 0.089 |
| Child-Pugh | 1.03 | 0.59-1.80 | 0.905 |
WBC: White blood cell count; HB: Hemoglobin; PLT: Platelet count; INR: International normalized ratio; APTT: Activated partial thromboplastin time; ALT: Alanine transaminase; ALB: Albumin; TBil: Total bilirubin; HCO3-: Bicarbonate; Crea: Creatinine.
The cumulative overall survival rates of ruptured HCC patients with different BCLC stages differed significantly. The median survival times in BCLC stages A, B and C were 251, 175 and 40 d, respectively (P < 0.001) (Figure 1). The cumulative overall survival rates of patients receiving anti-tumor treatment before HCC rupture also differed. The mean survival time in patients receiving anti-tumor treatment before HCC rupture was 74.9 d, and the mean survival time in patients who did not undergo therapy before HCC rupture was 492.3 d (P < 0.001) (Figure 2). In addition, the cumulative overall survival rates of patients receiving anti-tumor treatment in the follow-up period differed. The mean survival time in patients who underwent therapy during the follow-up period was 645.5 d, and the mean survival time of patients who did not undergo therapy was 198.6 d (P < 0.001) (Figure 3).
Figure 1.
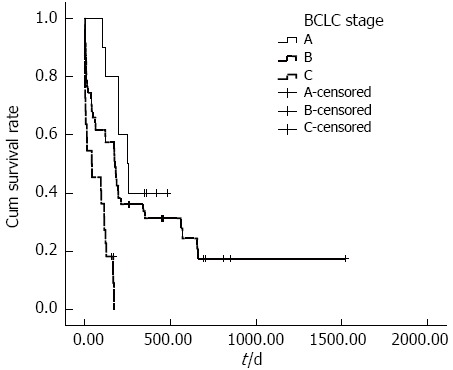
Cumulative survival according to Barcelona-Clinic Liver Cancer stage. Cumulative survival rates of patients with ruptured HCC according to BCLC stage significantly differed; the median survival times of patients with HCC rupture and BCLC stage A, B and C disease were 251, 175 and 40 d, respectively (P < 0.001). BCLC: Barcelona-Clinic Liver Cancer; HCC: Hepatocellular carcinoma.
Figure 2.
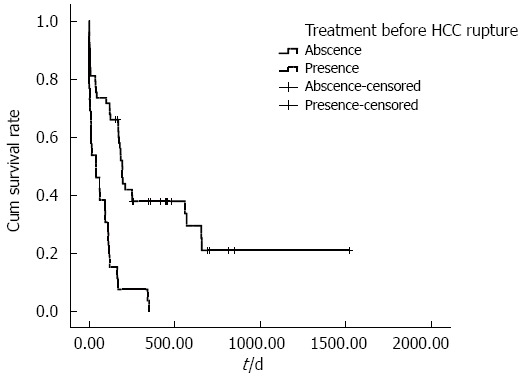
Cumulative survival according to treatment of hepatocellular carcinoma before tumor rupture. The cumulative survival rate of patients who underwent anti-tumor therapy before HCC rupture was significantly reduced compared with patients who did not undergo therapy before HCC rupture. The mean survival time of patients receiving anti-tumor treatment before HCC rupture was 74.9 d, whereas the mean survival time of patients without therapy before HCC rupture was 492.3 d (P < 0.001). HCC: Hepatocellular carcinoma.
Figure 3.
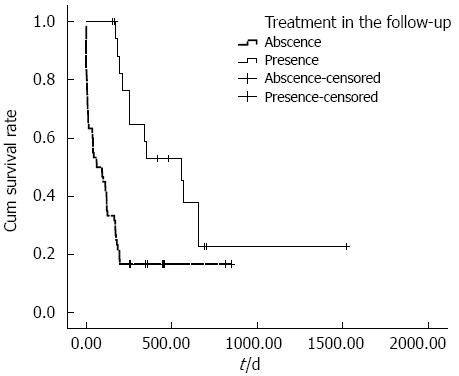
Cumulative survival according to further therapy after hepatocellular carcinoma rupture. The cumulative survival rate of patients who underwent therapy after HCC rupture was significantly increased compared with patients who did not undergo therapy after HCC rupture. The mean survival time of patients with therapy during the follow-up period was 645.5 d, whereas the mean survival time of patients without therapy was 198.6 d (P < 0.001). HCC: Hepatocellular carcinoma.
The cut-off point for tumor size was 8.85 cm for 30-day mortality of HCC ruptured patients, and the area under the ROC curve (AUC) was 0.813 (P < 0.001). The sensitivity and specificity of this analysis were 81.8% and 78.9%, respectively.
DISCUSSION
Spontaneous HCC rupture is a life-threatening complication that can cause sudden death. Its incidence has been reported to be 3%-15% making it a rare event in Western countries[8]. HCC rupture may be attributed to increased intratumoral pressure, occlusion of the vessels by tumor thrombus, rapid growth of the tumor, and necrosis. In addition, tumor size and superficial location serve as the risk factors[9,10]. Other characteristics, such as coagulation impairment caused by cirrhosis and hypertension, may also have a role[11].
HCC rupture is a dangerous condition in the clinic with a poor prognosis; the 30-d mortality has been reported to range from 30% to 70%[12-14]. In our cohort study, the 30-d mortality due to HCC rupture was 27.8%. A number of investigators have concluded that TAE or surgery is an effective therapy for patients with HCC rupture[3,6]. TAE is an effective and minimally invasive procedure for ruptured HCC and achieves immediate hemostasis; it is also the major therapy for patients with unresectable HCC. Surgical treatments for patients with HCC rupture include surgical hepatic resection and perihepatic packing[8,15-17]. Surgical hepatic resection is a curative treatment that requires small tumors or a small numbers of tumors. To prevent life-threatening complications, such as liver failure after TAE or surgery, patients must typically exhibit good reserved hepatic function and tolerable coagulopathy. However, ruptured HCC is an emergency event that is accompanied by the loss of hepatic and coagulatory functions[18,19]. The damage may be reduced by pharmacological treatment; thus, the treatment modality for HCC rupture can also be altered from conservative to TAE or surgery. To reduce the bias produced by treatment modality at HCC rupture, TAE or surgery within 48 h after tumor rupture is considered to be an effective treatment, and other treatment approaches that were changed from conservative treatment greater than 48 h after tumor rupture were considered to be conservative treatment modalities. We therefore obtained the unique result that the treatment modality at tumor rupture was not associated with survival (P = 0.086).
Our results revealed, for the first time, that treatment during the follow-up period was an independent predictor for overall survival (HR = 0.21, 95%CI: 0.07-0.67, P = 0.008); the overall survival rates of patients with ruptured HCCs who underwent anti-tumor therapy in the follow-up period were significantly increased compared with patients who did not undergo anti-tumor therapy. TAE effectively achieves hemodynamic stability. In addition, TAE increases the 30-d survival in HCC ruptured patients[3,7], and prolonged survival can be achieved through curative liver resection[2,11]. Therefore, staged hepatectomy followed by TAE in selected patients has been suggested in the literature[18,20]. From our perspective, TAE, staged hepatectomy and sorafenib in the follow-up period are better than no treatment in the follow-up period, and the subsequent treatment of ruptured HCC patients is more important than the treatment modality at the time of tumor rupture. The results of the present study showed similar findings, where treatment modality at the time of tumor rupture was not related to survival, whereas treatment during the follow-up period was an important factor related to long-term survival.
In this study, patients who underwent anti-tumor therapy before HCC rupture had poor survival (HR = 4.36, 95%CI: 1.27-14.99, P = 0.019). These therapies included transhepatic arterial chemoembolization (TACE) (19), surgical treatments (2), radiofrequency ablations (2), TACE combined with oxaliplatin chemotherapy (2), and TACE combined with sorafenib (1). Because the number of patients who underwent surgical treatment, radiofrequency, TACE combined with oxaliplatin or sorafenib is too small, these patients constitute a variable in our analysis. The overall survival rate of patients with HCC rupture who did not undergo anti-tumor therapies before tumor rupture was significantly increased compared with those who did. TAE, surgery and sorafenib are the most accepted therapies for HCC in selected patients[21-24], but these approaches also damage the liver. Angiograms in patients with a history of TAE or sorafenib typically reveal a thinner hepatic artery[25-28], and all anti-tumor therapies have the adverse effect of reducing liver function reserve[29,30]. Consequently, spontaneous HCC rupture is more dangerous in those patients with a prior history of anti-tumor therapies. Three patients had HCC rupture within 24 h after TAE in our study, and 2 patients died of uncontrolled hemorrhagic shock. The increasing pressure in a tumor and the poor elasticity of ischemic tumors make hemorrhage more difficult to control.
The univariate analysis revealed that survival significantly differed in patients with different BCLC stages. Multivariate analysis indicated that BCLC stage was an independent predictor for HCC rupture, but the HR was 0.10 in stage B and 0.19 in stage C (stage A was used as a control). The reason the HRs were less than 1.0 is that the percent survival of the patients in the three BCLC stages differed significantly; 40.0% for stage A (4 surviving cases in 10 patients), 23.4% for stage B (11 surviving cases in 47 patients), and 9.1% for stage C (2 surviving cases in 22 patients). Given the small number of stage A cases, the mean survival time was reduced (303.6 d in stage A, 418.8 d in stage B and 65.7 d in stage C). Therefore, the median survival time is crucial in the relationship between survival and BCLC stage, with the median survival times of 251, 175 and 40 d for stage A, B and C patients, respectively. Therefore, advanced HCC is an independent predictor of poor survival.
Tumor length and number have been considered as predictors of ruptured HCC in a previous study[11], and these values are likely to reflect the volume of the tumor cell. Similarly, we found that the lesion length and number affected overall survival (HR = 1.46 and 1.37, respectively). The cut-off point for tumor size was 8.85 cm for 30-d mortality in HCC ruptured patients. We also demonstrated that elevated ALT and decreased ALB are associated with poor survival. Elevated Crea levels are not significantly associated with poor survival; however, given that the 95%CI was greater than 1.0, elevated Crea levels are likely to reflect liver and renal injuries at the time of HCC rupture. Elevated HCO3- levels were positively associated with poor survival; in our study, the average HCO3- level in blood samples collected immediately after admission was 23.3 ± 5.6 mmol/L (range: 8-32 mmol/L). As a result of stress reactions and hemorrhage, most patients exhibited relatively elevated HCO3- levels in the blood, but not serious acidosis. Another dangerous factor for ruptured HCC patients in our study was age; our observed HR for age was 0.96, similar to that in other studies[6,7]. However, the HRs which ranged from 0.89 to 1.18 revealed that age, ALT, ALB and HCO3- only play a small part in the overall survival of ruptured HCC patients.
Our HR for ALT was 1.003; the manuscript requested two decimal points, thus the HR was written as 1.00. Other factors with HRs very close to 1 included ALB and HCO3-. Library data, such as ALT, ALB, and HCO3-, can be easily and artificially corrected during medical therapy. In contrast, other factors, such as BCLC stage, treatment before rupture, and treatment after rupture cannot be immediately changed. Therefore, the clinical significance of factors, such as ALT, for which the HR is close to 1, is limited even though statistical significance was detected.
Our study has several limitations. First, the number of samples was small given the low incidence of the disease, and this small sample size may produce bias. Second, the presence of cirrhosis may affect hepatic function, therefore the parameters in the multivariate analysis, including ALB, Tbil and cirrhosis, may have produced bias.
In conclusion, spontaneous rupture of HCC is a life-threatening condition with a poor prognosis. Receiving anti-tumor therapy during the follow-up period, no history of anti-tumor therapies administered before HCC rupture, small tumor length and number, and early BCLC stage are the most crucial predictors associated with satisfactory overall survival. Patient age, ALT level, ALB level, and HCO3- level are independent predictors that play only a small role in overall survival. We believe that the present study provides useful information for doctors in the assessment of spontaneous HCC rupture.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is a common tumor, and spontaneous rupture of HCC is a life-threatening complication. Therefore, identifying the essential predictors of survival after HCC rupture is very important.
Research frontiers
Several possible predictors, including age, tumor characteristics, tumor stage, hemoglobin levels, hepatic function, renal function, and treatment modality at the time of tumor rupture, have been investigated, but treatment before HCC rupture and during the follow-up period have not been evaluated.
Innovations and breakthroughs
The present study revealed that receiving anti-tumor therapy in the follow-up period without a history of anti-tumor therapy before HCC rupture, small tumor length and number, and early Barcelona-Clinic Liver Cancer stage are the most crucial predictors associated with satisfactory overall survival.
Applications
The present study may provide useful information for doctors in the assessment of spontaneous HCC rupture.
Terminology
Because the factors related to poor survival in patients with HCC rupture are variable, the Cox regression hazard model is useful for identifying independent factors and establishing the importance of these factors in the clinic.
Peer-review
This is a good retrospective study that identifies the risk factors for HCC rupture. Compared with existing reports, this study highlights the role of anti-tumor therapies before and after HCC rupture. The results are applicable to clinical practice.
Footnotes
Supported by National High-tech Research Foundation of China, No. 2012AA022701.
Ethics approval: This study was approved by the Research Ethics Committee of the First Hospital of China Medical University (Number: 2013-112-2).
Informed consent: All study participants provided written informed consent prior to study enrollment.
Conflict-of-interest: The authors declare that they have no conflicts of interest.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 11, 2014
First decision: January 8, 2015
Article in press: February 11, 2015
P- Reviewer: Perini MV, Zhang Q S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Zhang DN
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Tarantino L, Sordelli I, Calise F, Ripa C, Perrotta M, Sperlongano P. Prognosis of patients with spontaneous rupture of hepatocellular carcinoma in cirrhosis. Updates Surg. 2011;63:25–30. doi: 10.1007/s13304-010-0041-8. [DOI] [PubMed] [Google Scholar]
- 3.Kim JY, Lee JS, Oh DH, Yim YH, Lee HK. Transcatheter arterial chemoembolization confers survival benefit in patients with a spontaneously ruptured hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2012;24:640–645. doi: 10.1097/MEG.0b013e3283524d32. [DOI] [PubMed] [Google Scholar]
- 4.Hsueh KC, Fan HL, Chen TW, Chan DC, Yu JC, Tsou SS, Chang TM, Hsieh CB. Management of spontaneously ruptured hepatocellular carcinoma and hemoperitoneum manifested as acute abdomen in the emergency room. World J Surg. 2012;36:2670–2676. doi: 10.1007/s00268-012-1734-6. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mashat FM, Sibiany AM, Kashgari RH, Maimani AA, Al-Radi AO, Balawy IA, Ahmad JE. Spontaneous rupture of hepatocellular carcinoma. Saudi Med J. 2002;23:866–870. [PubMed] [Google Scholar]
- 6.Jin YJ, Lee JW, Park SW, Lee JI, Lee DH, Kim YS, Cho SG, Jeon YS, Lee KY, Ahn SI. Survival outcome of patients with spontaneously ruptured hepatocellular carcinoma treated surgically or by transarterial embolization. World J Gastroenterol. 2013;19:4537–4544. doi: 10.3748/wjg.v19.i28.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin BS, Park MH, Jeon GS. Outcome and prognostic factors of spontaneous ruptured hepatocellular carcinoma treated with transarterial embolization. Acta Radiol. 2011;52:331–335. doi: 10.1258/ar.2010.100369. [DOI] [PubMed] [Google Scholar]
- 8.Battula N, Madanur M, Priest O, Srinivasan P, O’Grady J, Heneghan MA, Bowles M, Muiesan P, Heaton N, Rela M. Spontaneous rupture of hepatocellular carcinoma: a Western experience. Am J Surg. 2009;197:164–167. doi: 10.1016/j.amjsurg.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Reso A, Ball CG, Sutherland FR, Bathe O, Dixon E. Rupture and intra-peritoneal bleeding of a hepatocellular carcinoma after a transarterial chemoembolization procedure: a case report. Cases J. 2009;2:68. doi: 10.1186/1757-1626-2-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka A, Takeda R, Mukaihara S, Hayakawa K, Shibata T, Itoh K, Nishida N, Nakao K, Fukuda Y, Chiba T, et al. Treatment of ruptured hepatocellular carcinoma. Int J Clin Oncol. 2001;6:291–295. doi: 10.1007/s10147-001-8030-z. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Q, Li J, Yan JJ, Huang L, Wu MC, Yan YQ. Predictors and clinical outcomes for spontaneous rupture of hepatocellular carcinoma. World J Gastroenterol. 2012;18:7302–7307. doi: 10.3748/wjg.v18.i48.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu CL, Fan ST, Lo CM, Tso WK, Poon RT, Lam CM, Wong J. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001;19:3725–3732. doi: 10.1200/JCO.2001.19.17.3725. [DOI] [PubMed] [Google Scholar]
- 13.Kirikoshi H, Saito S, Yoneda M, Fujita K, Mawatari H, Uchiyama T, Higurashi T, Imajo K, Sakaguchi T, Atsukawa K, et al. Outcomes and factors influencing survival in cirrhotic cases with spontaneous rupture of hepatocellular carcinoma: a multicenter study. BMC Gastroenterol. 2009;9:29. doi: 10.1186/1471-230X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki T, Kokudo N, Matsuyama Y, Izumi N, Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O, et al. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg. 2014;259:532–542. doi: 10.1097/SLA.0b013e31828846de. [DOI] [PubMed] [Google Scholar]
- 15.Chen ZY, Qi QH, Dong ZL. Etiology and management of hemmorrhage in spontaneous liver rupture: a report of 70 cases. World J Gastroenterol. 2002;8:1063–1066. doi: 10.3748/wjg.v8.i6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida H, Mamada Y, Taniai N, Mizuguchi Y, Kakinuma D, Ishikawa Y, Kanda T, Matsumoto S, Bando K, Akimaru K, et al. Long-term results of elective hepatectomy for the treatment of ruptured hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:178–182. doi: 10.1007/s00534-007-1239-0. [DOI] [PubMed] [Google Scholar]
- 17.Leowardi C, Hormann Y, Hinz U, Wente MN, Hallscheidt P, Flechtenmacher C, Buchler MW, Friess H, Schwarzbach MH. Ruptured angiosarcoma of the liver treated by emergency catheter-directed embolization. World J Gastroenterol. 2006;12:804–808. doi: 10.3748/wjg.v12.i5.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimada R, Imamura H, Makuuchi M, Soeda J, Kobayashi A, Noike T, Miyagawa S, Kawasaki S. Staged hepatectomy after emergency transcatheter arterial embolization for ruptured hepatocellular carcinoma. Surgery. 1998;124:526–535. [PubMed] [Google Scholar]
- 19.Yamagata M, Maeda T, Ikeda Y, Shirabe K, Nishizaki T, Koyanagi N. Surgical results of spontaneously ruptured hepatocellular carcinoma. Hepatogastroenterology. 1995;42:461–464. [PubMed] [Google Scholar]
- 20.Li WH, Cheuk EC, Kowk PC, Cheung MT. Survival after transarterial embolization for spontaneous ruptured hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:508–512. doi: 10.1007/s00534-009-0094-6. [DOI] [PubMed] [Google Scholar]
- 21.Imai N, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Hayashi K, Hirooka Y, Goto H. Transarterial chemoembolization for hepatocellular carcinoma: A review of techniques. World J Hepatol. 2014;6:844–850. doi: 10.4254/wjh.v6.i12.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, Arakawa S, Yoshida R, Isetani M. Recent advances in liver resection for hepatocellular carcinoma. Front Surg. 2014;1:21. doi: 10.3389/fsurg.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:4115–4127. doi: 10.3748/wjg.v20.i15.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 25.Maeda N, Osuga K, Mikami K, Higashihara H, Onishi H, Nakaya Y, Tatsumi M, Hori M, Kim T, Tomoda K, et al. Angiographic evaluation of hepatic arterial damage after transarterial chemoembolization for hepatocellular carcinoma. Radiat Med. 2008;26:206–212. doi: 10.1007/s11604-007-0216-5. [DOI] [PubMed] [Google Scholar]
- 26.Panaro F, Ramos J, Gallix B, Mercier G, Herrero A, Niampa H, Pageaux GP, Navarro F. Hepatic artery complications following liver transplantation. Does preoperative chemoembolization impact the postoperative course? Clin Transplant. 2014;28:598–605. doi: 10.1111/ctr.12358. [DOI] [PubMed] [Google Scholar]
- 27.Sun H, Zhu MS, Wu WR, Shi XD, Xu LB. Role of anti-angiogenesis therapy in the management of hepatocellular carcinoma: The jury is still out. World J Hepatol. 2014;6:830–835. doi: 10.4254/wjh.v6.i12.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747–1752. doi: 10.1002/cncr.10407. [DOI] [PubMed] [Google Scholar]
- 29.Ma TC, Shao HB, Xu Y, Xu K. Three treatment methods via the hepatic artery for hepatocellular carcinoma - a retrospective study. Asian Pac J Cancer Prev. 2013;14:2491–2494. doi: 10.7314/apjcp.2013.14.4.2491. [DOI] [PubMed] [Google Scholar]
- 30.Lu W, Li YH, Yu ZJ, He XF, Chen Y, Zhao JB, Zhu ZY. A comparative study of damage to liver function after TACE with use of low-dose versus conventional-dose of anticancer drugs in hepatocellular carcinoma. Hepatogastroenterology. 2007;54:1499–1502. [PubMed] [Google Scholar]


