Abstract
AIM: To identify the risk factors for organ failure (OF) in cholangitis with bacteriobilia.
METHODS: This study included 182 patients with acute cholangitis who underwent percutaneous transhepatic biliary drainage between January 2005 and April 2013. We conducted a retrospective analysis of comprehensive clinical and laboratory data.
RESULTS: There were 24 cases (13.2%) of OF and five deaths (2.7%). Bile culture was positive for microbial growth in 130 out of 138 (94.2%) patients. In multivariate analysis of 130 patients with positive bile cultures, significant predictive factors for OF were the presence of extended-spectrum beta-lactamase (ESBL) organisms in blood cultures, pre-existing renal dysfunction, and choledocholithiasis as an etiology, with odds ratios of 15.376, 6.319, and 3.573, respectively. We developed a scoring system with a regression coefficient of each significant variable. The OF score was calculated using the following equation: (2.7 × ESBL organisms in blood cultures) + (1.8 × pre-existing renal dysfunction) + (1.3 × choledocholithiasis). This scoring system for predicting OF was highly specific (99.1%) and had a positive predictive value of 86.2%.
CONCLUSION: ESBL organisms in blood cultures, pre-existing renal dysfunction, and choledocholithiasis are risk factors for OF in cholangitis with bacteriobilia. The OF scoring system may aid clinicians to identify a poor prognosis group.
Keywords: Acute cholangitis, Bacteriobilia, Bile culture, Organ failure
Core tip: There has been no study of the prognostic factors in acute cholangitis with bacteriobilia. The current study identified three risk factors for organ failure in cholangitis with bacteriobilia: extended-spectrum beta-lactamase organisms in blood cultures, pre-existing renal dysfunction, and choledocholithiasis as an etiology. In addition, a organ failure scoring system created by these risk factors may aid clinicians to identify a poor prognosis group.
INTRODUCTION
Acute cholangitis occurs mainly by bacterial infection in an obstructed biliary system, and choledocholithiasis has been reported as the leading cause[1,2]. The range of its severity varies, from mild to life-threatening, with a mortality rate of approximately 5%-10%[3]. Initial therapy includes administration of empiric broad-spectrum antibiotic and prompt biliary decompression[4]. If appropriate treatment is not provided, acute cholangitis may cause organ failure, including septic shock, which could cause a significant increase in the mortality rate to 88%-100%[5]. Therefore, the choice of appropriate antibiotics is very important and identification of the causative microorganism is an essential step in the management of acute cholangitis.
Bile cultures provide an opportunity to detect the causative microorganism and to establish antibiotic susceptibility testing and bacterial resistance profiling. In previous studies, positive rates of bile cultures among patients with acute cholangitis ranged from 59% to 93% and were higher than blood cultures[6-9]. This high sensitivity of bile cultures is physiologically plausible because the material for microbiological analysis is obtained directly from the site of inflammation.
According to previous studies on bacteremic cholangitis, organ failure has been reported as an important prognostic factor of mortality[10-12]. In addition, Lee et al[11] investigated several factors associated with organ failure in bacteremic cholangitis. Although some studies have reported correlation of a positive bile culture (bacteriobilia) with increased incidence of post-operative infective complications[13,14], the risk factors of organ failure have not yet been identified in cholangitis with bacteriobilia.
The aim of this study was to identify the risk factors for organ failure in acute cholangitis with bacteriobilia and to develop a prognostic scoring system that could be used to predict organ failure using the risk factors.
MATERIALS AND METHODS
Selection of the study population
This retrospective study initially included all patients (n = 411) with a discharge diagnosis of acute cholangitis who underwent PTBD at Seoul National University Hospital between January 2005 and April 2013, using information contained in medical charts and computerized records. To ensure statistical independence in the analyses, if multiple episodes of acute cholangitis occurred in the same patient (n = 49), only the first episode of acute cholangitis was included. In addition, patients were excluded for the following reasons: no definite or suspected diagnosis of acute cholangitis using the updated Tokyo guidelines for acute cholangitis and acute cholecystitis (TG13)[15] (n = 56), non-PTBD insertion (n = 43), underwent PTBD or bile culture after organ failure (n = 22), occurrence of organ failure from other causes (n = 7), and no initiation or completion of treatment in our institute (n = 52). A flow chart showing patient selection for the study is seen in Figure 1. Finally, 182 patients with acute cholangitis who underwent PTBD were included in the analysis. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-1308-086-514).
Figure 1.

Flow chart of patient selection for the study. PTBD: Percutaneous transhepatic biliary drainage.
Definitions of events
The definite or suspected diagnosis of acute cholangitis was defined according to TG13 diagnostic criteria for acute cholangitis[15]. Bacteriobilia was defined as the presence of microorganisms in the bile, documented by at least one positive bile culture. Unsuccessful biliary decompression was defined as a reposition or additional insertion after initial PTBD insertion. Septic shock was defined as persistent sepsis-induced hypotension despite adequate fluid resuscitation[16]. Sepsis-induced hypotension was defined as a systolic blood pressure of less than 90 mmHg or a reduction of more than 40 mmHg from baseline in the absence of other causes of hypotension[16].
Organ failures assessed at emergency department admission and during hospitalization manifested as[10,17]: (1) septic shock; (2) acute renal failure (ARF)-serum creatinine level of greater than 3 mg/dL or, in the case of pre-existing renal dysfunction, doubling of previous serum creatinine values[18,19]; (3) altered consciousness level-Glasgow Coma Scale score of less than 12 or a decrease in the score of at least 3 if primary central nervous system injury is present; and (4) acute respiratory distress-pulse oxygen saturation of less than 90%.
Data collection and analysis
The following data were collected for analysis. The clinical and demographic variables included age, sex, smoking, alcohol, body temperature, Charcot’s triad, symptom to door time (time from symptom onset until arrival at the hospital), Charlson comorbidity index score[20], pre-existing renal dysfunction, TG13 severity assessment criteria for acute cholangitis[15], and bile culture time (time from PTBD insertion until bile sample collection). Etiological variables were choledocholithiasis, benign biliary stricture, malignant biliary obstruction, and procedure-related causes. Microbiological and laboratory variables included causative microorganisms in blood or bile cultures, white blood cell count, total bilirubin, alkaline phosphatase, albumin, and C-reactive protein at admission. Treatment and outcome variables were unsuccessful biliary decompression, visit to decompression time (time from arrival at the hospital until intervention for biliary decompression), initial antibiotic resistance for microorganisms of blood or bile cultures, and length of hospital stay.
The above mentioned variables were analyzed for identification of risk factors for organ failure in patients with positive bile cultures.
Statistical analysis
In univariate analyses, the Mann-Whitney U test and the χ2 test with Fisher’s exact test were used for comparison of continuous or categorical variables, respectively. A logistic regression test analysis was performed using the stepwise method. All significant variables in the univariate analysis were entered in the multivariate analysis. Then, logistic regression coefficients of the factors were ascertained to develop equations (organ failure score) to predict organ failure. Finally, receiver operating characteristic (ROC) curves were constructed for the organ failure score to determine a specific threshold value that would optimize its predictive value. P < 0.05 for two-sided tests was considered statistically significant. In this study, odds ratios (ORs) are reported together with their 95%CI. All statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, United States).
RESULTS
Clinical and microbiological characteristics of the patients
A total of 182 patients (61.0% male, median 65 years, range 22-91 years) were enrolled. A summary of the demographic and clinical characteristics of the 182 patients is shown in Table 1. According to the TG13[15], the severities of acute cholangitis at admission were mild in 73 patients, moderate in 64, and severe in 45. The most common etiology was malignant biliary obstruction (48.4%), followed by choledocholithiasis (32.4%), benign biliary stricture (17%), and procedure-related causes (2.2%). The causes of PTBD insertion in 59 patients with choledocholithiasis were esophageal stricture (n = 3), gastric outlet obstruction (n = 7), previous gastric surgery (n = 27) and severe cardiopulmonary disease (n = 22). All patients received intravenous antibiotics within the initial 2 h and underwent biliary decompression through PTBD within the initial 48 h. All blood cultures (n = 182) were performed before the use of antibiotics. Unsuccessful biliary decompression occurred in 14 (7.7%) patients, of whom six patients required repositioning of the PTBD catheter and eight patients required insertion of new PTBD catheters. Except minor bleeding, no major complications of the PTBD procedure occurred in any patient. There were 24 cases (13.2%) of organ failure, including septic shock (n = 13), ARF (n = 8), and acute respiratory distress (n = 3). The median time taken until organ failure from the patient’s initial arrival at the hospital was 14 h (range 4-312 h). The overall hospital mortality rate was 2.7% (n = 5) and all patients expired from septic shock. The mean length of the hospital stay was 25.3 ± 22.6 and 13.1 ± 11.6 d in patients with and without organ failure, respectively (P = 0.001).
Table 1.
Baseline characteristics of patients with acute cholangitis who underwent percutaneous transhepatic biliary drainage (n = 182)
| Characteristic | n (%) |
| Age ≥ 65 yr | 95 (52.2) |
| Sex (male/female) | 111/71 |
| Heavy smoker 1 | 12 (12.1) |
| Heavy alcohol drinker 2 | 17 (9.3) |
| Body temperature > 38 or < 36 °C | 61 (33.5) |
| Positive Charcot’s triad | 23 (12.6) |
| Symptom to door time (h), mean ± SD | 107.2 ± 171.2 |
| CCIs ≥ 4 | 90 (49.5) |
| Pre-existing renal dysfunction | 14 (7.7) |
| Etiology | |
| Choledocholithiasis | 59 (32.4) |
| Benign biliary stricture | 31 (17.0) |
| Malignant biliary obstruction | 88 (48.4) |
| Procedure-related | 4 (2.2) |
| Laboratory finding | |
| WBC count > 12000 or < 4000/mm3 | 70 (38.5) |
| Total bilirubin ≥ 5 mg/dL | 59 (32.4) |
| Alkaline phosphatase ≥ 250 IU/L | 106 (58.2) |
| Albumin ≤ 2.8 g/dL | 59 (32.4) |
| C-reactive protein ≥ 5 mg/dL | 120 (65.9) |
| The severity of acute cholangitis3 | |
| Mild | 73 (40.1) |
| Moderate | 64 (35.2) |
| Severe | 45 (24.7) |
| Performance of bile cultures | 138 (75.8) |
| During PTBD insertion | 58 |
| Within 24 h after PTBD insertion | 80 |
| Positive blood culture | 55/182 (30.2) |
| Unsuccessful biliary decompression4 | 14 (7.7) |
| Reposition | 6 |
| Additional insertion | 8 |
| Organ failure | 24 (13.2) |
| Septic shock | 13 |
| Acute renal failure | 8 |
| Acute respiratory distress | 3 |
| Death | 5 (2.7) |
Values are presented as number or number (%).
Heavy smoker is defined as an individual with 20 or more pack-years (1 pack year = 1 pack per day for one year) of use;
Heavy drinker is defined as an individual currently drinking alcoholic beverages in a daily amount of ≥ 80 g (male) or ≥ 40 g (female);
TG13 severity assessment criteria for acute cholangitis (at admission);
Reposition or additional insertion after initial PTBD insertion. PTBD: Percutaneous transhepatic biliary drainage; CCIs: Charlson comorbidity index score; WBC: White blood cell.
All bile cultures were performed during PTBD insertion (n = 58) or within 24 h after PTBD insertion (n = 80). Bile culture was positive for microbial growth in 130 out of 138 (94.2%) patients. Monomicrobial growth (50.8%) was slightly more frequent than polymicrobial growth (49.2%). A total of 221 microorganisms were isolated, comprising 18 different species (Table 2). The most frequently encountered microorganisms were Enterococcus species (24.4%) and Escherichia coli (21.3%).
Table 2.
Distribution of different microorganisms in positive bile cultures
| Microorganism | n (%) |
| Enterococcus species | 54 (24.4) |
| Escherichia coli | 47 (21.3) |
| Pseudomonas species | 27 (12.2) |
| Klebsiella species | 26 (11.8) |
| Citrobacter species | 18 (8.1) |
| Streptococcus species | 12 (5.4) |
| Staphylococcus species | 11 (5.0) |
| Enterobacter species | 7 (3.2) |
| Proteus species | 4 (1.8) |
| Stenotrophomonas maltophilia | 4 (1.8) |
| Acinetobacter species | 3 (1.4) |
| Aeromonas species | 2 (< 1) |
| Morganella morganii | 1 (< 1) |
| Corynebacterium species | 1 (< 1) |
| Chromobacterium violaceum | 1 (< 1) |
| Shewanella putrefaciens | 1 (< 1) |
| Leuconostoc pseudomesenteroides | 1 (< 1) |
| Candia albicans | 1 (< 1) |
Risk factors for organ failure in patients with bacteriobilia who underwent PTBD because of acute cholangitis
A total of 130 patients with positive bile cultures were analyzed to determine the risk factors for organ failure in acute cholangitis with bacteriobilia. Organ failure occurred in 22 (16.9%) patients and did not occur in 108 (83.1%) patients. Univariate analysis identified four variables showing significant (P < 0.05) association with organ failure. In multivariate analysis, variables showing significant association with organ failure in patients with bacteriobilia included the presence of ESBL organisms in blood cultures, pre-existing renal dysfunction, and choledocholithiasis, with ORs of 15.376, 6.319, and 3.573, respectively (Table 3).
Table 3.
Univariate and multivariate analysis of risk factors for organ failure in patients with bacteriobilia who underwent percutaneous transhepatic biliary drainage because of acute cholangitis (n = 130) n (%)
| Factor |
Univariate analysis |
Multivariate analysis |
|||
| Patients with OF (n = 22) | Patients without OF (n = 108) | P value | OR (95%CI) | P value | |
| Age ≥ 65 yr | 14 (63.6) | 57 (52.8) | 0.351 | ||
| Male/female | 11/11 | 64/44 | 0.423 | ||
| Heavy smoker1 | 0 (0.0) | 10 (9.3) | 0.211 | ||
| Heavy alcohol drinker2 | 1 (4.5) | 6 (5.6) | 1.000 | ||
| Body temperature > 38 or < 36 °C | 8 (36.4) | 33 (30.6) | 0.593 | ||
| Positive Charcot’s triad | 3 (13.6) | 13 (12.0) | 0.734 | ||
| Symptom to door time (h)1 | 96.6 ± 88.8 | 99.4 ± 175.2 | 0.106 | ||
| CCIs ≥ 4 | 10 (45.5) | 65 (60.2) | 0.202 | ||
| Pre-existing renal dysfunction | 7 (31.8) | 4 (3.7) | 0.0009 | 6.319 (1.348-29.629) | 0.0199 |
| Etiology | |||||
| Choledocholithiasis | 11 (50.0) | 25 (23.1) | 0.0109 | 3.573 (1.195-10.686) | 0.0239 |
| Benign biliary stricture | 2 (9.1) | 17 (15.7) | 0.528 | ||
| Malignant biliary obstruction | 9 (40.9) | 62 (57.4) | 0.157 | ||
| Procedure-related | 0 (0.0) | 4 (3.7) | 1.000 | ||
| Laboratory finding | |||||
| WBC > 12000 or < 4000/mm3 | 13 (59.1) | 44 (40.7) | 0.114 | ||
| Total bilirubin ≥ 5 mg/dL | 10 (45.5) | 32 (29.6) | 0.148 | ||
| ALP ≥ 250 IU/L | 14 (63.6) | 60 (55.6) | 0.485 | ||
| Albumin ≤ 2.8 g/dL | 13 (59.1) | 36 (33.3) | 0.0239 | ||
| C-reactive protein ≥ 5 mg/dL | 16 (76.2) | 74 (69.8) | 0.557 | ||
| The severity of acute cholangitis3 | |||||
| Mild | 5 (22.7) | 40 (37.0) | 0.198 | ||
| Moderate | 7 (31.8) | 41 (39.9) | 0.586 | ||
| Severe | 10 (45.5) | 27 (25.0) | 0.053 | ||
| Bile culture-Causative microorganism | |||||
| Escherichia coli | 4 (18.2) | 15 (13.9) | 0.740 | ||
| Klebsiella species | 2 (9.1) | 7 (6.5) | 0.648 | ||
| Pseudomonas species | 1 (4.5) | 10 (9.3) | 0.689 | ||
| Enterobacter species | 0 (0.0) | 2 (1.9) | 1.000 | ||
| Enterococcus species | 5 (22.7) | 9 (8.3) | 0.062 | ||
| Other organism | 2 (9.1) | 9 (8.3) | 1.000 | ||
| Multiorganism | 8 (36.4) | 56 (51.8) | 0.185 | ||
| Bile culture-ESBL | 3 (13.0) | 11 (9.6) | 0.705 | ||
| Bile culture-MRSA | 1 (4.3) | 3 (2.6) | 0.528 | ||
| Bile culture-Antibiotic resistance4 | 10 (45.5) | 52 (48.1) | 0.818 | ||
| Positive blood culture | 8 (36.4) | 36 (33.3) | 0.784 | ||
| Blood culture-Causative microorganism | |||||
| Escherichia coli | 5 (62.5) | 18 (50.0) | 0.542 | ||
| Klebsiella species | 1 (12.5) | 9 (25.0) | 1.000 | ||
| Pseudomonas species | 1 (12.5) | 5 (13.9) | 1.000 | ||
| Other organism | 0 (0.0) | 3 (8.3) | 1.000 | ||
| Multiorganism | 1 (12.5) | 1 (2.8) | 0.311 | ||
| Blood culture-ESBL | 3 (13.6) | 2 (1.9) | 0.0349 | 15.376 (1.748-135.267) | 0.0149 |
| Blood culture-Antibiotic resistance5 | 3 (13.6) | 5 (4.6) | 0.133 | ||
| Same microorganisms6 | 4 (50.0) | 16 (44.4) | 0.747 | ||
| Unsuccessful biliary decompression7 | 4 (18.2) | 8 (7.4) | 0.121 | ||
| Visit to decompression time (h)8 | 21.6 ± 13.0 | 16.7 ± 11.3 | 0.103 | ||
Heavy smoker is defined as an individual with 20 or more pack-years (1 pack year = 1 pack per day for one year) of use;
Heavy drinker is defined as an individual currently drinking alcoholic beverages in a daily amount of ≥ 80 g (male) or ≥ 40 g (female);
TG13 severity assessment criteria for acute cholangitis (at admission);
Antibiotic susceptibility of microorganisms in bile culture;
Initial antibiotic resistance of microorganisms in blood culture;
The same microbial growth in bile cultures among patients with positive blood cultures;
Reposition or additional insertion after initial PTBD insertion;
Values are presented as mean ± SD;
Statistically significant. PTBD: Percutaneous transhepatic biliary drainage; OF: Organ failure; CCIs: Charlson comorbidity index score; WBC: White blood cell; ALP: Alkaline phosphatase; ESBL: Extended-spectrum beta-lactamase; OR: Odds ratio.
Proposal of organ failure scoring system in patients with bacteriobilia who underwent PTBD due to acute cholangitis
The regression coefficients (standard error) for ESBL organisms in blood cultures, pre-existing renal dysfunction, and choledocholithiasis in the multiple logistic regression model to predict the risk of organ failure were 2.733 (1.109), 1.844 (0.788), and 1.273 (0.559), respectively. Therefore, the regression equation for predicting organ failure was proposed as the following: Organ failure score = (2.7 × ESBL organisms in blood cultures) + (1.8 × pre-existing renal dysfunction) + (1.3 × choledocholithiasis). This equation could be used to provide a numerical score that gives prognostic information for acute cholangitis with bacteriobilia. When dichotomizing the organ failure score to a value ≥ 2.9 with ROC curves, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for organ failure in patients with bacteriobilia who underwent PTBD because of acute cholangitis were 22.7%, 99.1%, 86.2%, 83.3%, and 86.3 %, respectively (Figure 2).
Figure 2.
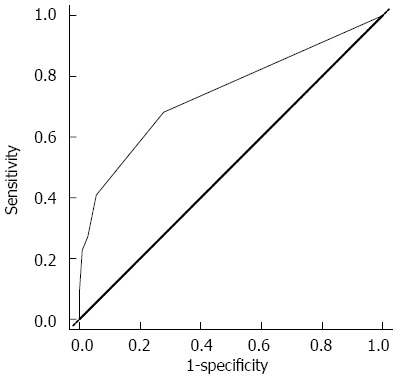
Receiver operating characteristic curves of organ failure score to predict organ failure in patients with bacteriobilia who underwent percutaneous transhepatic biliary drainage because of acute cholangitis. The area under the receiver operating characteristic curves was 0.744 (95%CI: 0.615-0.873), and the best cut-off point of the organ failure score was 2.9 (sensitivity 22.7%, specificity 99.1%, positive predictive value 86.2%, negative predictive value 83.3%).
DISCUSSION
Previous studies have reported that bile culture for microbiological analysis may become a valuable diagnostic tool because it leads to more adequate therapy in patients with cholangitis[21,22]. Although these studies focused on the microbial profile and antibiotic sensitivity pattern in bile cultures, they did not identify prognostic factors in cholangitis with bacteriobilia. In the current study, multivariate analysis identified three risk factors for organ failure in acute cholangitis with bacteriobilia: ESBL organisms in blood cultures, pre-existing renal dysfunction, and choledocholithiasis as an etiology. In addition, we proposed a scoring system to predict organ failure in acute cholangitis with bacteriobilia. This organ failure scoring system was highly specific and had a good positive predictive value.
According to previous studies on acute cholangitis, the most commonly isolated microorganisms were Enterococcus species, Escherichia coli, and Klebsiella species[6,22-24]. These results are consistent with our findings. In addition, our bile samples were collected by PTBD, which is less prone to contamination by intestinal bacteria than endoscopic retrograde cholangiopancreatography (ERCP). These findings support the view that bile culture results in our study had a higher reliability than those reported in previous prospective studies[21,22], which included bile samples through ERCP. Of particular interest, the results of our study showed a high bacterial colonization rate in bile (94%). This phenomenon may be explained by our selected patient cohort, which includes many patients with prior biliary tract manipulations (79/138; 57.2%). Negm et al[22] reported that risk factors of bacteriobilia include biliary stenting and repeated biliary interventions. In particular, biliary stenting is associated with bacteriobilia because stenting of the common bile duct remains a cause of ascending cholangitis[25]. These findings are consistent with our results.
Gotthardt et al[26] recently reported an association of bacteriobilia with outcome in patients who underwent endoscopic treatment for biliary complications after liver transplantation; however, its association with clinical prognosis in acute cholangitis remains unclear. Furthermore, the prognostic factors for cholangitis with bacteriobilia have not yet been identified. The current study identified three risk factors for organ failure in acute cholangitis with bacteriobilia. Among them, the presence of ESBL organisms in blood cultures was the most significant risk factor. Lee et al[11] reported an association of the presence of ESBL organisms in blood cultures with organ failure in bacteremic cholangitis. Although there was no statistical significance because of the small number of ESBL patients in our study, inappropriate initial antibiotic use was more frequent in patients with organ failure than in those without organ failure (3/3; 100% vs 0/2; 0%, P = 0.100). Detection of ESBL organisms takes a few days in current clinical practice; however, new methods for rapid detection of ESBL organisms in blood cultures have recently emerged[27-30]. These facts support the clinical relevance of our result, because detection of ESBL patients during the early period of hospitalization is possible using newly emerging methods.
Previous studies have reported that the creatinine level is a prognostic factor in various conditions, including cholangitis[31-33]. Kent et al[34] reported that pre-existing renal disease promotes sepsis-induced acute kidney injury and is associated with worse outcome. In the current study, among 14 patients with existing renal dysfunction, organ failure occurred in seven patients (50%), including chronic renal failure (n = 5) and end stage renal disease (n = 2). These data indicated a close association of pre-existing renal failure with progression to ARF related to organ failure.
Arima et al[35] reported that bacterial cholangitis caused by impacted bile duct stones could become a serious condition in elderly patients, despite emergency biliary decompression, which is consistent with our finding. In our study, among patients with choledocholithiasis, higher rates of impacted bile duct stone were observed in patients with organ failure than in those without organ failure (8/11; 72.5% vs 7/25; 28%, P = 0.025). Although the mechanism is unclear, the abrupt increment of intrabiliary pressure is probably a key factor, which may cause a higher incidence of septicemia and endotoxemia by adversely affecting the defensive mechanisms such as bile flow, Kupffer cell functions, and secretory IgA production[36].
In the current study, the organ failure scoring system developed with three significant factors was highly specific and positively predictive for predicting organ failure when dichotomizing the score to a value ≥ 2.9. This scoring system and its cut-off value of ≥ 2.9 would allow clinicians to identify the group with poor prognosis even after biliary decompression. However, this scoring system has low sensitivity. This finding may be explained by the very low incidence of organ failure. Therefore, we think that the high specificity and the positive predictive value are more worthwhile than high sensitivity in the organ failure scoring system.
The limitations of this study were the small sample size and retrospective design without a systemized management protocol in a single center. Therefore, a prospective randomized multicenter study should be conducted in the future to confirm our results and validate the organ failure score. Nevertheless, to the best of our knowledge, our work is the first to report prognostic factors in cholangitis with bacteriobilia.
In conclusion, ESBL organisms in blood cultures, pre-existing renal dysfunction, and choledocholithiasis are risk factors for organ failure in acute cholangitis with bacteriobilia. The organ failure scoring system may aid clinicians to recognize a poor prognosis group that could be monitored closely in an intensive care setting.
COMMENTS
Background
Although some studies have reported correlation of bacteriobilia with increased incidence of post-operative infective complications, there has been no study of prognostic factors in cholangitis with bacteriobilia.
Research frontiers
Previous studies focused on the microbial profile and antibiotic sensitivity pattern in bile cultures. The current research hotspot was to identify the risk factors for organ failure in acute cholangitis with bacteriobilia and to develop a prognostic scoring system that can be used to predict organ failure using the risk factors.
Innovations and breakthroughs
The current study identified three risk factors for organ failure in acute cholangitis with bacteriobilia: extended-spectrum beta-lactamase (ESBL) organisms in blood cultures, pre-existing renal dysfunction, and choledocholithiasis as an etiology. In addition, the authors proposed a scoring system to predict organ failure in acute cholangitis with bacteriobilia: Organ failure score = (2.7 × ESBL organisms in blood cultures) + (1.8 × pre-existing renal dysfunction) + (1.3 × choledocholithiasis). This organ failure scoring system was highly specific and positively predictive.
Applications
The authors proposed a scoring system to predict organ failure in acute cholangitis with bacteriobilia. This organ failure scoring system may aid clinicians to recognize a poor prognosis group that could be monitored closely in an intensive care setting.
Terminology
Bacteriobilia was defined as the presence of microorganisms in the bile, documented by at least one positive bile culture.
Peer-review
This is a good descriptive study in which the authors identified the risk factors for organ failure in cholangitis with bacteriobilia. The conclusion has good diagnostic value and clinical significance, because the organ failure scoring system introduced by the authors may aid clinicians to identify a poor prognosis group.
Footnotes
Ethics approval: The study was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-1308-086-514).
Informed consent: Not applicable (due to the retrospective study using information contained in medical charts and computerized records).
Conflict-of-interest: No conflict-of-interest exists for any of the authors.
Data sharing: The technical appendix, statistical code, and dataset are available from the corresponding author at [gidoctor@snuh.org]. Consent was not obtained but the presented data are anonymized and risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 16, 2014
First decision: February 10, 2015
Article in press: March 31, 2015
P- Reviewer: Liaskou E, Luo HS, Romanelli GR, Zhang ZM S- Editor: Qi Y L- Editor: Stewart G E- Editor: Zhang DN
References
- 1.Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg. 1993;165:399–404. doi: 10.1016/s0002-9610(05)80930-4. [DOI] [PubMed] [Google Scholar]
- 2.Beger HG, Schwarz A. Review article: Spectrum of biliary infections in the West and in the East. HPB Surg. 1995;8:215–222. doi: 10.1155/1995/74384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai EC, Mok FP, Tan ES, Lo CM, Fan ST, You KT, Wong J. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582–1586. doi: 10.1056/NEJM199206113262401. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal N, Sharma BC, Sarin SK. Endoscopic management of acute cholangitis in elderly patients. World J Gastroenterol. 2006;12:6551–6555. doi: 10.3748/wjg.v12.i40.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saik RP, Greenburg AG, Farris JM, Peskin GW. Spectrum of cholangitis. Am J Surg. 1975;130:143–150. doi: 10.1016/0002-9610(75)90362-1. [DOI] [PubMed] [Google Scholar]
- 6.Chang WT, Lee KT, Wang SR, Chuang SC, Kuo KK, Chen JS, Sheen PC. Bacteriology and antimicrobial susceptibility in biliary tract disease: an audit of 10-year’s experience. Kaohsiung J Med Sci. 2002;18:221–228. [PubMed] [Google Scholar]
- 7.Tanaka A, Takada T, Kawarada Y, Nimura Y, Yoshida M, Miura F, Hirota M, Wada K, Mayumi T, Gomi H, et al. Antimicrobial therapy for acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:59–67. doi: 10.1007/s00534-006-1157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvador VB, Lozada MC, Consunji RJ. Microbiology and antibiotic susceptibility of organisms in bile cultures from patients with and without cholangitis at an Asian academic medical center. Surg Infect (Larchmt) 2011;12:105–111. doi: 10.1089/sur.2010.005. [DOI] [PubMed] [Google Scholar]
- 9.Westphal JF, Brogard JM. Biliary tract infections: a guide to drug treatment. Drugs. 1999;57:81–91. doi: 10.2165/00003495-199957010-00007. [DOI] [PubMed] [Google Scholar]
- 10.Lee CC, Chang IJ, Lai YC, Chen SY, Chen SC. Epidemiology and prognostic determinants of patients with bacteremic cholecystitis or cholangitis. Am J Gastroenterol. 2007;102:563–569. doi: 10.1111/j.1572-0241.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee BS, Hwang JH, Lee SH, Jang SE, Jang ES, Jo HJ, Shin CM, Park YS, Kim JW, Jung SH, et al. Risk factors of organ failure in patients with bacteremic cholangitis. Dig Dis Sci. 2013;58:1091–1099. doi: 10.1007/s10620-012-2478-8. [DOI] [PubMed] [Google Scholar]
- 12.Melzer M, Toner R, Lacey S, Bettany E, Rait G. Biliary tract infection and bacteraemia: presentation, structural abnormalities, causative organisms and clinical outcomes. Postgrad Med J. 2007;83:773–776. doi: 10.1136/pgmj.2007.064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard TJ, Yu J, Greene RB, George V, Wairiuko GM, Moore SA, Madura JA. Influence of bactibilia after preoperative biliary stenting on postoperative infectious complications. J Gastrointest Surg. 2006;10:523–531. doi: 10.1016/j.gassur.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Sivaraj SM, Vimalraj V, Saravanaboopathy P, Rajendran S, Jeswanth S, Ravichandran P, Vennilla R, Surendran R. Is bactibilia a predictor of poor outcome of pancreaticoduodenectomy? Hepatobiliary Pancreat Dis Int. 2010;9:65–68. [PubMed] [Google Scholar]
- 15.Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Yokoe M, et al. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos) J Hepatobiliary Pancreat Sci. 2013;20:24–34. doi: 10.1007/s00534-012-0561-3. [DOI] [PubMed] [Google Scholar]
- 16.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 17.Bone RC, Fisher CJ, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–658. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 18.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 19.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Kaya M, Beştaş R, Bacalan F, Bacaksız F, Arslan EG, Kaplan MA. Microbial profile and antibiotic sensitivity pattern in bile cultures from endoscopic retrograde cholangiography patients. World J Gastroenterol. 2012;18:3585–3589. doi: 10.3748/wjg.v18.i27.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negm AA, Schott A, Vonberg RP, Weismueller TJ, Schneider AS, Kubicka S, Strassburg CP, Manns MP, Suerbaum S, Wedemeyer J, et al. Routine bile collection for microbiological analysis during cholangiography and its impact on the management of cholangitis. Gastrointest Endosc. 2010;72:284–291. doi: 10.1016/j.gie.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 23.Maluenda F, Csendes A, Burdiles P, Diaz J. Bacteriological study of choledochal bile in patients with common bile duct stones, with or without acute suppurative cholangitis. Hepatogastroenterology. 1989;36:132–135. [PubMed] [Google Scholar]
- 24.Csendes A, Burdiles P, Maluenda F, Diaz JC, Csendes P, Mitru N. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch Surg. 1996;131:389–394. doi: 10.1001/archsurg.1996.01430160047008. [DOI] [PubMed] [Google Scholar]
- 25.Herzog T, Belyaev O, Muller CA, Mittelkotter U, Seelig MH, Weyhe D, Felderbauer P, Schlottmann R, Schrader H, Schmidt WE, et al. Bacteribilia after preoperative bile duct stenting: a prospective study. J Clin Gastroenterol. 2009;43:457–462. doi: 10.1097/MCG.0b013e318186b19b. [DOI] [PubMed] [Google Scholar]
- 26.Gotthardt DN, Weiss KH, Rupp C, Bode K, Eckerle I, Rudolph G, Bergemann J, Kloeters-Plachky P, Chahoud F, Büchler MW, et al. Bacteriobilia and fungibilia are associated with outcome in patients with endoscopic treatment of biliary complications after liver transplantation. Endoscopy. 2013;45:890–896. doi: 10.1055/s-0033-1344713. [DOI] [PubMed] [Google Scholar]
- 27.Colodner R, Reznik B, Gal V, Yamazaki H, Hanaki H, Kubo R. Evaluation of a novel kit for the rapid detection of extended-spectrum beta-lactamases. Eur J Clin Microbiol Infect Dis. 2006;25:49–51. doi: 10.1007/s10096-005-0074-y. [DOI] [PubMed] [Google Scholar]
- 28.Cagatay AA, Kocagoz T, Eraksoy H. Dio-Sensimedia: a novel culture medium for rapid detection of extended spectrum beta-lactamases. BMC Infect Dis. 2003;3:22. doi: 10.1186/1471-2334-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ercis S, Sancak B, Kocagöz T, Kocagöz S, Hasçelik G, Bolmström A. Rapid 4 to 6 hour detection of extended-spectrum beta-lactamases in a routine laboratory. Scand J Infect Dis. 2007;39:781–785. doi: 10.1080/00365540701367751. [DOI] [PubMed] [Google Scholar]
- 30.Weinbren MJ, Borthwick MA. Rapid detection of extended-spectrum beta-lactamase (ESBL)-producing organisms in blood culture. J Antimicrob Chemother. 2005;55:131–132. doi: 10.1093/jac/dkh502. [DOI] [PubMed] [Google Scholar]
- 31.Tai DI, Shen FH, Liaw YF. Abnormal pre-drainage serum creatinine as a prognostic indicator in acute cholangitis. Hepatogastroenterology. 1992;39:47–50. [PubMed] [Google Scholar]
- 32.Aung A, Alqudihy S, Rybicki L, Platt A, Davis MP. Does serum albumin and creatinine predict survival of inpatient palliative care patients? Am J Hosp Palliat Care. 2014;31:862–866. doi: 10.1177/1049909113501851. [DOI] [PubMed] [Google Scholar]
- 33.Kertai MD, Boersma E, Bax JJ, van den Meiracker AH, van Urk H, Roelandt JR, Poldermans D. Comparison between serum creatinine and creatinine clearance for the prediction of postoperative mortality in patients undergoing major vascular surgery. Clin Nephrol. 2003;59:17–23. doi: 10.5414/cnp59017. [DOI] [PubMed] [Google Scholar]
- 34.Doi K, Leelahavanichkul A, Hu X, Sidransky KL, Zhou H, Qin Y, Eisner C, Schnermann J, Yuen PS, Star RA. Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int. 2008;74:1017–1025. doi: 10.1038/ki.2008.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arima N, Uchiya T, Hishikawa R, Saito M, Matsuo T, Kurisu S, Umeki M, Kita Y, Koyama T, Hatta T. [Clinical characteristics of impacted bile duct stone in the elderly] Nihon Ronen Igakkai Zasshi. 1993;30:964–968. doi: 10.3143/geriatrics.30.964. [DOI] [PubMed] [Google Scholar]
- 36.Sung JY, Costerton JW, Shaffer EA. Defense system in the biliary tract against bacterial infection. Dig Dis Sci. 1992;37:689–696. doi: 10.1007/BF01296423. [DOI] [PubMed] [Google Scholar]


