Abstract
Objective
Serum concentrations of the hepatokine fibroblast growth factor (FGF) 21 are elevated in obesity, type-2 diabetes, and the metabolic syndrome. We asked whether FGF21 levels differ between subjects with metabolically healthy vs. unhealthy obesity (MHO vs. MUHO), opening the possibility that FGF21 is a cross-talker between liver and adipose tissue in MUHO. Furthermore, we studied the effects of chronic FGF21 treatment on adipocyte differentiation, lipid storage, and adipokine secretion.
Methods
In 20 morbidly obese donors of abdominal subcutaneous fat biopsies discordant for their whole-body insulin sensitivity (hereby classified as MHO or MUHO subjects), serum FGF21 was quantified. The impact of chronic FGF21 treatment on differentiation, lipid accumulation, and adipokine release was assessed in isolated preadipocytes differentiated in vitro.
Results
Serum FGF21 concentrations were more than two-fold higher in MUHO as compared to MHO subjects (457 ± 378 vs. 211 ± 123 pg/mL; p < 0.05). FGF21 treatment of human preadipocytes for the entire differentiation period was modestly lipogenic (+15%; p < 0.05), reduced the expression of key adipogenic transcription factors (PPARG and CEBPA, −15% and −40%, respectively; p < 0.01 both), reduced adiponectin expression (−20%; p < 0.05), markedly reduced adiponectin release (−60%; p < 0.01), and substantially increased leptin (+60%; p < 0.01) and interleukin-6 (+50%; p < 0.001) release.
Conclusions
The hepatokine FGF21 exerts weak lipogenic and anti-adipogenic actions and marked adiponectin-suppressive and leptin and interleukin-6 release-promoting effects in human differentiating preadipocytes. Together with the higher serum concentrations in MUHO subjects, our findings reveal FGF21 as a circulating factor promoting the development of metabolically unhealthy adipocytes.
Keywords: FGF21, Hepatokine, Adiponectin, Adipokine, Secretome, Type-2 diabetes
Abbreviations: AMPK, AMP-activated protein kinase; BMI, body mass index; C/EBP-α, CCAAT/enhancer-binding protein-α; CIDEA, cell death-inducing DNA fragmentation factor-like effector a; ERK, extracellular signal-regulated kinase; FGF, fibroblast growth factor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; hasc, human abdominal subcutaneous; IL-6, interleukin-6; MHO, metabolically healthy obesity; MUHO, metabolically unhealthy obesity; qPCR, quantitative polymerase chain reaction; PGC-1α, PPAR-γ coactivator-1α; PPAR-γ, peroxisome proliferator-activated receptor-γ; rh, recombinant human; UCP-1, uncoupling protein-1
Highlights
-
•
Metabolically healthy and unhealthy obese subjects differ in FGF21 blood levels.
-
•
FGF21 alters the secretome of human preadipocytes differentiated in vitro.
-
•
FGF21 exerts potent adiponectin-suppressive effects.
-
•
FGF21 and adiponectin may constitute a humoral vicious cycle between liver and fat.
1. Introduction
Fibroblast growth factor (FGF) 21, FGF19 (murine homologue: FGF15), and FGF23 constitute a subfamily of FGFs with hormone-like rather than growth factor functions [1].
In mice, FGF21 is produced by liver, pancreas, skeletal muscle, brown and white adipose tissue, and major stimuli for FGF21 release are fasting and ketogenic diets, cold exposure, and exercise [2]. In murine models of obesity and diabetes, and less evident or even absent in normal mice, pharmacological administration or transgenic overexpression of FGF21 exerts beneficial metabolic effects, i.e., reduction of body weight and liver fat, improvement of insulin sensitivity, hyperlipidaemia, and hyperglycaemia, and this is most probably due to the induction of thermogenesis and stimulation of energy expenditure [3–5].
In humans, FGF21's role in physiology and metabolic disease is more complicated and far from being understood: circulating FGF21 is mainly produced by the liver and is independent of nutritional status [1]; furthermore, its plasma concentrations are elevated in abdominal obesity, fatty liver disease, hyperlipidaemia, insulin resistance, type-2 diabetes, metabolic syndrome, and coronary artery disease [6–10]. Since this is in sharp contrast to data obtained in mice and the existence of a postulated FGF21-resistant state in humans is still insufficiently proven, there is a clear need for further studies to better understand FGF21's role in humans.
We previously succeeded in describing metabolically healthy and unhealthy obesity (MHO and MUHO) with hepatic fat content and inflammation representing major determinants of these states [11,12]. In a very recent metabolomics study, we have shown that human abdominal subcutaneous (hasc) preadipocytes differentiated in vitro to adipocytes reveal intra- and extracellular metabolite signatures that also allow discriminating MUHO from MHO [13]. Thus, these cells reveal stable, mitotically inherited properties that may reflect and/or contribute to these states.
In this study, we asked whether serum FGF21 concentrations differ between MHO and MUHO subjects. This would open the possibility that FGF21 acts as a cross-talker between the liver and adipose tissue affecting (pre)adipocyte properties in a way that could explain metabolic features of MUHO versus MHO. Therefore, we studied the effects of chronic FGF21 treatment on typical adipocyte functions, such as adipose conversion, lipid storage, and adipokine secretion, in hasc preadipocytes differentiated in vitro.
2. Material and methods
2.1. Study participants
The 20 hasc adipose tissue donors reported earlier [13] were morbidly obese (body mass index (BMI) > 40 kg/m² all), well matched for gender, age, and body fat content (as measured by bioelectrical impedance), but discordant for their whole-body insulin sensitivity (as estimated by a five-point oral glucose tolerance test-derived insulin sensitivity index calculated as 10,000/[fasting glucose {mmol/L} x fasting insulin {pmol/L} x mean glucose {mmol/L} x mean insulin {pmol/L}]1/2). According to their insulin sensitivity, they were stratified into ten MHO (insulin-sensitive; insulin sensitivity index ≥5.0 × 1019 L2 x mol−2) and ten MUHO (insulin-resistant; insulin sensitivity index ≤3.5 × 1019 L2 x mol−2) subjects, and the clinical characteristics of the two groups were reported recently [13]. The study adhered to The Code of Ethics of the World Medical Association (Declaration of Helsinki). All participants gave informed written consent to the study, and the study protocol was approved by the local ethics board.
2.2. Preadipocyte culture, differentiation, and treatment
Preadipocytes were isolated from the 20 hasc adipose tissue explants as described earlier [14] and expanded in α-MEM/Ham's nutrient mixture F12 (1:1) containing 20% fetal calf serum, 1% chicken embryo extract (Sera Laboratories, Haywards Heath, UK), 100 IU/mL penicillin, 0.1 mg/mL streptomycin, 0.5 μg/mL fungizone, and 2 mmol/L glutamine. Second-pass cells were used for experiments. At confluence, adipose conversion was induced by shifting the cells into DMEM/Ham's nutrient mixture F12 (1:1), 5% fetal calf serum, 17 μmol/L pantothenate, 1 μmol/L biotin, 2 μg/mL apo-transferrin, 1 μmol/L human insulin, 1 μmol/L dexamethasone, 100 IU/mL penicillin, 0.1 mg/mL streptomycin, 0.5 μg/mL fungizone, and 2 mmol/L glutamine (differentiation medium) supplemented with 0.5 mmol/L 3-isobutyl-1-methyl-xanthine, 2 nmol/L triiodo-thyronine, and 50 μmol/L indomethacin for seven days. Thereafter, the cells were allowed to terminally differentiate for another 11 days in differentiation medium alone. Differentiating preadipocytes were left untreated or were chronically treated for the whole 18-day period with 50 ng/mL recombinant human (rh)FGF21 (PeproTech, Rocky Hill, NJ, USA). Culture media and supplements were obtained from Lonza (Basel, Switzerland) and Biochrom (Berlin, Germany).
2.3. Staining of intracellular neutral lipids
Differentiation of preadipocytes to adipocytes was qualitatively monitored by microscopy after Oil Red O staining according to Greenberger et al. [15]. For quantification of intracellular neutral lipids, Oil Red O was extracted with isopropanol and photometrically measured at 500 nm as described earlier [16].
2.4. RNA isolation and real-time quantitative polymerase chain reaction (qPCR)
(Pre)adipocytes were harvested with Qiazol lysis reagent (Qiagen, Hilden, Germany). Total RNA was isolated with miRNeasy columns (Qiagen), treated with RNase-free DNase I, and reverse transcribed into cDNA using Qiagen's QuantiTect reverse transcription kit. Real-time qPCR was performed in technical duplicates with QuantiTect primer assays on a LightCycler™ 480 II (Roche Diagnostics, Mannheim, Germany). All mRNA data were normalized to the housekeeping gene RPS13 using the ΔCt method.
2.5. Enzyme-linked immunosorbent assays
FGF21 in serum and adiponectin, leptin, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in the conditioned cell culture media were measured with immunoassays from R&D Systems (Wiesbaden-Nordenstadt, Germany) according to the manufacturers' instructions.
2.6. Protein extraction and immunoblotting
Cellular protein was extracted using RIPA buffer (50 mmol/L Tris–HCl pH 7.4, 150 mmol/L NaCl, 1% NP-40, 0.25% Na-deoxycholate, 1 mmol/L phenyl-methyl-sulfonyl-fluoride, 1 mmol/L dithiothreitol) containing a protease and phosphatase inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany). Cell lysates were sonicated and cleared by centrifugation. Protein concentration was determined with the RC DC kit from Bio-Rad (Hercules, CA, USA). Equal amounts of protein (30 μg/lane) were loaded onto 10-% sodium dodecyl sulfate polyacrylamide gels. After electrophoresis, proteins were transferred to polyvinylidene fluoride membranes and incubated with antibodies against phospho-threonine 172 of the catalytic α-subunit of AMP-activated protein kinase (AMPK), AMPKα protein, phosphorylated extracellular signal-regulated kinases (ERK) 1 and 2, ERK1 and 2 protein, or glyceraldehyde 3-phosphate dehydrogenase (GAPDH), respectively. All primary antibodies were from Cell Signaling Technologies (Danvers, MA, USA), the secondary anti-rabbit antibody was from Santa Cruz Biotechnology (Dallas, TX, USA). Proteins were visualized by electrochemiluminescence (PerkinElmer, Waltham, MA, USA).
2.7. Genome-wide gene expression analysis
Total RNA was isolated as described. The Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany) was used to assess RNA quality, and only high-quality RNA (RNA integrity number >9) was used for microarray analysis. Total RNA (30 ng) was amplified using the Ovation PicoSL WTA System V2 in combination with the Encore Biotin Module (NuGEN, Leek, The Netherlands). Amplified cDNA was hybridized on Affymetrix Human Gene ST 1.0 arrays (Affymetrix, Santa Clara, CA, USA). Staining (Fluidics script FS450_0007) and scanning was done according to the Affymetrix expression protocol including minor modifications as suggested in the Encore Biotin protocol (NuGEN). Expression console (Affymetrix) was used for quality control and to obtain annotated normalized robust multi-array analysis gene level data (standard settings including sketch-quantile normalization). Statistical analyses were performed by utilizing the statistical programming environment R implemented in CARMAweb [17]. Genewise testing for differential expression was done employing the limma t-test (paired) and Benjamini-Hochberg correction for multiple testing (false discovery rate <10%). Gene Ontology and Kyoto Encyclopedia of Genes and Genomes term and pathway enrichment analyses were performed with GePS (Genomatix, Germany) and significant terms (p < 0.01) were determined. In addition, Ingenuity Pathway Software (http://www.ingenuity.com) was used to identify enriched pathways. Array data have been submitted to GEO (GSE67279).
2.8. Further statistical analyses
To approximate normal distribution, serum FGF21 concentrations were loge-transformed prior to statistical analysis. Differences in serum FGF21 between the MHO and MUHO groups were tested by multiple linear regression analysis with FGF21 concentration as outcome variable, the nominal MHO/MUHO classification as independent variable, and gender, age, and BMI as confounding variables. All in vitro data, except microarray data (see above), were analyzed by two-group comparisons (FGF21-treated vs. untreated) using two-tailed paired Student's t-test. A p-value <0.05 was considered statistically significant. For these analyses, the statistical software package JMP 10.0 (SAS Institute, Cary, NC, USA) was used.
3. Results
3.1. FGF21 concentrations in MHO and MUHO subjects
After adjustment for the putative confounders gender, age, and BMI by multiple linear regression modeling, serum FGF21 concentrations were more than twice as high in MUHO subjects as compared to MHO subjects (unadjusted 457 ± 378 vs. 211 ± 123 pg/mL, means ± SD; adjusted data presented in Figure 1; p = 0.023).
Figure 1.
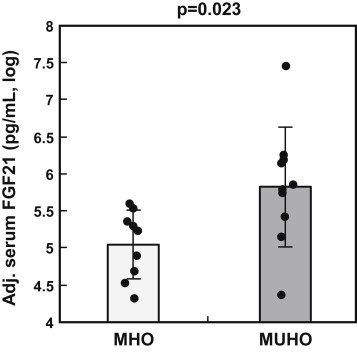
Serum FGF21 concentrations in MHO and MUHO subjects Data adjusted for gender, age, and BMI are shown as log-transformed individual data and means ± SD (N = 10 per group). Adjustment was performed by multiple linear regression modeling. MHO – metabolically healthy obesity; MUHO – metabolically unhealthy obesity.
3.2. FGF21 responsiveness of differentiating hasc preadipocytes
The FGF21 receptor complex, composed of FGF receptor-1 and β-klotho, was readily detectable on the gene expression level as determined by qPCR (mean Cp-values in undifferentiated preadipocytes: FGFR1 22.8, KLB 33.9). To further provide evidence that hasc preadipocytes are responsive to rhFGF21, differentiating preadipocytes (from day 8 of differentiation) were incubated for 10 min with 50 ng/mL (2.6 nmol/L) rhFGF21, a concentration that is above the physiological range (reported to reach up to 3 ng/mL [1]), but well within the pharmacological range reported for novel FGF21 analogues/mimetics (17.5–150 ng/mL [18]), and below the high concentrations usually used in in vitro settings (≥1 μg/mL [19–21]). This acute treatment induced robust phosphorylation of AMPKα, ERK1, and ERK2 (Figure 2), well-known FGF21 downstream signaling events [19,22]. Therefore, we used 50 ng/mL rhFGF21 in all subsequent experiments.
Figure 2.
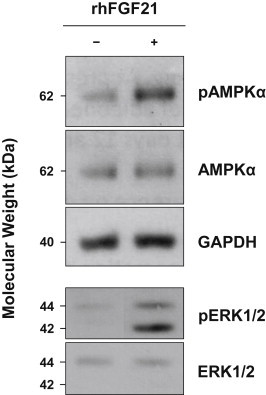
FGF21 signaling in differentiating hasc preadipocytes Cells from day 8 of the differentiation protocol were left untreated (−) or were treated (+) for 10 min with rhFGF21 (50 ng/mL). Immunoblotting was performed with primary antibodies against phospho-threonine 172 of AMPKα, AMPKα protein, phosphorylated ERK1 and ERK2, and ERK1/2 protein. A primary antibody against GAPDH was used as loading control. One immunoblot representative of three biological replicates is shown. AMPK – AMP-activated protein kinase; ERK – extracellular signal-regulated kinase; hasc – human abdominal subcutaneous; GAPDH – glyceraldehyde 3-phosphate dehydrogenase; rh – recombinant human.
3.3. Effect of chronic FGF21 treatment on lipid accumulation
As shown by qualitative Oil Red O staining (Figure 3A) and photometric quantification of the intracellular Oil Red O content over time (Figure 3B), triglyceride accumulation was significantly increased by rhFGF21 during the early stages of differentiation. This effect, however, was modest (+15%, days 4 and 8) and diminished to a statistical trend at later time-points (p = 0.09, days 12 and 18; Figure 3B).
Figure 3.
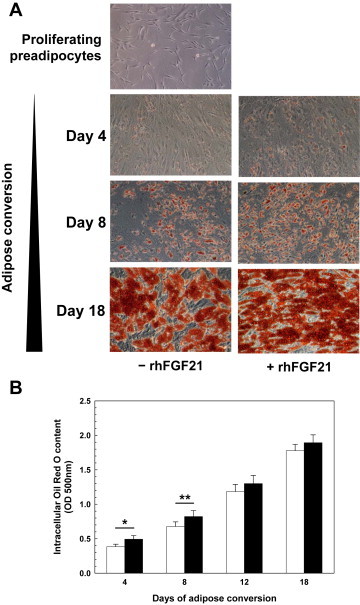
FGF21 effect on lipid accumulation in differentiating hasc preadipocytes (A) Triglyceride accumulation was visualized by Oil Red O staining of proliferating preadipocytes (topmost panel) and of differentiating adipocytes (from days 4, 8, and 18) left untreated (left column of images) or chronically treated with rhFGF21 (right column of images). Microscopical pictures of Oil Red O-stained cultures representative of 20 biological replicates are shown. (B) The Oil Red O content of cultures was photometrically quantified at 500 nm after stain extraction with isopropanol (means ± SEM; N = 20). Black bars – rhFGF21-treated cultures; white bars – untreated controls. hasc – human abdominal subcutaneous; OD – optical density; rh – recombinant human. *p < 0.05, **p < 0.01.
3.4. Effects of chronic FGF21 treatment on white and brown adipocyte marker gene expression
Chronic rhFGF21 treatment significantly impaired the expression of the key adipogenic transcription factors peroxisome proliferator-activated receptor-γ (PPAR-γ; -15%, day 18; Figure 4A) and CCAAT/enhancer-binding protein-α (C/EBP-α; up to −40%, days 8–18; Figure 4B). In addition, rhFGF21 reduced the expression of the adipokine adiponectin (up to −20%; days 4 and 18; Figure 4C), whereas leptin expression remained unaffected (p > 0.08). Unexpectedly, the brown adipocyte marker genes encoding uncoupling protein-1 (UCP-1), PPAR-γ coactivator-1α (PGC-1α), and cell death-inducing DNA fragmentation factor-like effector a (CIDEA) were differently regulated with UCP-1 being 2-fold induced by rhFGF21 (days 12 and 18; Figure 4D) and PGC-1α and CIDEA being repressed by up to −40% and −50%, respectively (all time-points; Figure 4E,F). Similar regulations of the brown adipocyte marker genes were seen when rhFGF21 was sub-chronically added for three days to adipocytes (from day 18) that were differentiated in the absence of rhFGF21 (data not shown). It should be noted that the UCP-1 expression levels were very low (mean Cp-value on day 0: 35.9; mean Cp-value on day 18: 31.4). Moreover, we did not obtain higher expression levels using isoproterenol, a well-known and potent inducer of the gene. Therefore, we concluded that hasc preadipocytes represent a cell type with very limited capacity to brown. In accordance with this, we did not detect any convincing signal for UCP-1 protein by immunocytochemistry.
Figure 4.
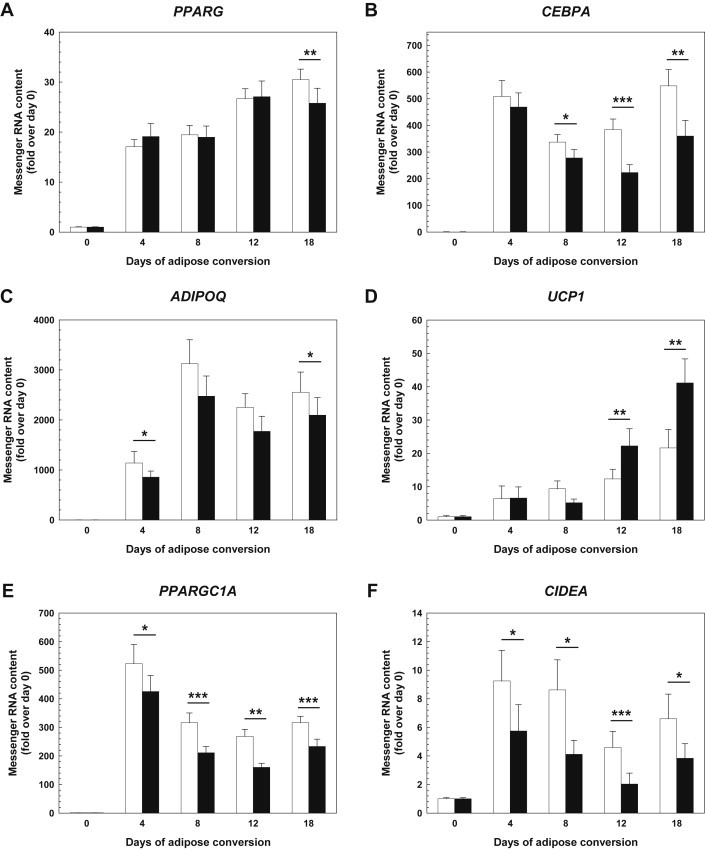
FGF21 effects on white and brown adipocyte marker gene expression in differentiating hasc preadipocytes The mRNA expression of PPARG encoding PPAR-γ (A), CEBPA encoding C/EBP-α (B), ADIPOQ encoding adiponectin (C), UCP1 encoding UCP-1 (D), PPARGC1A encoding PGC-1α (E), and CIDEA encoding CIDEA (F) was monitored by real-time qPCR (means ± SEM; N = 20). Black bars – rhFGF21-treated cultures; white bars – untreated controls. C/EBP-α – CCAAT/enhancer-binding protein α; CIDEA – cell death-inducing DNA fragmentation factor-like effector a; hasc – human abdominal subcutaneous; PGC-1α – PPAR-γ coactivator 1α; PPAR-γ – peroxisome proliferator-activated receptor γ; qPCR – quantitative polymerase chain reaction; rh – recombinant human; UCP-1 – uncoupling protein 1. *p < 0.05, **p < 0.01, ***p < 0.001.
3.5. Effects of chronic FGF21 treatment on adipokine secretion
Secretion of the major adipokines adiponectin, leptin, IL-6, and TNF-α upon chronic treatment with rhFGF21 was assessed in 48-hour conditioned culture media at different time-points after start of differentiation. Adiponectin release into the medium was markedly reduced (up to −60%; days 8 and 18; Figure 5A), whereas that of leptin (up to +60%; days 12 and 18; Figure 5B) and interleukin-6 (up to +50%; all time-points; Figure 5C) was substantially increased by rhFGF21 treatment. TNF-α secreted by preadipocytes of day 0 ranged at the detection limit (<5 pg/mL) and became undetectable at later time-points both in the absence and presence of rhFGF21 (data not shown). To see whether the rhFGF21-induced increase in IL-6 release was due to enhanced IL-6 gene expression, we performed qPCR. In accordance with the release data, IL-6 gene expression was induced by rhFGF21 (up to +45%; days 4, 8, and 18; Figure 5D).
Figure 5.
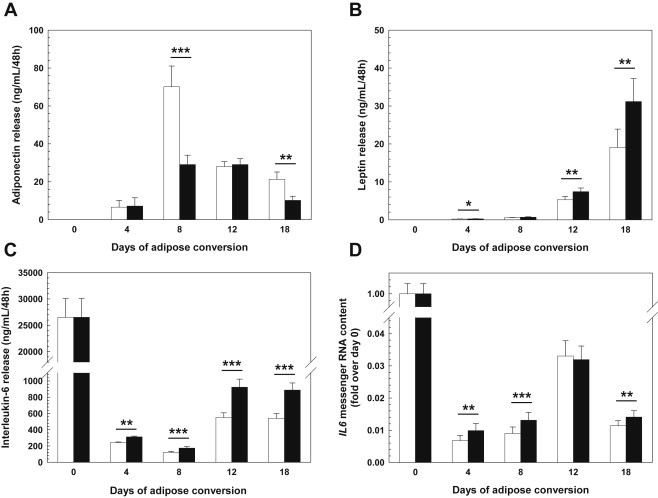
FGF21 effects on adipokine release and interleukin-6 gene expression in differentiating hasc preadipocytes The concentrations of adiponectin (A), leptin (B), and interleukin-6 (C) in media conditioned for 48 hours by differentiating preadipocytes was measured by enzyme-linked immunosorbent assays (means ± SEM; N = 20). The mRNA expression of IL6 encoding interleukin-6 (D) was monitored by real-time qPCR (means ± SEM; N = 20). Black bars – rhFGF21-treated cultures; white bars – untreated controls. hasc – human abdominal subcutaneous; rh – recombinant human. *p < 0.05, **p < 0.01, ***p < 0.001.
Upon a closer look at the temporal course of adiponectin release, as depicted in Figure 5A, it appeared as if FGF21 not only reduces overall adiponectin release but also alters the shape of the release curve: in the absence of chronic FGF21, adiponectin release appeared biphasic with a prominent peak on day 8 (first phase) and half-maximum levels on day 12 that modestly further declined from day 12 on (second phase); chronic FGF21 treatment changed the curve into a bell-shaped one with a markedly diminished, broader, and delayed peak between days 8 and 12; as a result of this, there is a crossing point of the curves on day 12.
3.6. Role of candidate genes/pathways underlying FGF21-induced adiponectin suppression
Adiponectin is a major secretory product of adipocytes and an important insulin-sensitizing adipokine [23]. Therefore, we tried to further decipher the adiponectin-suppressive effect of chronic FGF21 treatment. Even though the repressive effect of FGF21 on adiponectin expression may be explained by impaired PGC-1α expression (and consequently reduced activity of the adiponectin-inducing transcription factor PPAR-γ), the effect size of adiponectin gene repression was modest (−20%) and did not correspond to the marked adiponectin release-suppressive effect of FGF21 (−60%). Hence, we asked whether chronic rhFGF21 treatment affects the expression of genes involved in cellular protein synthesis, protein degradation, or exocytosis. Using whole-genome microarray gene expression analysis and subsequent single-gene as well as pathway analyses based on Gene Ontology and Kyoto Encyclopedia of Genes and Genomes terms did not reveal any significant rhFGF21-induced changes in these pathways (Supplementary Figure S1, Supplementary Tables S1 and S2). This whole-genome gene expression analysis, however, confirmed the IL-6-inducing (+50%; day 8) and PGC-1α-repressing (−30%; day 8) effects of rhFGF21 (+49% and −29%; Supplementary Table S1). As a second hypothesis, we tested whether the enhanced IL-6 expression of FGF21-treated cells might affect, in an auto-/paracrine mode, adiponectin release. Neither the chronic use of a neutralizing anti-IL-6 antibody nor that of a pharmacological inhibitor of IL-6 signaling (small molecule STAT3 inhibitor stattic) significantly influenced rhFGF21-dependent suppression of adiponectin release (data not shown).
4. Discussion
In the present study of morbidly obese human subjects, we asked whether FGF21 is a potential cross-talker between the liver, where it originates in humans, and adipose tissue, affecting human (pre)adipocyte properties in a way that may discriminate MUHO from MHO.
In humans, FGF21 concentrations in blood directly correlate with BMI, overall body fat mass, visceral fat mass, pericardial fat mass, and ectopic lipid deposition [10,24–31]. On the other hand, anorexia nervosa is associated with lower plasma FGF21 levels [32], and acute fat loss due to bariatric surgery was also reported to reduce plasma FGF21 [33]. Here we show that circulating FGF21 levels are more than two-fold higher in MUHO as compared to body fat-matched MHO subjects, pointing to an adiposity-independent role of FGF21 in insulin resistance in humans. This is in keeping with previous studies reporting BMI- and body fat mass-independent associations of plasma FGF21 levels with hepatic and whole-body insulin resistance [6,26,27]. This finding opened the possibility that pathophysiologically high plasma concentrations of the human hepatokine FGF21, as measured in the morbidly obese subjects of the MUHO group, can exert metabolically detrimental effects and thus may contribute to the MUHO phenotype.
To explore whether chronically elevated concentrations of FGF21 alter human (pre)adipocyte properties, we applied a moderately supraphysiological concentration (50 ng/mL) for 18 days and studied its impact on adipose conversion, lipid storage, and adipokine secretion of differentiating hasc preadipocytes. We detected modest lipogenic and anti-adipogenic effects of this treatment. Most impressively however, chronic FGF21 treatment markedly altered the release of the classical adipokines adiponectin, leptin, and IL-6: FGF21 potently suppressed adiponectin release and enhanced leptin and IL-6 release with the effects on adiponectin and IL-6 being, at least in part, reflected by FGF21's impact on the expression of the respective genes. Since the FGF21 concentration used in these experiments is well within the range of pharmacological blood concentrations reached with FGF21 analogues/mimetics [18], the described unfavorable FGF21 effects on (pre)adipocyte properties could be of particular relevance for the clinical use of these novel compounds currently in development for the treatment of diabetes. Even though the adiponectin-suppressive effect of FGF21 observed in this study is in agreement with results from several human cross-sectional and intervention studies showing an inverse relationship between plasma FGF21 and plasma adiponectin concentrations [29,31–35], a recent 28-day clinical trial with LY2405319, an FGF21 variant engineered to improve biopharmaceutical properties, revealed treatment-induced elevations of plasma adiponectin [18]. The origin of this discrepancy is unclear but may be due to the structural peculiarities of LY2405319 resulting from the introduction of an additional disulfide bond, deletion of four N-terminal amino acids, and elimination of an O-linked glycosylation site [36]. With respect to physiology and to adiponectin's well-known insulin-sensitizing properties, adiponectin suppression by the natural form of human FGF21, as observed in our study in differentiating hasc preadipocytes, could be a possible reason for FGF21's association with insulin resistance in humans.
As to the molecular mechanism(s) underlying adiponectin suppression, the observed FGF21-induced repression of adiponectin gene expression may, of course, be relevant. Adiponectin gene repression could, in turn, result from impaired PGC-1α gene expression as PGC-1α is an essential coactivator of the transcription factor PPAR-γ, a well-described transcriptional inducer of the adiponectin gene [37,38]. However, the repressive effect of FGF21 on adiponectin gene expression was modest compared to the FGF21-induced reduction in adiponectin release. Thus, additional mechanisms might contribute to FGF21-dependent adiponectin suppression. In this context, we assessed whether IL-6, which is induced and released upon FGF21 treatment, acts as an auto-/paracrine negative feedback regulator of adiponectin release, as was hypothesized in earlier studies [39,40]. However, our attempts to interfere with IL-6 signaling were without any effect on adiponectin release. Our results obtained with a neutralizing anti-IL-6 antibody and an inhibitor of IL-6 signaling are certainly insufficient to ultimately exclude a role of adipocyte-derived IL-6 in FGF21-induced adiponectin suppression, and further studies are needed to clarify this issue. Finally, we tested whether adiponectin suppression is due to FGF21-induced alterations in the expression of genes involved in more general pathways, such as protein synthesis, protein degradation, and/or exocytosis. Since our whole-genome microarray analysis was well-powered (N = 20) and did not reveal any significant FGF21 effects on these pathways, we suggest that other, still unknown, mechanisms may contribute to FGF21-dependent adiponectin suppression.
Even though mechanistically unresolved, FGF21-induced adiponectin suppression may establish a novel hypothesis as depicted in Figure 6: fatty liver, a hallmark of MUHO [11,41], produces elevated levels of circulating FGF21 [10,42]. FGF21, in turn, suppresses adiponectin production in adipose tissue as suggested by our data herein, and this is in line with the reduced serum adiponectin levels of our MUHO subjects [11,13] as well as with the inverse relationship between FGF21 and adiponectin levels observed in several cross-sectional and longitudinal studies [29,31–35]. As FGF21 is repressed by low- and high-molecular-weight forms of adiponectin in primary human hepatocytes [43] and adiponectin knockdown vice versa results in increased hepatic FGF21 expression [44], FGF21-induced hypoadiponectinaemia may further enhance hepatic FGF21 production and establish a vicious cycle aggravating whole-body insulin resistance.
Figure 6.
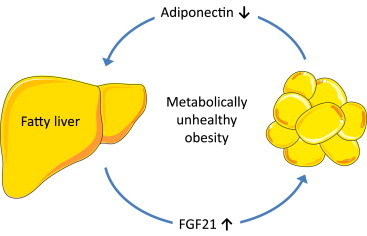
Hypothetical model describing the role of FGF21 and adiponectin in metabolically unhealthy obesity Fatty liver, a hallmark of metabolically unhealthy obesity, produces elevated levels of circulating FGF21 which, in turn, suppress adiponectin production in adipose tissue. As adiponectin acts as a physiological inhibitor of hepatic FGF21 expression, hypoadiponectinaemia results in unblocked FGF21 production in the liver. This establishes a vicious cycle that aggravates whole-body insulin resistance.
One might argue that a bias of our work is that we do not discuss our data with respect to the wealth of findings about FGF21 in mice. However, we are convinced that such a discussion, against the background of obvious species-specific differences with respect to the sites of FGF21 production, the regulation of FGF21 expression, the relationship of circulating FGF21 levels with pathophysiological states, and certain FGF21 effects (as was recently reviewed in [2]), is not helpful as long as we do not know the exact molecular basis of these discrepancies. Therefore, the primary focus of our work was to get closer to an understanding of FGF21's role in humans by discussing our human in vivo and in vitro findings in the light of what is known about FGF21 in humans.
5. Conclusions
The hepatokine FGF21 exerts weak lipogenic and anti-adipogenic effects and marked adiponectin-suppressive and leptin and interleukin-6 release-promoting effects in cultured hasc adipocytes. Together with the markedly higher serum concentrations in MUHO subjects, our findings point to FGF21 as a circulating factor promoting the development of metabolically unhealthy adipocytes and hypoadiponectinaemia. These metabolically unfavorable FGF21 effects could be of particular relevance for the clinical use of FGF21 analogues/mimetics currently in pharmaceutical development.
Conflict of interest
The authors have no conflicts of interest related to this study.
Acknowledgments
We thank Carina Hermann, Christina Lukas, and Bernd Rädle for excellent technical assistance. The study was supported in part by a grant (01GI0925) from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.). The work was also supported by grants from the Helmholtz Portfolio Theme ‘Metabolic Dysfunction and Common Disease’ and the Helmholtz Alliance ‘ICEMED’ to Johannes Beckers. Norbert Stefan is currently funded by a Heisenberg-Professorship from the German Research Foundation (STE 1096/3-1).
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Heatmap displaying FGF21-induced alterations in gene expression Data are shown as small colored boxes representing ratios (FGF21 versus control) of 733 probe sets significantly regulated upon FGF21 treatment (false discovery rate <10%, difference >1.1-fold and <-1.1-fold, average expression >20 in at least one group). Light red – most strongly up-regulated; dark red – modestly up-regulated; dark blue – modestly down-regulated; light blue – most strongly down-regulated.
References
- 1.Angelin B., Larsson T.E., Rudling M. Circulating fibroblast growth factors as metabolic regulators–a critical appraisal. Cell Metabolism. 2012;16:693–705. doi: 10.1016/j.cmet.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Iglesias P., Selgas R., Romero S., Diez J.J. Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. European Journal of Endocrinology. 2012;167:301–309. doi: 10.1530/EJE-12-0357. [DOI] [PubMed] [Google Scholar]
- 3.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coskun T., Bina H.A., Schneider M.A., Dunbar J.D., Hu C.C., Chen Y. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 5.Berglund E.D., Li C.Y., Bina H.A., Lynes S.E., Michael M.D., Shanafelt A.B. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150:4084–4093. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semba R.D., Sun K., Egan J.M., Crasto C., Carlson O.D., Ferrucci L. Relationship of serum fibroblast growth factor 21 with abnormal glucose metabolism and insulin resistance: the Baltimore Longitudinal Study of Aging. Journal of Clinical Endocrinology and Metabolism. 2012;97:1375–1382. doi: 10.1210/jc.2011-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobbert T., Schwarz F., Fischer-Rosinsky A., Pfeiffer A.F., Mohlig M., Mai K. Fibroblast growth factor 21 predicts the metabolic syndrome and type 2 diabetes in Caucasians. Diabetes Care. 2013;36:145–149. doi: 10.2337/dc12-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen Y., Ma X., Zhou J., Pan X., Hao Y., Zhou M. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovascular Diabetology. 2013;12:124. doi: 10.1186/1475-2840-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavez A.O., Molina-Carrion M., Abdul-Ghani M.A., Folli F., Defronzo R.A., Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32:1542–1546. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dushay J., Chui P.C., Gopalakrishnan G.S., Varela-Rey M., Crawley M., Fisher F.M. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefan N., Kantartzis K., Machann J., Schick F., Thamer C., Rittig K. Identification and characterization of metabolically benign obesity in humans. Archives of Internal Medicine. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 12.Stefan N., Haring H.U. The metabolically benign and malignant fatty liver. Diabetes. 2011;60:2011–2017. doi: 10.2337/db11-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohm A., Halama A., Meile T., Zdichavsky M., Lehmann R., Weigert C. Metabolic signatures of cultured human adipocytes from metabolically healthy versus unhealthy obese individuals. PLoS One. 2014;9:e93148. doi: 10.1371/journal.pone.0093148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schling P., Mallow H., Trindl A., Loffler G. Evidence for a local renin angiotensin system in primary cultured human preadipocytes. International Journal of Obesity and Related Metabolic Disorders. 1999;23:336–341. doi: 10.1038/sj.ijo.0800821. [DOI] [PubMed] [Google Scholar]
- 15.Greenberger J.S. Corticosteroid-dependent differentiation of human marrow preadipocytes in vitro. In Vitro. 1979;15:823–828. doi: 10.1007/BF02618309. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez-Zacarias J.L., Castro-Munozledo F., Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 17.Rainer J., Sanchez-Cabo F., Stocker G., Sturn A., Trajanoski Z. CARMAweb: comprehensive R- and bioconductor-based web service for microarray data analysis. Nucleic Acids Research. 2006;34:W498–W503. doi: 10.1093/nar/gkl038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaich G., Chien J.Y., Fu H., Glass L.C., Deeg M.A., Holland W.L. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metabolism. 2013;18:333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Arner P., Pettersson A., Mitchell P.J., Dunbar J.D., Kharitonenkov A., Ryden M. FGF21 attenuates lipolysis in human adipocytes - a possible link to improved insulin sensitivity. FEBS Letters. 2008;582:1725–1730. doi: 10.1016/j.febslet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 20.Mashili F.L., Austin R.L., Deshmukh A.S., Fritz T., Caidahl K., Bergdahl K. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes/Metabolism Research and Reviews. 2011;27:286–297. doi: 10.1002/dmrr.1177. [DOI] [PubMed] [Google Scholar]
- 21.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & Development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chau M.D., Gao J., Yang Q., Wu Z., Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hara K., Yamauchi T., Kadowaki T. Adiponectin: an adipokine linking adipocytes and type 2 diabetes in humans. Current Diabetes Reports. 2005;5:136–140. doi: 10.1007/s11892-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 24.Taniguchi H., Tanisawa K., Sun X., Cao Z.B., Oshima S., Ise R. Cardiorespiratory fitness and visceral fat are key determinants of serum fibroblast growth factor 21 concentration in Japanese men. Journal of Clinical Endocrinology and Metabolism. 2014;99:E1877–E1884. doi: 10.1210/jc.2014-1877. [DOI] [PubMed] [Google Scholar]
- 25.Mutanen A., Heikkila P., Lohi J., Raivio T., Jalanko H., Pakarinen M.P. Serum FGF21 increases with hepatic fat accumulation in pediatric onset intestinal failure. Journal of Hepatology. 2014;60:183–190. doi: 10.1016/j.jhep.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Giannini C., Feldstein A.E., Santoro N., Kim G., Kursawe R., Pierpont B. Circulating levels of FGF-21 in obese youth: associations with liver fat content and markers of liver damage. Journal of Clinical Endocrinology and Metabolism. 2013;98:2993–3000. doi: 10.1210/jc.2013-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y., Lim S., Hong E.S., Kim J.H., Moon M.K., Chun E.J. Serum FGF21 concentration is associated with hypertriglyceridaemia, hyperinsulinaemia and pericardial fat accumulation, independently of obesity, but not with current coronary artery status. Clinical Endocrinology (Oxford) 2014;80:57–64. doi: 10.1111/cen.12134. [DOI] [PubMed] [Google Scholar]
- 28.Reinehr T., Woelfle J., Wunsch R., Roth C.L. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: a longitudinal analysis. Journal of Clinical Endocrinology and Metabolism. 2012;97:2143–2150. doi: 10.1210/jc.2012-1221. [DOI] [PubMed] [Google Scholar]
- 29.Tan B.K., Hallschmid M., Adya R., Kern W., Lehnert H., Randeva H.S. Fibroblast growth factor 21 (FGF21) in human cerebrospinal fluid: relationship with plasma FGF21 and body adiposity. Diabetes. 2011;60:2758–2762. doi: 10.2337/db11-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X., Yeung D.C., Karpisek M., Stejskal D., Zhou Z.G., Liu F. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 31.Cuevas-Ramos D., Almeda-Valdes P., Gomez-Perez F.J., Meza-Arana C.E., Cruz-Bautista I., Arellano-Campos O. Daily physical activity, fasting glucose, uric acid, and body mass index are independent factors associated with serum fibroblast growth factor 21 levels. European Journal of Endocrinology. 2010;163:469–477. doi: 10.1530/EJE-10-0454. [DOI] [PubMed] [Google Scholar]
- 32.Dostalova I., Kavalkova P., Haluzikova D., Lacinova Z., Mraz M., Papezova H. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. Journal of Clinical Endocrinology and Metabolism. 2008;93:3627–3632. doi: 10.1210/jc.2008-0746. [DOI] [PubMed] [Google Scholar]
- 33.Haluzikova D., Lacinova Z., Kavalkova P., Drapalova J., Krizova J., Bartlova M. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity. (Silver. Spring) 2013;21:1335–1342. doi: 10.1002/oby.20208. [DOI] [PubMed] [Google Scholar]
- 34.Novotny D., Vaverkova H., Karasek D., Lukes J., Slavik L., Malina P. Evaluation of total adiponectin, adipocyte fatty acid binding protein and fibroblast growth factor 21 levels in individuals with metabolic syndrome. Physiological Research. 2014;63:219–228. doi: 10.33549/physiolres.932602. [DOI] [PubMed] [Google Scholar]
- 35.Wang D., Zhu W., Li J., An C., Wang Z. Serum concentrations of fibroblast growth factors 19 and 21 in women with gestational diabetes mellitus: association with insulin resistance, adiponectin, and polycystic ovary syndrome history. PLoS One. 2013;8:e81190. doi: 10.1371/journal.pone.0081190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharitonenkov A., Beals J.M., Micanovic R., Strifler B.A., Rathnachalam R., Wroblewski V.J. Rational design of a fibroblast growth factor 21-based clinical candidate, LY2405319. PLoS One. 2013;8:e58575. doi: 10.1371/journal.pone.0058575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Combs T.P., Wagner J.A., Berger J., Doebber T., Wang W.J., Zhang B.B. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 38.Maeda N., Takahashi M., Funahashi T., Kihara S., Nishizawa H., Kishida K. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 39.Fasshauer M., Kralisch S., Klier M., Lossner U., Bluher M., Klein J. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochemical and Biophysical Research Communications. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 40.Sopasakis V.R., Sandqvist M., Gustafson B., Hammarstedt A., Schmelz M., Yang X. High local concentrations and effects on differentiation implicate interleukin-6 as a paracrine regulator. Obesity Research. 2004;12:454–460. doi: 10.1038/oby.2004.51. [DOI] [PubMed] [Google Scholar]
- 41.Stefan N., Haring H.U., Hu F.B., Schulze M.B. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes & Endocrinology. 2013;1:152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 42.Li H., Fang Q., Gao F., Fan J., Zhou J., Wang X. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. Journal of Hepatology. 2010;53:934–940. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 43.Wanninger J., Liebisch G., Eisinger K., Neumeier M., Aslanidis C., Voggenreiter L. Adiponectin isoforms differentially affect gene expression and the lipidome of primary human hepatocytes. Metabolites. 2014;4:394–407. doi: 10.3390/metabo4020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M., Zhang L., Wang C., Liu H., Boden G., Yang G. Liraglutide increases FGF-21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PLoS One. 2012;7:e48392. doi: 10.1371/journal.pone.0048392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heatmap displaying FGF21-induced alterations in gene expression Data are shown as small colored boxes representing ratios (FGF21 versus control) of 733 probe sets significantly regulated upon FGF21 treatment (false discovery rate <10%, difference >1.1-fold and <-1.1-fold, average expression >20 in at least one group). Light red – most strongly up-regulated; dark red – modestly up-regulated; dark blue – modestly down-regulated; light blue – most strongly down-regulated.


