Abstract
AIM: To investigate the effect of all-trans-retinoic acid (ATRA) on arsenic trioxide (As2O3)-induced apoptosis of human hepatoma, breast cancer, and lung cancer cells in an attempt to find a better combination therapy for solid tumors.
METHODS: Human hepatoma cell lines HepG2, Hep3B, human breast cancer cell line MCF-7, and human lung adenocarcinoma cell line AGZY-83-a were treated with As2O3 together with ATRA. Cell survival fraction was determined by MTT assay, cell viability and apoptosis were measured by annexin V-fluorescein isothiocyanate (FITC) and PI staining, and intracellular glutathione (GSH) and glutathione-S-transferase (GST) activities were determined using commercial kits.
RESULTS: Cytotoxicity of ATRA was low. ATRA (0.1, 1, and 10 μmol/L) could synergistically potentiate As2O3 to exert a dose-dependent inhibition of growth and to induce apoptosis in each of the cell lines. HepG2 and Hep3B with low intracellular GSH or GST activities were remarkably sensitive to As2O3 or As2O3+ATRA, while AGZY-83-a with higher GSH or GST activities was less sensitive to As2O3 or As2O3+ATRA. Treatment with 2 μmol/L As2O3 for 72 h significantly decreased intracellular GSH and GST levels in each of the cell lines, and 1 μmol/L ATRA alone reduced minimal intracellular GSH and GST levels. ATRA potentiated the effect of As2O3 on intracellular GSH levels, but intracellular GST levels were not significantly affected by the combination of As2O3 and ATRA for 72 h as compared to As2O3 alone.
CONCLUSION: ATRA can strongly potentiate As 2O3-induced growth-inhibition and apoptosis in each of the cell lines, and two drugs can produce a significant synergic effect. The sensitivity to As2O3 or As2O3+ATRA is inversely proportional to intracellular GSH or GST levels in each of the cell lines. The GSH redox system may be the possible mechanism by which ATRA synergistically potentiates As2O3 to exert a dose-dependent inhibition of growth and to induce apoptosis.
Keywords: Arsenic trioxide, All-trans-retinoic acid, Hepatocellular carcinoma, Cancer of breast and lung, Glutathione, Glutathione-S-transferase
INTRODUCTION
Liver, breast, and lung cancers are the most common malignant diseases in the human race. Surgical resection or radiation therapy is potentially curative for localized diseases. Advanced liver, breast, and lung cancers are associated with a poor prognosis, and conventional chemotherapies and radiation therapy are still of limited effectiveness. Innovative approaches for advanced disease are necessary.
During the last decade, investigators using As2O3 alone or the combination of all-trans-retinoic acid (ATRA) and As2O3 for patients with acute promyelocytic leukemia (APL), have achieved great success[1-3]. Because of the many pathways involved in mediating the effects of arsenic, the potential exists for synergism with other agents to provide enhanced therapeutic benefits. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of APL[4,5].
Other studies have also shown that As2O3 or ATRA alone has antiproliferative and apoptotic activities in some solid tumors, including human hepatoma and breast cancer[6-8]. Because of the drug resistance of solid tumors to As2O3, it has not been widely used in the treatment of solid tumors. Therefore, this study was to investigate the effect of ATRA on As2O3-induced cell apoptosis in human hepatoma, breast cancer, and lung cancer in an attempt to find a better combination therapy for solid tumors. To our knowledge, this is the first report on the effects of the combination of As2O3 and ATRA for solid tumors.
The glutathione (GSH) redox system is known to modulate the growth-inhibitory and apoptotic effect of As2O 3. Different kinds of malignant cells have different GSH levels. It was reported that the GSH redox system is relative to sensitivity of malignant cells to As2O3[9]. Elevated GSH levels are associated with the chemoresistance of malignant cells. Optimal therapies for chemoresistant malignant cells should overcome or bypass the increased intracellular GSH levels. Glutathione-S-transferase (GST), an enzyme involved in metabolic detoxification of a variety of xenobiotics, is increased in an arsenic-resistant CHO cell line[10,11]. Therefore, we also measured intracellular GSH and GST levels in malignant cells, when they were treated in the absence or presence of As2O3, ATRA, or As2O3+ATRA.
MATERIALS AND METHODS
Materials
Human hepatoma cell lines HepG2, Hep3B, human breast cancer cell line MCF- 7 (American Type Culture Collection, Rockville, MD, USA) and human lung adenocarcinoma cell line AGZY-83-a (Harbin Medical University) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mmol/L L- glutamine, and 10% heat-inactivated fetal bovine serum in a humidified atmosphere of 50 mL/L CO2 at 37°C. Cells in logarithmic growth were seeded at 1×105 cells/mL for studies performed in duplicate and repeated at least thrice. As2O3 solution (0.1%) was purchased from Harbin Medical University (Harbin, China). ATRA, MTT, and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Cell proliferation and cell survival rate tested with MTT
Cell proliferation was measured by a MTT assay. For proliferation assays, cells were plated onto 96-well plates (2×103 cells/well, 200 μL cell suspension per well) and cultured overnight to allow for cell attachment. Cells were then treated with drugs of different concentrations in the absence (control) or presence of As2O3 (0.5, 1, 1.5, and 2 μmol/L), ATRA (0.1, 1, and 10 μmol/L), or As2O3+ATRA. All groups had concentrations of four compound wells. After incubation for 72 h, 20 μL of 0.5% MTT was added to each well and incubated for another 4 h. The supernatant was discarded and 150 μL of DMSO was added. When the stain was dissolved, the optical density ABS (absorbance) value of each well was read on Minireader II at 490 nm. Cell survival rate was calculated with the following equation: average A value of experimental group/ average A value of control group×100%. Each experiment was repeated at least thrice.
Quantitation of cell viability and apoptotic cells
Cell viability and apoptosis were measured by annexin V-fluorescein isothiocyanate (FITC) (Becton Dickinson) and PI staining. Cells were plated onto six-well dishes (1×105 cells/dish) and grown overnight to allow for cell attachment. They were then treated in the absence (control) or presence of As2O3 (2 μmol/L), ATRA (1 and 10 μmol/L), or As2O3+ATRA (1 and 10 μmol/L) for 72 h. After the indicated time, cells were harvested, washed once in PBS and stained with annexin V-FITC (Biovision) and PI (2 mg/mL) according to manufacturer’s instructions. Samples were acquired on a FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA) and analyzed with CellQuest software (Becton Dickinson).
Intracellular glutathione measurements
Intracellular GSH levels were determined in all cell lines. Cells were plated onto six-well dishes (1×105 cells/dish) and grown overnight to allow for cell attachment. They were then treated in the absence (control) or presence of As2O3 (2 μmol/L), ATRA (1 μmol/L), or As2O3+ATRA for 72 h. After the indicated time, cells were harvested, and intracellular GSH was measured using the GSH assay kit (Calbiochem, San Diego, CA, USA). Briefly, cells were pelleted, resuspended in 500 μL ice-cold 5% metaphosphoric acid, and homogenized with a Teflon pestle and an overhead stirrer. After centrifugation at 3 000 r/min for 10 min at 4°C, 60 μL supernatant, 60 μL solution R1, 2 μL solution R2, and 1 mL solution R3 were combined according to the manufacturer’s instructions. Samples were incubated at room temperature for 5 min in the dark, and the final absorbance was measured at 412 nm and compared to a GSH standard curve. To quantitate the total protein, the pellet from the above centrifugation was resuspended in 1 mol/L NaOH, and the protein concentration was measured using the Bio-Rad DC protein assay (Bio-Rad, Hercules, CA, USA). Intracellular GSH was normalized to total protein content.
Measurement of intracellular GST
GST activity was determined using commercial kits (Calbiochem, San Diego, CA, USA). GST activity was measured using 1-chloro- 2,4-dinitrobenzene (CDNB) and GSH as substrates. Cells were plated onto six-well dishes (1×105 cells/dish) and grown overnight to allow for cell attachment. They were then treated in the absence (control) or presence of As2O3 (2 μmol/L), ATRA (1 μmol/L), or As2O3+ATRA for 72 h. After the indicated time, cells were harvested. The cell pellets were resuspended in 300 μL of 100 mmol/L potassium phosphate buffer, pH 6.8, sonicated for 10 s at 4°C, and centrifuged at 14 000 g for 30 min. Supernatant was used for GST measurement according to the manufacturer’s instructions. The absorbance at 412 nm was continuously recorded for 2 min. The pellet was dissolved in 1 mol/L NaOH and analyzed for protein by Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). The GST content was expressed as nanomoles per milligram protein per minute.
RESULTS
Statistical analysis
Data were presented graphically as mean±SD. Treatment groups were compared by independent t-test or paired t test as appropriate, with P reported in each figure legend. Statistical analyses were performed using SPSS 10.1 software.
Effects of ATRA and As2O3 on growth inhibition in each cell line
To examine the possible relationship between serum concentration and growth-inhibitory effects of the two drugs, cells were treated with drugs of different concentrations in the absence (control) or presence of As2O3 (0.5, 1, 1.5, and 2 μmol/L), ATRA (0.1, 1, and 10 μmol/L), or As2O3+ATRA for 72 h, and then cell growth was measured by MTT assays.
The ATRA alone at 0.1, 1, and 10 μmol/L could only moderately inhibit cell growth in each of the cell lines. As2O3 exerted a dose-dependent growth inhibition of the HepG2, Hep3B, MCF-7, AGZY-83-a cells (Figures 1A-D).
Figure 1.
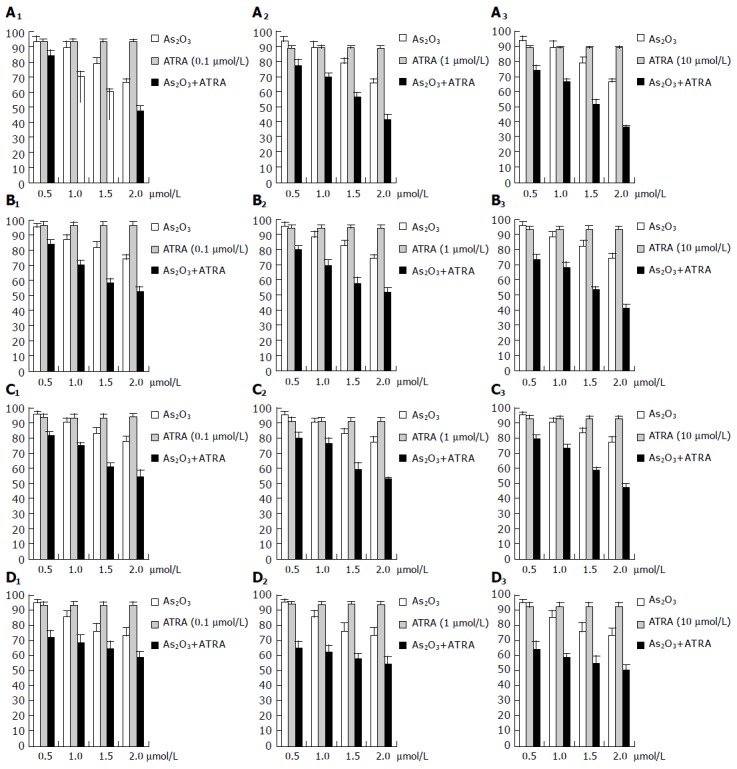
Effects of ATRA and As2O3 on growth inhibition in cancer cell lines HepG2 (A), Hep3B (B), MCF-7 (C), and AGZY-83-a (D).
Interestingly, the combination of ATRA (0.1, 1, and 10 μmol/L) and As2 O3 had at least an additive effect on growth inhibition in each of the cell lines. ATRA (0.1, 1, and 10 μmol/L) could synergistically potentiate As2O 3 to exert a dose-dependent growth inhibition in each of the cell lines. High concentration of ATRA (10 μmol/L) exerted greater synergistic effects on growth inhibition than low concentration (0.1 μmol/L, Figures 1A-D).
Effects of two drugs on apoptosis in each cell line
Cytotoxicity of ATRA was low. The apoptotic rate of 1 or 10 μmol/L of ATRA approached to that of control in each of the cell lines, and the difference was not significant (P>0.05). ATRA greatly potentiated the apoptosis induced by As2 O3. Exposure of the cells to the combination of As2O3 (2 μmol/L) and ATRA (10 μmol/L) for 72 h synergistically induced apoptosis in HepG2 (61.1 ± 5.6%), Hep3B (57.1 ± 4.9%), MCF -7 (45.5 ± 3.0%), AGZY-83-a (40.8 ± 5.6%) respectively, as measured by annexin V-FITC and PI staining as compared to exposure of the cells to either agent alone (Figure 2A). A similar, dramatic effect was observed, when they were treated in the absence (control) or presence of As2 O3 (2 μmol/L), ATRA (1 μmol/L), or As2O3+ATRA for 72 h, with HepG2 being 48.4 ± 4.1%, Hep3B being 49.3 ± 4.2%, MCF-7 being 40.2 ± 3.3%, AGZY-83-a being 32.9 ± 3.0% respectively (Figure 2B). All these findings indicated that ATRA could synergistically potentiate As2O3 to induce apoptosis in each of the cell lines.
Figure 2.
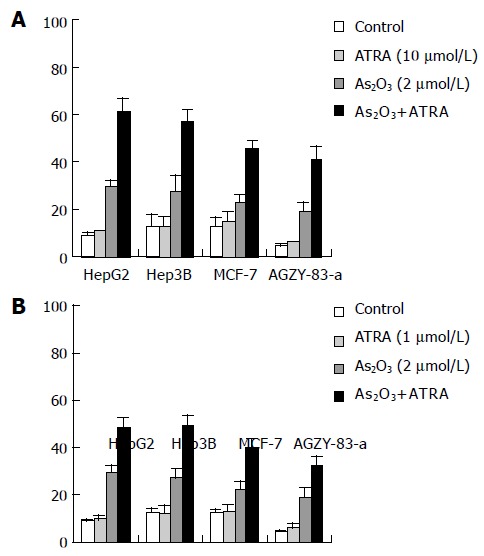
Effects of combined ATRA (10 mol/L: A, 1 mol/L: B) and As2O3, and either agent alone on apoptosis in cancer cell lines.
Changes of intracellular GSH and GST
HepG2 and Hep3B with low intracellular GSH or GST activities, were remarkably sensitive to 2 μmol/L As2O3 or As2O3 (2 μmol/L)+ATRA (10 μmol/L), the apoptotic rate was 29.3 ± 3.2% and 61.1 ± 5.6%, respectively. AGZY-83-a with higher GSH or GST activities was less sensitive to As2O3 or As 2O3+ATRA, the apoptotic rate was 19.0 ± 3.8% and 40.8 ± 5.6%, respectively (Table 1). The sensitivity to As2O3 or As2O3+ATRA was inversely proportional to intracellular GSH or GST levels in each of the cell lines. A similar, dramatic effect was observed when they were treated in the presence of As2O3 (2 μmol/L), or As2O3 (2 μmol/L)+ATRA (1 μmol/L) for 72 h (Table 1).
Table 1.
Basal activities of GSH or GST and As2O3+ ATRA or As2O3-induced apoptosis in different cell lines (mean±SD)
| Cell lines | GSH | GST | As2O3 |
Apoptosis (%) |
|
| As2O3+ATRA (10 μmol/L) | As2O3+ ATRA (1 μmol/L) | ||||
| HepG2 | 60.7 ± 7.1 | 22.3 ± 1.4 | 29.3 ± 3.2 | 61.1 ± 5.6 | 48.4 ± 4. 1 |
| Hep3B | 62.4 ± 5.4 | 21.4 ± 2.8 | 27.3 ± 6.5 | 57.1 ± 4.9 | 49.3 ± 4.2 |
| MCF-7 | 76.0 ± 5.1 | 31.0 ± 2.6 | 22.6 ± 3.3 | 45.5 ± 3.0 | 40.2 ± 3.3 |
| AGZY-83-a | 104.8 ± 8.1 | 50.6 ± 4.5 | 19.0 ± 3.8 | 40.8 ± 5.6 | 32.9 ± 3.0 |
Dramatic changes of intracellular GSH or GST activities were observed when the cell lines were treated in the absence (control) or presence of As2O 3 (2 μmol/L), ATRA (1 μmol/L), or As 2O3 +ATRA for 72 h. Treatment with 2 μmol/L As2O3 for 72 h significantly decreased the intracelluar GSH and GST levels in each of the cell lines, and 1 μmol/L ATRA alone reduced minimal intracellular GSH and GST levels. ATRA potentiated the effect of As2O3 on intracellular GSH levels, but intracellular GST levels were not significantly affected by the combination of As2O3 (2 μmol/L) and ATRA (1 μmol/L) for 72 h as compared to As O alone (Tables 2 and 3).
Table 2.
Effect of As2O3 on intracellular GSH levels (mean±SD)
| Cell lines | Control | ATRAa | As2O3c | As2O3+ATRAe |
| HepG2 | 60.7 ± 7.1 | 54.4 ± 4.9 | 46.7 ± 9.0 | 26.8 ± 4.4 |
| Hep3B | 62.4 ± 5.4 | 55.6 ± 0.5 | 35.3 ± 3.0 | 25.9 ± 3.6 |
| MCF-7 | 76.0 ± 5.1 | 69.9 ± 1.7 | 52.5 ± 2.4 | 44.1 ± 3.8 |
| AGZY-83-a | 104.8 ± 8.1 | 99.8 ± 9.4 | 89.8 ± 5.6 | 80.4 ± 1.0 |
P > 0.05,
P < 0.05 vs the control cells,
P < 0.05 vs As2O3-treated cells.
Table 3.
Changes in intracellular GST activities (mean±SD)
| Cell lines | Control | ATRAa | As2O3c | As2O3+ATRAe |
| HepG2 | 22.3 ± 1.4 | 21.2 ± 1.7 | 5.5 ± 1.8 | 8.6 ± 2.3 |
| Hep3B | 21.4 ± 2.8 | 20.4 ± 4.4 | 8.6 ± 1.8 | 10.3 ± 3.4 |
| MCF-7 | 31.0 ± 2.6 | 29.7 ± 3.0 | 16.4 ± 1.6 | 19.3 ± 1.4 |
| AGZY-83-a | 50.6 ± 4.5 | 49.3 ± 4.7 | 36.3 ± 0.9 | 39.3 ± 3.1 |
P > 0.05,
P < 0.05 vs the control cells,
P > 0.05 vs As2O3-treated cells.
DISCUSSION
Our in vitro studies showed that the combination of As2O3 and ATRA was statistically superior to either As2O3 or ATRA alone in the treatment of the three cell lines. Furthermore, ATRA (0.1, 1, and 10 μmol/L) could synergistically potentiate As2O3 to exert a dose-dependent growth inhibition in each of the cell lines. Cell survival rate could be reduced from 89.5 ± 3.9% to 70.5 ± 3.3% in HepG2 cells exposed to 1 μmol/L As2O 3 or the combination of 1 μmol/L As2O3 and 0.1 μmol/L ATRA for 72 h.
Apoptosis is important for the development and homeostasis of multicellular organisms. Specific therapies have been designed to enhance the susceptibility of human cancers to apoptosis. We showed that the combination of As2 O3 and ATRA dramatically and significantly increased the number of apoptotic cells in each of the cell lines, especially the human hepatoma cell lines HepG2 and Hep3B.
Drug resistance of cancer cells and toxicity of cell apoptotic agents are the major factors contributing to the failure of chemotherapy. At least four distinct mechanisms contribute to the chemoresistance. Cellular response to steroids typically depends on the expression of the glucocorticoid receptor, and resistance to steroid therapies is classically associated with downregulation or loss of glucocorticoid receptor expression in some malignant cells[12,13]. The overexpression of drug efflux pumps, such as mdr gene product P-glycoprotein (PgP), is also a common trait of chemoresistant malignant cells[14,15]. In addition, it was reported that the expression of the antiapoptotic protein Bcl-xL is higher in some chemoresistant malignant cells[16]. Furthermore, increased expression or activity of GSH and GSH-related enzymes confers resistance to antineoplastic agents[17,18]. GSH is the major auto-oxidant of the cells, and functions to scavenge free radicals and to detoxify toxins and chemotherapeutic agents. GSH can bind to arsenic by the formation of a transient As(GS)3 complex[19].
The GSH redox system is known to modulate the growth-inhibitory and apoptotic effect of As2 O3. Different kinds of malignant cells have different GSH levels. It was reported that the GSH redox system is relative to sensitivity of malignant cells to As2O3[9]. Elevated GSH levels are associated with the chemoresistance of malignant cells. Optimal therapies for chemoresistant malignant cells should overcome or bypass the increased intracellular GSH levels. It is known that GST, an enzyme involved in metabolic detoxification of a variety of xenobiotics, is increased in an arsenic-resistant CHO cell line[10]. Our study showed that HepG2 and Hep3B with low intracellular GSH or GST activities were remarkably sensitive to As2O3 or As2O3+ATRA, while AGZY-83-a with higher GSH or GST activities was less sensitive to As2O3 or As2O3+ATRA. The sensitivity to As2O3 or As 2O3+ATRA was inversely proportional to GSH or GST levels in each of the cell lines.
Arsenite can decrease GSH levels which can result in DNA damage as a result of increased intracellular reactive oxygen molecules. Our intracellular GSH and GST assays showed that treatment with 2 μmol/L As2O3 for 72 h significantly decreased intracellular GSH and GST levels in each of the cell lines, and 1 μmol/L ATRA alone reduced minimal intracellular GSH and GST levels (Tables 2 and 3). ATRA potentiated the effect of As2O3 on intracellular GSH levels, but intracellular GST levels were not significantly affected by the combination of As2O3 (2 μmol/L) and ATRA (1 μmol/L) for 72 h as compared to As2O3 alone. These findings indicate that GSH redox system may be the possible mechanism by which ATRA synergistically potentiates As2O3 to induce apoptosis or to exert a dose-dependent inhibition of growth. Either As2O3 alone or in combination with ATRA may become a useful adjuvant therapy for liver, breast, and lung cancers.
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
Supported by the Key Scientific and Technological Projects of Heilongjiang Province during the 10th Five-Year Plan Period, No. GB05C401-10
References
- 1.Avvisati G, Lo Coco F, Mandelli F. Acute promyelocytic leukemia: clinical and morphologic features and prognostic factors. Semin Hematol. 2001;38:4–12. [PubMed] [Google Scholar]
- 2.Fenaux P, Chomienne C, Degos L. All-trans retinoic acid and chemotherapy in the treatment of acute promyelocytic leukemia. Semin Hematol. 2001;38:13–25. doi: 10.1016/s0037-1963(01)90002-2. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Chen GQ, Shen ZX, Chen SJ, Wang ZY. Treatment of acute promyelocytic leukemia with arsenic compounds: in vitro and in vivo studies. Semin Hematol. 2001;38:26–36. doi: 10.1053/shem.2001.20863. [DOI] [PubMed] [Google Scholar]
- 4.Lallemand-Breitenbach V, Guillemin MC, Janin A, Daniel MT, Degos L, Kogan SC, Bishop JM, de The H. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J Exp Med. 1999;189:1043–1052. doi: 10.1084/jem.189.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rego EM, He LZ, Warrell RP, Wang ZG, Pandolfi PP. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARalpha and PLZF-RARalpha oncoproteins. Proc Natl Acad Sci USA. 2000;97:10173–10178. doi: 10.1073/pnas.180290497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T, Wang SS, Hong L, Wang XL, Qi QH. Arsenic trioxide induces apoptosis of rat hepatocellular carcinoma cells in vivo. J Exp Clin Cancer Res. 2003;22:61–68. [PubMed] [Google Scholar]
- 7.Vernhet L, Allain N, Le Vée M, Morel F, Guillouzo A, Fardel O. Blockage of multidrug resistance-associated proteins potentiates the inhibitory effects of arsenic trioxide on CYP1A1 induction by polycyclic aromatic hydrocarbons. J Pharmacol Exp Ther. 2003;304:145–155. doi: 10.1124/jpet.102.042176. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Kong L, Zhao J, Yang P. Arsenic trioxide in the mechanism of drug resistance reversal in MCF-7/ADM cell line of human breast cancer. Zhonghua ZhongLiu ZaZhi. 2002;24:339–343. [PubMed] [Google Scholar]
- 9.Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–277. [PubMed] [Google Scholar]
- 10.Lee TC, Wei ML, Chang WJ, Ho IC, Lo JF, Jan KY, Huang H. Elevation of glutathione levels and glutathione S-transferase activity in arsenic-resistant Chinese hamster ovary cells. In Vitro Cell Dev Biol. 1989;25:442–448. doi: 10.1007/BF02624629. [DOI] [PubMed] [Google Scholar]
- 11.Lo JF, Wang HF, Tam MF, Lee TC. Glutathione S-transferase pi in an arsenic-resistant Chinese hamster ovary cell line. Biochem J. 1992;288(Pt 3):977–982. doi: 10.1042/bj2880977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moalli PA, Pillay S, Weiner D, Leikin R, Rosen ST. A mechanism of resistance to glucocorticoids in multiple myeloma: transient expression of a truncated glucocorticoid receptor mRNA. Blood. 1992;79:213–222. [PubMed] [Google Scholar]
- 13.Gomi M, Moriwaki K, Katagiri S, Kurata Y, Thompson EB. Glucocorticoid effects on myeloma cells in culture: correlation of growth inhibition with induction of glucocorticoid receptor messenger RNA. Cancer Res. 1990;50:1873–1878. [PubMed] [Google Scholar]
- 14.Epstein J, Xiao HQ, Oba BK. P-glycoprotein expression in plasma-cell myeloma is associated with resistance to VAD. Blood. 1989;74:913–917. [PubMed] [Google Scholar]
- 15.Dalton WS, Durie BG, Alberts DS, Gerlach JH, Cress AE. Characterization of a new drug-resistant human myeloma cell line that expresses P-glycoprotein. Cancer Res. 1986;46:5125–5130. [PubMed] [Google Scholar]
- 16.Tu Y, Renner S, Xu F, Fleishman A, Taylor J, Weisz J, Vescio R, Rettig M, Berenson J, Krajewski S, et al. BCL-X expression in multiple myeloma: possible indicator of chemoresistance. Cancer Res. 1998;58:256–262. [PubMed] [Google Scholar]
- 17.Gupta V, Singh SV, Ahmad H, Medh RD, Awasthi YC. Glutathione and glutathione S-transferases in a human plasma cell line resistant to melphalan. Biochem Pharmacol. 1989;38:1993–2000. doi: 10.1016/0006-2952(89)90499-1. [DOI] [PubMed] [Google Scholar]
- 18.Bellamy WT, Dalton WS, Gleason MC, Grogan TM, Trent JM. Development and characterization of a melphalan-resistant human multiple myeloma cell line. Cancer Res. 1991;51:995–1002. [PubMed] [Google Scholar]
- 19.Scott N, Hatlelid KM, MacKenzie NE, Carter DE. Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem Res Toxicol. 1993;6:102–106. doi: 10.1021/tx00031a016. [DOI] [PubMed] [Google Scholar]


