Abstract
AIM: To elucidate the biological function of HBV core antigen (HBcAg) on pathogenesis of hepatitis B, a novel gene C12 coding for protein with unknown function interacting with HBcAg in hepatocytes was identified and characterized.
METHODS: HBcAg bait plasmid pGBKT7-HBcAg was constructed and transformed into yeast AH109, then the transformed yeast was mated with yeast Y187 containing liver complementary DNA (cDNA) library plasmid in 2×YPDA medium. Diploid yeast was plated on synthetic dropout nutrient medium (SD/-Trp-Leu-His-Ade) and synthetic dropout nutrient medium (SD/-Trp-Leu-His-Ade) containing X-α -gal for screening twice. After extracting and sequencing of plasmid from blue colonies, we isolated a cDNA clone encoding a novel protein designated as C12 that directly interacted with HBcAg. The interaction between HBcAg and C12 was verified again by re-mating. pEGFP-N1-C12 fluorescent protein fusion gene was transfected in 293 and L02 cell, and observed by fluorescent microscope. MTT reduction assay was used to study the action of C12 protein effect on metabolism of mammal cell. Yeast two-hybrid and cDNA microarray were performed to search binding protein and differential expression genes regulated by C12 protein.
RESULTS: C12 gene was screened and identified by yeast two-hybrid system 3. The interaction between HBcAg and the novel protein coded by the new gene C12 was further confirmed by re-mating. After 48 h, fluorescence of fusion protein could be observed steadily in the 293 and L02 cell plasma. Under MTT assay, we found that the expression of C12 did not influence the growth of liver cells. Seventeen differential expression genes in HepG2 cells transfected with C12 protein expression plasmid by cDNA microarray, of which 16 genes were upregulated and 1 gene was downregulated by C12 protein. Twenty-one colonies containing 16 different genes coding for C12 protein binding proteins were isolated by yeast two-hybrid, there were 2 new genes with unknown function.
CONCLUSION: The novel protein C12 is located in cell plasma, and its overexpression has no significant effect on the metabolism of liver cell. It interacts with many proteins in hepatocytes and may be involved in many processes of gene expression.
Keywords: Hepatitis B virus core antigen, Yeast two-hybrid, cDNA microarray, MTT assay
INTRODUCTION
There are more than 400 million carriers of HBV worldwide who are at high risk for the development of chronic liver diseases, but the precise mechanism of virus involvement remains elusive[1,2]. Identification of cellular HBV core antigen (HBcAg) interaction protein would provide insights into the mechanism of HBV cellular effects[3-5]. Based on the yeast two-hybrid screening and bio-information assay, we found a novel gene named C12 coding for protein binding with HBcAg, and located in cell plasma. After the finishing of human genome project, we obtained mass information of chromosome DNA including many sequences of coding gene with unknown functions. While DNA constitutes the information archive of the genome, it is the proteins that actually serve as the functional effectors of cellular processes. How to elucidate the structure, biological function and medical significance of protein coding by novel genes, are the most difficult and complex work in future. By means of MTT reduction assay, complementary DNA (cDNA) microarray and yeast two -hybrid technique, the potential biological function of C12 protein was initially investigated in the context of effect on cell metabolism and gene expression, the binding protein in hepatocytes. We expected the results would bring us some valuable information and pave the way for further study.
MATERIALS AND METHODS
Plasmid construction
The HBcAg gene from a known HBV DNA sequence (subtype ayw) was amplified by PCR (P1 3’-GAA TTC ATG GAC ATC GAC CCT TAT AA-5’, P2 3’-CTG CAG AAC ATT GAG ATT CCC GAG AT-5’) and cloned into the DNA-binding domain vector pGBKT7 (Clontech, Palo Alto, CA, USA). This plasmid contains TRP1 gene, permitting it to grow in trp medium. A normal human adult liver cDNA stretch library was cloned into the activation domain vector pACT2 and transformed into yeast strain Y187 to build pretransformed cDNA liver cell library (Clontech Co., USA); pACT2 plasmid contain Leu2 gene (Clontech Co., USA), permitting it to grow in leu-medium[6-9].
Western blotting
The plasmid pGBKT7-HBcAg containing full length of HBcAg gene was constructed by insertion of HBcAg gene in-frame into EcoRI/BamHI site, and could directly express DNA binding domain, c-myc and core fusion protein was transformed into yeast strain AH109 by using lithium acetate method. Yeast protein of AH109 was extracted and Western blotting was performed to confirm the expression of the fusion protein by using c-myc mAb. Transformed AH109 was cultured on quadrupole dropout media to exclude the auto-activity.
Two-hybrid library screen and cDNA isolation
One large (2-3 mm), fresh ( < 2 mo old) colony of AH109 containing bait plasmid was inoculated into 50 mL of SD/-Trp and incubated at 30°C overnight (16-24 h) with shaking at 250-270 r/min. Then the cells were spun down by centrifuging the entire 50- mL culture at 1 000 r/min for 5 min and supernatant was decanted, the cell pellet was resuspended in the residual liquid by vortexing. The entire AH109 (bait) culture and the 1-mL library were combined and cultured in a 2-L sterile flask and 45 mL of 2× YPDA/ Kan was added and swirled gently. After 20-h mating, the cells were spun down, resuspended and then spread on 50 large (150-mm) plates, containing 200 mL of SD/-Ade/ -His/-Leu/-Trp. After 6-18 d of growth, the yeast colonies were transferred onto the plates containing X-α -gal to check for expression of the MEL1 reporter gene (blue colonies). Blue colonies were considered to be true positives and were discarded. Yeast plasmid DNA was isolated with lyticase method (Clontech), and transformed into E. coli by using electroporation; transformants were plated on ampicillin LB selection media and analyzed by DNA sequencing.
DNA sequencing and computation
cDNA inserts were sequenced with the Sequenase version 2.0 sequencing kit (Boya Biochemical Corp, China) based on the dideoxy method. Homology searches for the DNA sequences thus determined and their deduced amino acid sequences were performed at the National Center for Biotechnology Information with the BLAST network service.
RT-PCR and re-mating
Based on the information of GenBank, PCR primers of the novel gene C12 were designed (P3 5’-GAA TTC ATG ATA AGT GAA GGC GGA TG-3’, P4 5’-GGA TCC CTA TTG TTA ACA GAG GAC T-3’), and the full-length gene of C12 was amplified by using reverse transcription polymerase chain reaction (RT-PCR) by using genomic DNA from HepG2 cell line as the template. C12 gene was inserted into yeast expression vector pGADT7 and transformed into yeast strain Y187, then re-mated with AH109 containing pGBKT7-HBcAg. After mating, the diploid yeasts were plated on QDO covered with X-α -gal for further verification[10].
Sub-cellular localization
Full-length C12 gene was amplified by PCR with primers (P5 5’-GAA TTC ATG ATA AGT GAA GGC GGA TG-3’, P6 5’-GGA TCC TTG TTA ACA GAG GAC TTT T-3’) and inserted into green fluorescent protein (GFP) expression vector pEGFP-N1 (Promega Co., USA) . The human liver cell line L02 and African green monkey renal cell line 293 was incubated in DMEM medium with 10% fetal bovine serum (FBS), 200 µmol/L L-glutamine, penicillin, streptomycin, and plated at a density of 1×104 /well in 35-mm dishes. Purified plasmid pEGFP-N1-C12 was transfected into 293 and L02 cell by using Lipofectamine PLUS (Gibco Co., USA) and observed under fluorescent microscope after 48-h incubation.
MTT reduction assay
L02 and Bel7402 cells were respectively divided into four groups; they were empty cell group, liposomes group, pEGFP-N1 group, and pEGFP-N1 -C12 group. Each group included six wells and each well was incubated with 1×104 cells. Deionized water, liposomes, pEGFP-N1, and pEGFP-N1-C12 were transfected into corresponding groups and incubated for 18 h in DMEM medium with 10% FBS at 37°C (Roche Co., USA). Cells were incubated for next 4 h with DMEM without FBS, and 750 µL DMSO was added in each well followed by discarding the medium and washing with 1× PBS. After mixing steadily, the absorbance of samples was measured (detection wavelength 550 nm, reference wavelength 650 nm) to quantify cell number.
Screening of binding protein
pGBKT7-C12 bait plasmid was constructed by inserting C12 full-length gene into yeast expression vector pGBKT7 and transfected into AH109 cell. Positive AH109 cell mated with pretransformed cDNA liver cell library Y187 cell for screening twice. Blue colonies were discarded and analyzed by DNA sequencing and bioinformatic assay.
cDNA microarray assay
HepG2 cell line transfected with pcDNA3.1(-)- C12 and pcDNA3.1(-) were incubated at DMEM medium for 48 h. mRNA from these cells were extracted by using Quick Prep micro mRNA Purification Kit (Amersham Pharmacia Co., USA). Cy3-dUTP was labeled into cDNA of pcDNA3.1(-) group and Cy5-dUTP was labeled into cDNA of pcDNA3.1 (-)-C12 group during the RT-PCR procedure. These cDNA was hybridized with cDNA chip containing 1 152 cDNA and amplified by PCR with common primer. After dotting, hydrating, dehydrating, ultraviolet joining, and washing with SDS, water and sodium borocaptate solution, samples were degenerated in water at 95°C for 5 min and hybridized with mixed probe for 15-17 h. Followed by washing, drying at room temperature, specimens were analyzed by ScanArray 3000 (General Scanning, positive standard: Cy5/ Cy3 > 2.0, negative standard: Cy5/Cy3 < 0.5).
RESULTS
Expression of the “bait” fusion protein
Yeast strain AH109 transformed with pGBKT7-HBcAg could stably express the fusion protein at high level (Figure 1). The transformed AH109 could only grow on SD/-Trp medium but not on QDO medium. Thus, the transformed yeast could be used for yeast hybrid analysis.
Figure 1.
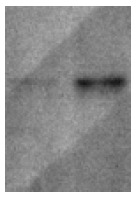
Western blotting showed the expression of HBV core protein in yeast. Lane 1: 5-µL sample; lane 2: 10-µL sample.
Two-hybrid library screen and re-mating
In order to unravel the molecular mechanisms of HBcAg, we adopted the yeast two-hybrid system to identify and characterize cellular targets of HBcAg. An adult human liver cDNA library containing cDNAs fused to the GAL4 activation domain in yeast vector pCAT2 was used to screen for clones interacting with HBcAg. Plasmid pACT2-cDNA was recovered from the true positive clone and was sequenced. The result of our screening led to the identification of a clone containing gene without homologs in GenBank. This cDNA was 591 bases long (Figure 3), which contained an open reading frame (ORF) able to encode a protein of 197 amino acids. The protein was named C12. Sequence homology searches in GenBank revealed that it was a novel gene. The entire ORF coding for C12 was obtained from HepG2 cells and subcloned into yeast plasmid pGADT7 to be expressed as a fusion protein with DNA activation domain of GAL4. pGADT7-C12 was transfected into Y187 and re-mated with AH109 transfected with pGBKT7 -HBcAg to verify the reliability of the interaction between them.
Figure 3.

Nucleic and amino acid sequences of C12 cDNA.
Sub-cellular localization
After the full-length gene of C12 was cloned into pEGFP-N1 (Figure 2), the fluorescent protein expression plasmid pEGFP-N1-C12 was transfected into 293 and L02 cells and incubated for 48 h. When we observed under the fluorescent microscope, the steady, powerful green fluorescent signal could be observed in most cells (Figures 4A and B). The result indicated that the C12 protein could be expressed in these cells and mainly localized in the cell plasma.
Figure 2.
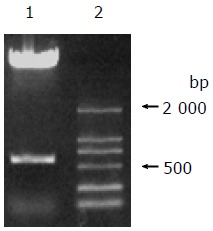
pEGFP-N1-C12 digested by EcoRI and BamHI. Lane 1: pEGFP-N1-C12; lane 2: DNA marker.
Figure 4.
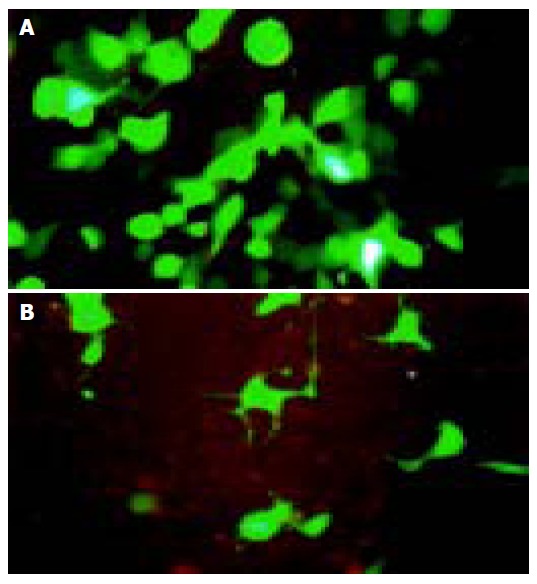
A pEGFP-N1-C12 expression in 293 cell line; B: pEGFP-N1-C12 expression in L02 cell line.
MTT reduction assay
MTT reduction assay was performed in Bel7402 and L02 cell line transfected with deionized water, liposomes, pEGFP- N1 and pEGFP-N1-C12 respectively. The results showed that the optical density in Bel7402 cell line groups were 0.547 ± 0.076, 0.575 ± 0.122, 0.470 ± 0.124, 0.503 ± 0.093, while the optical density in L02 cell line groups were 0.216 ± 0.028, 0.201 ± 0.01, 0.197 ± 0.011, 0.205 ± 0.024. It revealed that the expression of C12 gene in both cells did not have inhibitory or promotional effect on the growth of these cells, compared with deionized water, liposomes and empty vector (P > 0.01).
Screening of binding protein
Yeast strain AH109 transformed with pGBKT7-C12 could grow on SD/ -Trp medium and could not grow on QDO medium, the result showed that C12 protein have no autoactivation in yeast cell and could be used for yeast two-hybrid screening. After being mated with pretransformed cDNA library strain Y187 and screened on QOD, 21 blue clones were obtained. Plasmid in these clones were cloned into Escherichia coli and analyzed by DNA sequencing. After comparing these sequences in GenBank, 16 different genes were screened. Fourteen genes were homolog of known genes, two genes did not find homolog in GenBank (Table 1)[11].
Table 1.
Binding protein of C12 protein screened by yeast two-hybrid
| Homeobox gene | Homogeneity % | Clones |
| Cathepsin B | 99 | 1 |
| Apolipoprotein M | 98 | 1 |
| Cytochrome C oxidase II | 97–99 | 3 |
| Receptor JAK (EPHB4) variation factor | 99 | 1 |
| KH splicing regulation protein (KSRP) | 98 | 1 |
| Phosphatidylinositol deacylase | 97 | 1 |
| poly-glucose Q transcriptional variation factor 1 | ||
| Ferritin light chain | 99 | 2 |
| Ornithine decarboxylase 1 | 99 | 1 |
| Metallothionein 2A | 98–100 | 3 |
| Blood coagulation factor IX | 100 | 1 |
| Acetolactate synthase | 99 | 1 |
| Calpain | 98 | 1 |
| Exo-nucleus triphosphate diplo-phosphoinostide | 99 | 1 |
| hydrolase 5 | ||
| Plasma globulin inhibitive factor H4 | 99 | 1 |
| Novel gene | 100 | 2 |
cDNA microarray assay
Eight paddy genes from 1 152 cDNA on the chip that served as negative control showed very weak hybrid signal, which confirmed the reliability of the experiment. Total mRNA of HepG2 cells transfected with pcDNA3.1(-) and pcDNA3.1(-)-C12 were purified, respectively, then labeled with Cy3-dUTP and Cy5-dUTP during RT-PCR procedure. Cy5 fluorescein showed red fluorescence and Cy3 fluorescein showed green fluorescence. The difference of color showed the difference of gene expression level, such as yellow showed that there was no difference between the two groups. Based on the criteria, 16 differential expression genes upregulated by C12 and 1 gene downregulated by C12 were screened (Table 2). These genes were involved in cell signal conduction, cell proliferation, differentiation, and generation of carcinoma[12].
Table 2.
Differential expression genes upregulated by C12 gene
| GenBank number | Homeobox gene |
| 208 | Insulin receptor (INSR), |
| 5500 | SUMO-1 activating enzyme subunit 1 (SAE1), |
| 6292 | Tumor susceptibility gene 101 (TSG101), |
| 12248 | Selenophosphate synthetase 2 (SPS2), |
| 6217 | Serine (or cysteine) proteinase inhibitor, clade I |
| (neuroserpin), member 2 (SERPINI2), | |
| 16614 | TRAF and TNF receptor-associated protein (TTRAP), |
| 6437 | ADP-ribosyltransferase-like 1 (ADPRTL1) |
| 33306 | Caspase 4, apoptosis-related cysteine protease |
| (CASP4), transcript variant gamma, | |
| L04731 | Translocation T (4:11) of ALL-1 gene |
| 2085 | Glutathione peroxidase 4 (phospholipid |
| hydroperoxidase) (GPX4), | |
| 4728 | DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide |
| 21 (DDX21), | |
| 2624 | Prefoldin 5 (PFDN5), transcript variant 1, |
| 6415 | Serine palmitoyl transferase, long chain base |
| subunit 1 (SPTLC1), | |
| 4127 | G protein pathway suppressor 1 (GPS1), |
| 14887 | Hypothetical protein from BCRA2 region |
| 660 | Transforming growth factor, beta 1 (TGF-β 1), |
DISCUSSION
The HBV infection is a public health problem worldwide, particularly in East Asia. The current therapy of HBV infection is mostly based on chemical agents and cytokines that have been shown to provide limited efficacy are also toxic to the human body[13-15]. Elucidating the interaction between hepatocytes and HBV was essential for finding new methods to inhibit the replication of HBV. The HBV nucleocapsid or core antigen (HBcAg) is extremely immunogenic during infection and after immunization. HBcAg has a unique three-dimensional folding that interacts with immunoglobulins outside the classical antibody-binding site, has the CD4+ T cell epitopes and the encapsidated nucleic acids that can efficiently interact and activate antigen presenting cells, especially naive B cells. HBcAg also performs pivotal functions by mediating release of (sub) viral particles, regulation of supercoiled DNA amplification, and transcriptional transactivation[16-19]. To assess its multiple functions and host-protein assistance involved, we initiated a two-hybrid screen using the HBcAg domain as bait and had identified a novel gene named C12 with unknown function. To study the biological function of C12 gene and its role in the HBV infection, we performed the following researches and hoped to get some valuable clues for the further investigation.
First of all, the full-length gene of C12 was amplified from HepG2 cell and verified by DNA sequencing. Gene of C12 was cloned into GFP expression vector pEGFP-N1 and transfected into 293 and L02 cell line. After incubating for 48 h, cells were observed under the fluorescent microscope. We found that these cells were stained with steady, strong green fluorescence in the whole cell, the result indicated that C12 protein mainly expressed in cell plasma. Eighteen hours after incubation with medium, cell number, as measured by absorbance (A), was quantified by using colorimetric MTT assay. The result showed that the optical density in Bel7402 and L02 cell line groups transfected with deionized water, liposomes, pEGFP-N1 and pEGFP-N1-C12 have no significant difference. This result could be explained as follows: C12 protein was a normal protein present in cells and may have the promotive or inhibitive effect on cell growth. It did not show significant effect because of the interference of network regulation of other proteins. The main expression of C12 protein does not intervene the growth and reproduction of normal cells.
Screening binding protein in hepatocytes, identification of interaction protein in the protein network, may provide valuable clues for the further research[20-23]. Based on the modified yeast two -hybrid technique, the “bait” plasmid pGBKT7-C12 was transformed into yeast strain AH109. After mating with liver cDNA library yeast strain Y187, the diploid yeast cells were plated on QDO media containing X-α -gal, 21 true positives were obtained. Sequencing analysis of isolated library plasmids, 16 different genes were found. Among them, cytochrome C oxidase, ornithine decarboxylase, and acetolactate synthase related to energy metabolism. C12 bind with these proteins indicating that C12 may participate in mitochondrion energy metabolism. KSRP was a factor that regulated the shearing process of enhancer exon[24,25]. Calpain, phosphatidylinositol deacylase poly-glucose Q transcriptional variation factor and receptor JAK (EPHB4) variation factor were important members involved in cellular signal transduction and cell cycle regulation. The interaction between C12 and these proteins suggest that C12 may serve in the above procedure. Ferritin related to hepatocellular damage and HCC[26]. Interaction between C12 and ferritin may play the bridge role in hepatocellular damage and HCC. C12 also take part in blood coagulation procedure for binding with coagulation factor IX. All of the above suggest that proteins found to binding C12 in the yeast two-hybrid system 3 appeared to reveal some probable functions of C12.
The cDNA microarray is a genomic technology, which is widely used in both basic and applied research. Recent advances in cDNA microarray technology have great impact on many areas of biomedical research and pharmacogenomics: discovering novel targets and genes, elucidating signatures of complex diseases, transcriptional profiling of models for diseases, and the development of individually optimized drugs based on differential gene expression patterns[17-29]. The commercial availability of several different precoated arrays and the ease of handling supported the broad distribution of this new technique. The basic protocol in this article involves the hybridization of complementary strands of fluorescent (Cy3 and Cy5) labeled cDNA from HepG2 cells transfected with expression plasmid pcDNA3.1(- )-C12 and empty vector pcDNA3.1(-) spotted onto a solid support and then detected by fluorescence scanning. Sixteen differential expression genes upregulated by C12 and 1 gene downregulated by C12 were screened (Table 2). These genes were involved in cell signal conduction, cell proliferation and differentiation, generation of carcinoma.
The preliminary data from the context studies suggest that the novel protein C12 is located in cell plasma, and its overexpression has no significant effect on the metabolism of liver cells; it interacted with many proteins in hepatocytes and may be involved in many processes of gene expression. These results stride the first step for the functional study of C12 gene and pave the way for the new gene biology research. We will further refine our understanding of currently known results as well as identify novel pathways and methods to elucidate the integral biological functions.
Footnotes
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
References
- 1.Li H, Wang L, Wang SS, Gong J, Zeng XJ, Li RC, Nong Y, Huang YK, Chen XR, Huang ZN. Research on optimal immunization strategies for hepatitis B in different endemic areas in China. World J Gastroenterol. 2000;6:392–394. doi: 10.3748/wjg.v6.i3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu LC, Gu CH. Mutation of hepatitis B virus and its associa-tion with liver diseases. Shijie Huaren Xiaohua Zazhi. 1999;7:978–979. [Google Scholar]
- 3.Milich DR. Do T cells "see" the hepatitis B core and e antigens differently? Gastroenterology. 1999;116:765–768. doi: 10.1016/s0016-5085(99)70203-9. [DOI] [PubMed] [Google Scholar]
- 4.Riedl P, Stober D, Oehninger C, Melber K, Reimann J, Schirmbeck R. Priming Th1 immunity to viral core particles is facilitated by trace amounts of RNA bound to its arginine-rich domain. J Immunol. 2002;168:4951–4959. doi: 10.4049/jimmunol.168.10.4951. [DOI] [PubMed] [Google Scholar]
- 5.Dong J, Cheng J, Wang Q, Huangfu J, Shi S, Zhang G, Hong Y, Li L, Si C. The study on heterogeneity of hepatitis B virus DNA. Zhonghua YiXue ZaZhi. 2002;82:81–85. [PubMed] [Google Scholar]
- 6.Lu YY, Li K, Cheng J, Wang L, Liu Y, Duan HJ, Zhang LX. Cloning and expression of hepatitis virus X gene in yeast. Shijie Huaren Xiaohua Zazhi. 2002;10:15–18. [Google Scholar]
- 7.Lu YY, Wang L, Cheng J, Li K, Lu Y, Zhang LX. Screening of HBcAg interacting proteins in hepatocytes with yeast-two hybrid technique. Shijie Huanren Xiaohua Zazhi. 2003;11:426–429. [Google Scholar]
- 8.Lu YY, Wang L, Li K, Liu Y, Cheng J, Zhang LX. Screening and cloning of HBeAg interacting proteins in hepatocytes. Shijie Huaren Xiaohua Zazhi. 2003;11:422–425. [Google Scholar]
- 9.Lu YY, Shao Q, Chen J, Cheng TY, Wang L, Liang YD, Liu Y, Zhang J, Li K, Zhang LX. Screening and identification of a novel hepatitis B virus e antigen binding protein E-19 in hepa-tocyte by yeast two-hybrid technique. Shijie Huaren Xiaohua Zazhi. 2003;11:1118–1121. [Google Scholar]
- 10.Lu YY, Chen TY, Cheng J, Shao Q, Liang YD, Wang L, Liu Y, Zhang J, Li K, Zhang LX. Screening and identification of a novel gene coding for Hepatitis B virus core antigen interact-ing protein C-12 in hepatocyte. Shijie Huaren Xiaohua Zazhi. 2003;11:1122–1125. [Google Scholar]
- 11.Liang YD, Lu YY, Cheng J, Li Q, Wang L, Wu J, Cheng ML. Screening of a new gene C-12 coding for a hepatitis B virus core antigen interacting protein in hepatocytes by yeast-two hybrid technique. Shijie Huaren Xiaohua Zazhi. 2003;11:1862–1865. [Google Scholar]
- 12.Lu YY, Liu Y, Cheng J, Ling YD, Chen TY, Shao Q, Wang L, Zhang LX. Gene expression profile of HepG2 cell transfected with a novel gene C-12 coding HBcAg binding protein. Shijie Huaren Xiaohua Zazhi. 2004;12:62–65. [Google Scholar]
- 13.Xu R, Cai K, Zheng D, Ma H, Xu S, Fan ST. Molecular therapeutics of HBV. Curr Gene Ther. 2003;3:341–355. doi: 10.2174/1566523034578294. [DOI] [PubMed] [Google Scholar]
- 14.Lagget M, Rizzetto M. Current pharmacotherapy for the treatment of chronic hepatitis B. Expert Opin Pharmacother. 2003;4:1821–1827. doi: 10.1517/14656566.4.10.1821. [DOI] [PubMed] [Google Scholar]
- 15.Lagget M, Rizzetto M. Treatment of chronic hepatitis B. Curr Pharm Des. 2002;8:953–958. doi: 10.2174/1381612024607054. [DOI] [PubMed] [Google Scholar]
- 16.Pumpens P, Grens E. HBV core particles as a carrier for B cell/T cell epitopes. Intervirology. 2001;44:98–114. doi: 10.1159/000050037. [DOI] [PubMed] [Google Scholar]
- 17.Hacker HJ, Deres K, Mildenberger M, Schröder CH. Antivirals interacting with hepatitis B virus core protein and core mutations may misdirect capsid assembly in a similar fashion. Biochem Pharmacol. 2003;66:2273–2279. doi: 10.1016/j.bcp.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Murray K, Shiau AL. The core antigen of hepatitis B virus as a carrier for immunogenic peptides. Biol Chem. 1999;380:277–283. doi: 10.1515/BC.1999.038. [DOI] [PubMed] [Google Scholar]
- 19.Vanlandschoot P, Cao T, Leroux-Roels G. The nucleocapsid of the hepatitis B virus: a remarkable immunogenic structure. Antiviral Res. 2003;60:67–74. doi: 10.1016/j.antiviral.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Gietz RD, Woods RA. Screening for protein-protein interactions in the yeast two-hybrid system. Methods Mol Biol. 2002;185:471–486. doi: 10.1385/1-59259-241-4:471. [DOI] [PubMed] [Google Scholar]
- 21.Nagpal S, Ghosn CR, Chandraratna RA. Identification of nuclear receptor interacting proteins using yeast two-hybrid technology. Applications to drug discovery. Methods Mol Biol. 2001;176:359–376. doi: 10.1385/1-59259-115-9:359. [DOI] [PubMed] [Google Scholar]
- 22.Serebriiskii IG, Toby GG, Finley RL, Golemis EA. Genomic analysis utilizing the yeast two-hybrid system. Methods Mol Biol. 2001;175:415–454. doi: 10.1385/1-59259-235-X:415. [DOI] [PubMed] [Google Scholar]
- 23.Zhen Z. Progress in proteomics. Sheng Wu Gong Cheng Xue Bao. 2001;17:491–493. [PubMed] [Google Scholar]
- 24.Min H, Turck CW, Nikolic JM, Black DL. A new regulatory protein, KSRP, mediates exon inclusion through an intronic splicing enhancer. Genes Dev. 1997;11:1023–1036. doi: 10.1101/gad.11.8.1023. [DOI] [PubMed] [Google Scholar]
- 25.Ring HZ, Vameghi-Meyers V, Nikolic JM, Min H, Black DL, Francke U. Mapping of the KHSRP gene to a region of conserved synteny on human chromosome 19p13.3 and mouse chromosome 17. Genomics. 1999;56:350–352. doi: 10.1006/geno.1998.5725. [DOI] [PubMed] [Google Scholar]
- 26.Cao Z, Bai Y, Yang X, Liu J, Li B, Li F. Study of iron metabolism abnormality in the hepatocyte damage of hepatitis B. Zhonghua GanZangBing ZaZhi. 2001;9:37–39. [PubMed] [Google Scholar]
- 27.Hackl H, Sanchez Cabo F, Sturn A, Wolkenhauer O, Trajanoski Z. Analysis of DNA microarray data. Curr Top Med Chem. 2004;4:1357–1370. doi: 10.2174/1568026043387773. [DOI] [PubMed] [Google Scholar]
- 28.Chittur SV. DNA microarrays: tools for the 21st Century. Comb Chem High Throughput Screen. 2004;7:531–537. doi: 10.2174/1386207043328454. [DOI] [PubMed] [Google Scholar]
- 29.Duncan RC, Salotra P, Goyal N, Akopyants NS, Beverley SM, Nakhasi HL. The application of gene expression microarray technology to kinetoplastid research. Curr Mol Med. 2004;4:611–621. doi: 10.2174/1566524043360221. [DOI] [PubMed] [Google Scholar]


