Abstract
AIM: Prior Helicobacter pylori (H pylori) infection has often been underestimated. These underestimations have misled physicians attempting to determine the significance between H pylori and certain gastrointestinal lesions such as intestinal metaplasia, atrophic gastritis, and gastric cancer. Our study endeavored to detect past H pylori infections accurately, easily, and rapidly with the newly developed immunoblot kit, Helico Blot 2.1.
METHODS: Thirty-three patients, including 25 H pylori infected and 8 uninfected cases, were enrolled in our study. All patients received consecutive gastroendoscopic examinations and 13C-urea breath test (UBT) tests at 6-or 12-mo intervals for up to 4 years. Serum samples were obtained from each patient at the same time. Intragastric H pylori infection was confirmed in accordance with the gold standard. Twenty-five H pylori-infected patients received triple therapies after initial bacterial confirmation, and were successful in eradicating their infections. Serially obtained sera were tested by means of Helico Blot 2.1.
RESULTS: Current infection marker detected by Helico Blot 2.1 was unreliable for representing ongoing H pylori infection. Only 35 and 37 ku antibodies of H pylori had significant seroconversion rates 1 year after having been cured. The seropositive rates of 116 ku (cytotoxin-associated antigen [CagA]) and Helico Blot 2.1 were nearly 100% during 4-year follow-up period. Both CagA antigen and Helico blot 2.1 could serve as indicators of long-term H pylori infection.
CONCLUSION: Helico Blot 2.1 can detect past H pylori infections for up to 4 years, and is the best method to date for detecting previous long-term H pylori infection.
Keywords: Helicobacter pylori, Long-term infection, Diagnosis, Serum
INTRODUCTION
Since Warren and Marshall reported Helicobacter pylori (H pylori) in 1983[1], this gram negative, spiral-shaped bacterium has been designated as a causative agent in many benign and malignant gastrointestinal diseases, such as peptic ulcer[2,3] and gastric cancer[4-7]. However, low intragastric H pylori infection rates have been reported in connection with certain precancerous lesions, such as intestinal metaplasia[8] and atrophic gastritis[9,10]. This has been attributed to inappropriate use of medications or intragastric environmental changes. Recent meta-analysis reports about serology studies have even argued that the association between H pylori and gastric cancer is relatively weak. Therefore, accurate detection of past H pylori infection is important in deciding the real prevalence rates of many gastrointestinal diseases.
Conventional ELISA serology study was previously known as the best method of determining past H pylori infection, because the IgG antibody decreases slowly and may remain detectable for months after bacterial cure[14-17]. The delay in fall in serum anti-H pylori IgG antibody following eradication therapy inspired the idea that serum antibodies to specific H pylori immunoreactive antigens could serve as tools in detecting past H pylori infections. The H pylori cytotoxin-associated antigen (CagA) is known to be more virulent[18-21], have a stronger immunoreactivity[22,23], and persist longer after H pylori eradication[24]. The other low molecular weight antigens of H pylori have also been helpful in detecting H pylori infection[25-27].
The primary goal of our study was to determine the feasibility and accuracy of a newly developed immunoblot device in the detection of past H pylori infections.
MATERIALS AND METHODS
A total of 33 patients were enrolled in this study. Of these, 25 (19 men, 6 women, mean age 57.5±14.3 years) were the H pylori positive study group, and 8 (2 men, 6 women, mean age 63.8±15.8 years) made up the H pylori negative control group. The 25 H pylori positive patients included 23 cases of peptic ulcers and 2 cases of early gastric cancers. There were eight H pylori negative patients as a control group, including four cases with peptic ulcers, two cases with gastro-esophageal regurgitation diseases, one case with gastritis, and one case with early gastric cancer. The H pylori positive study group was followed up for 18-45 mo, on an average of 30.0±8.9 mo. The negative control group was followed up for 26-48 mo, on an average of 34.6±7.1 mo. During the follow-up periods, all of the patients received gastroendoscopic examinations and biopsy- based H pylori studies at 6-mo or 1-year intervals. 13C-urea breath test (UBT) tests and sera collection for the diagnosis of H pylori infection were conducted at the same time. Exclusion criteria were the following: use of antibiotics or bismuth salts, or proton pump inhibitors therapy within the last 2 mo, previous anti-H pylori treatment, chronic use of corticosteroids or immunosuppressant drugs, prior gastric surgery, presence of a bleeding peptic ulcer, severe concomitant disease, and pregnancy or lactation.
The gold standard of identifying a H pylori infected patient is determined by positive culture results, or positive results in any two of the following: histology (hematoxylin and eosin, H&E stain), rapid urease test and 13C-UBT test. The negative H pylori-infected patient was identified by a negative culture result and at least two negative results in the following: histology, rapid urease test, and 13C-UBT test. All of the H pylori infected patients received H pylori eradication therapy with standard full-dose triple therapy regimen (omeprazole 20 mg, amoxicillin 500 mg, and clarithromycin 500 mg twice a day for 7 d).
The Helico Blot 2.1 kit (Genelabs Diagnostics, Singapore) was used to detect IgG antibodies to specific antigens of H pylori in accordance with manufacturer’s instructions. Helico Blot 2.1 kit is a qualitative serologic test using a Western blot made from bacterial lysate, and including a recombinant antigen of H pylori with a predictive value for indicating current H pylori infection. The corresponding bands to these specific antigens of H pylori are 116 ku (CagA), 89 ku (vacuolating-associated antigen [VacA]), 37, 35, 30 ku (Urease A), and 19.5 ku (Figure 1). The determinations of H pylori seropositivity on Helico Blot 2.1 and corresponding specific antigens were in accordance with the manufacturer’s criteria. The recommended criteria for determining a sample as H pylori seropositive on Helico Blot 2.1 kit is any one of the following conditions: (1) 116 ku (CagA) positive, where CagA has to be present with one or more of the following bands: 89 ku (VacA), 37, 35, 30 ku (Urease A), and 19.5 ku together, or with a current infection marker (CIM). (2) Presence of any one band at 89, 37 or 35 ku, with or without a CIM. (3) Presence of both 30 and 19.5 ku with or without a CIM. Each patient’s serum samples were analyzed at the same time to avoid interassay variation. Furthermore, three specialists who did not know about the study’s objectives were invited to examine and interpret the nitrocellulose strips to decrease the individual subjective bias (Figure 1).
Figure 1.
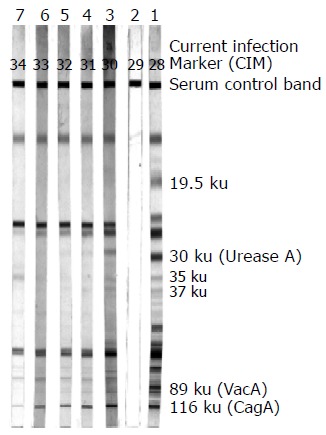
The nitrocellulose strips of Helico Blot 2.1 representative of immunoblot results: lane 1, positive control; lane 2, negative control; lanes 3-7, serial serum samples of a patient showing positive results.
Statistics
Due to the repeated measurements to determine antibody positive or negative status, the generalized estimating equation (GEE) was applied to statistically analyze intra- and inter-individual changes in specific antigens of H pylori during the follow-up periods (every 6 mo).
RESULTS
All 25 H pylori positive patients underwent successful bacterial eradication with triple therapy, as confirmed by gastroen-doscopic biopsy-based studies and 13C-UBT tests. A total of 112 sera samples obtained from the 25 patients in our study group were checked with Helico Blot 2.1. The test results are recorded in Tables 1 and 2, and depicted in Figure 2.
Table 1.
The results of Helico Blot 2.1 and various H pylori-specific antigens in 25 H pylori infected patients with a total of 112 sera samples
| HB2.11 | 116 ku (CagA) | 89 ku (VacA) | 37 ku | 35 ku | 30 ku (Urease A) | 19.5 ku | CIM2 | |
| Positive | 110/112 | 111/112 | 94/112 | 44/112 | 35/112 | 97/112 | 78/112 | 93/112 |
| sera (%) | -98 | -99 | -84 | -39 | -31 | -87 | -70 | -83 |
HB2.1: Helico Blot 2.1;
CIM: current infection marker.
Table 2.
The positive numbers and rates of various H pylori-specific antigens and Helico Blot 2.1 in 25 H pylori-infected patients at half-year intervals during a 4-year follow-up period (n, %).
| Pre-Hp Tx |
Post-Hp Tx |
||||||||
| 0.5 yr | 1 yr | 1.5 yr | 2 yr | 2.5 yr | 3 yr | 3.5 yr | 4 yr | ||
| CIM2 | 23 (92) | 21 (95) | 13 (93) | 7 (78) | 12 (80) | 9 (75) | 4 (80) | 3 (50) | 1 (25) |
| 19.5 ku | 23 (92) | 18 (82) | 7 (50) | 6 (67) | 8 (53) | 8 (67) | 4 (80) | 3 (50) | 1 (25) |
| 30 ku | 24 (96) | 21 (95) | 11 (79) | 8 (89) | 12 (80) | 10 (83) | 5 (100) | 4 (67) | 2 (50) |
| 35 ku | 11 (44) | 9 (41) | 4 (29) | 3 (33) | 3 (20) | 4 (33) | 0 (0) | 1 (17) | 0 (0) |
| 37 ku | 17 (68) | 11 (50) | 5 (36) | 2 (22) | 5 (33) | 2 (17) | 1 (20) | 1 (17) | 0 (0) |
| 89 ku | 22 (88) | 18 (82) | 13 (93) | 6 (67) | 13 (87) | 10 (83) | 4 (80) | 4 (67) | 4 (100) |
| 116 ku | 25 (100) | 22 (100) | 14 (100) | 9 (100) | 15 (100) | 12 (100) | 5 (100) | 6 (100) | 3 (75) |
| HB2.11 | 24 (96) | 22 (100) | 14 (100) | 9 (100) | 15 (100) | 11 (92) | 5 (100) | 6 (100) | 4 (100) |
| Total | 25 | 22 | 14 | 9 | 15 | 12 | 5 | 6 | 4 |
| case nos. | |||||||||
HB2.1: Helico Blot 2.1;
CIM: current infection marker; Pre-Hp Tx: before H pylori eradication therapy; Post-Hp Tx: after H pylori eradication therapy.
Figure 2.
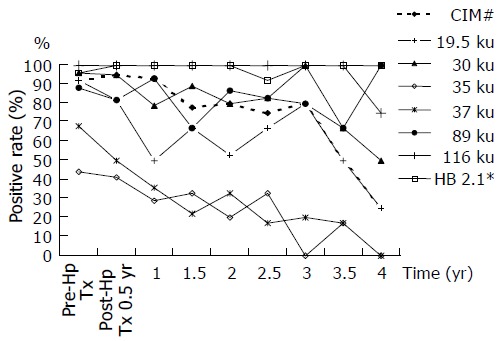
Fluctuating positive rates of various H pylori-specific antigens and Helico Blot 2.1 in 25 H pylori-infected patients were charted at half- year intervals during a 4-year follow- up period. The CIM was unreliable, because it maintained high positive rates for at least 3 years after H pylori eradication. Thirty-five and thirty-seven kilounits antibodies of H pylori showed significant seroconversion changes at 1 year after bacterial cure. The seropositive rates of 116 ku (CagA) and Helico Blot 2.1 were nearly 100% during the 4-year follow-up period.
Except for 37 and 35 ku, other H pylori specific antibodies (19.5, 30, 89, 116 ku, and even CIM) showed as seropositive for over half of the serum samples tested with Helico Blot 2.1 test during a 4-year follow-up period (Figure 2). Both Helico Blot 2.1 and 116 ku (CagA) antibody seropositive rates were nearly 100% (98% and 99% respectively, as shown in Table 1). Also, at least 79% of H pylori 30 ku antibody can be detected positively before the end of a 3-year follow-up. The 89 ku (VacA) was also at least 67% positive during the 4-year follow-up (Table 2 and Figure 2).
The seropositive rate of 19.5 ku antibody decreased significantly, but wide fluctuation decreased its reliability (Figure 2). The seroconversion rates of 35 and 37 ku antibodies were significant (GEE testing significance, P values as 0.008 and 0.001 respectively) till 1 year after bacterial cure, in spite of initial lower seropositive rates (35 ku, 44% down to 29% and 37 ku, 68% down to 36%, Table 2 and Figure 2). The positive rate of CIM decreased significantly between the 3rd and 4th years, but the small number of cases studied has influenced reliability (Figure 2).
The H pylori statuses in the negative control group were still negative as determined by the gold standard and Helico Blot 2.1 tests after a follow- up period similar to the study groups. Because of this, the data is not shown in tables or figures.
DISCUSSION
Although H pylori was designated as a human class I carcinogen already in 1994[28], the exact nature and strength of the association with gastric cancer has remained debatable[11-13].
Because H pylori infection are primarily acquired in child-hood[29,30], older subjects have had more time to develop mucosal atrophy and to loose their infection than younger subj -ects[9,10]. Also, decades may pass between initiation and detection of gastric cancers. H pylori may disappear spontaneously with progressing precancerous intragastric environmental change and invalidate various studies of its association with gastric cancer. The significance of detecting the past H pylori infection is to re-estimate the strength of the H pylori-gastric cancer relationship.
The CIM of Helico Blot 2.1 was developed and claimed to be able to detect ongoing H pylori infection. However, our study showed that the CIM band was oversensitive and unreliable, because it remained at a 75-80% positive rate for 3 years after H pylori eradication (Table 2). Our results differ from the conclusions of Oleastro’s et al[31] study of pediatric patients. There were two reasons for these discrepancies with our report. First were the different age groups of the studies. It is known that H pylori infection occurs beginning in childhood, and gradually accumulates as the patients get older[29,30]. Consequently, pediatric studies will have more true negative cases when compared with adult groups. Our adult patients may have had immunological memory due to past H pylori infection, and were mistaken for currently infected by the oversensitive CIM band. Second, these two studies had different designs. Oleastro’s study[31] was a cross-sectional design, not a long term follow-up study like ours. Their study checked serum H pylori antibody at only one time point, and the serial seroconversion changes could not be detected in cross-sectional study designs.
The seropositive rate of 116 ku (CagA) was the highest (up to 100% before the end of a 3.5-year follow-up period) among various antibodies to H pylori-specific antigens and nearly parallel to Helico Blot 2.1 (Table 2 and Figure 2). This phenomenon explains why the CagA antibody was the last antibody to disappear after H pylori eradication, and may serve as a single predictor in H pylori long-term infection. Our results showed that CagA antigen had the strongest immunoreactivity among various H pylori antigens, and was in agreement with numerous previous reports[22,24,32,33].
The roles of various low molecular weight antibodies of H pylori were ambiguous. The heat shock protein of H pylori was related to chronic gastric inflammation[34,35]. Mucosa-associated lymphoid lymphoma (MALToma) patients had higher prevalence rates of 19.5 and 30 ku antibodies of H pylori. After H pylori eradication, MALToma patients had significant negative seroconversion of 19.5, 30, and 35 ku antibodies[36]. The 35 ku antibody of H pylori also plays a role in peptic ulcers[37]. The immunoreaction with 30 ku (Urease A) of H pylori could provide a serological assessment of not only H pylori infection[25-27], but also of eradication[27]. In our study, the seroconversion of 35 and 37 ku was significant for up to 1 year after H pylori eradication.
According to our previous study[14], IgG seroconversion rates by conventional ELISA method were 42% and 94% at 6 and 12 mo after H pylori eradication, respectively. Although some papers[15,38] have proposed latent IgG seroconversion, many confounding factors, including small case numbers, different antigenic preparations, and variable cut-off values, made this conflict. Moreover, the follow-up periods of most studies were less than 2 years, which would mean that whether or not H pylori could be detected beyond 2 years after bacterial cure would be difficult to determine, and has rarely been addressed by the conventional ELISA method. Our study test kits can detect past intragastric H pylori infections for at least 3, or even 4 years after eradication.
The positive or negative results of Helico Blot 2.1 are given as the presence or absence of immunodetection of certain specific antigen bands, 116 ku (CagA), 89 ku (VacA), 37, 35, 30 ku (Urease A) and 19.5 ku, by the patient’s serum (Figure 1). Even the decay or disappearance of serum CagA antibody, the positive result of Helico Blot 2.1 could still be achieved by other recommended criteria (criteria 2 or 3). There is one serum sample in our 4th-year study shows negative CagA antibody and positive Helico Blot 2.1 results (Table 2). Besides, Helico Blot 2.1 can differentiate various H pylori strains according to the presence of corresponding specific immunoreactive bands. So, Helico Blot 2.1 has more advantages over CagA only in detecting the past H pylori infection.
We expected that Helico Blot 2.1 will improve the detection of H pylori infection in many GI and non-GI diseases, such as ischemic heart disease and chronic urticaria. The present role of H pylori in these non-GI diseases is debatable[39,40], and improvements in detecting past H pylori infections would further clarify the correlations between these diseases and H pylori.
In summary, Helico Blot 2.1 is the best method thus far of detecting past intragastric H pylori infections for up to 4 years after treatment. This newly developed device with Western blot method improves the detection of H pylori and further clarifies its role in many H pylori-related diseases.
Footnotes
Science Editor Wang XL and Zhu LH Language Editor Elsevier HK
Supported by Grants From the China American Petrochemical Co., Ltd. Foundation (CAPCO) and the National Science Council of the ROC (NSC-90-2314-B-037-044)
References
- 1.Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- 2.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 3.Olbe L, Fandriks L, Hamlet A, Svennerholm AM, Thoreson AC. Mechanisms involved in Helicobacter pylori induced duodenal ulcer disease: an overview. World J Gastroenterol. 2000;6:619–623. doi: 10.3748/wjg.v6.i5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 6.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 7.Wang RT, Wang T, Chen K, Wang JY, Zhang JP, Lin SR, Zhu YM, Zhang WM, Cao YX, Zhu CW, et al. Helicobacter pylori infection and gastric cancer: evidence from a retrospective cohort study and nested case-control study in China. World J Gastroenterol. 2002;8:1103–1107. doi: 10.3748/wjg.v8.i6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testoni P, Colombo E, Scelsi R, Cattani L, Bagnolo F, Lella F, Buizza M, Luinetti O. Tissue staining for Helicobacter pylori in intestinal metaplasia: correlation with its extension and histochemical subtypes. Ital J Gastroenterol. 1995;27:285–290. [PubMed] [Google Scholar]
- 9.Karnes WE, Samloff IM, Siurala M, Kekki M, Sipponen P, Kim SW, Walsh JH. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology. 1991;101:167–174. doi: 10.1016/0016-5085(91)90474-y. [DOI] [PubMed] [Google Scholar]
- 10.Testoni PA, Bonassi U, Bagnolo F, Colombo E, Scelsi R. In diffuse atrophic gastritis, routine histology underestimates Helicobacter pylori infection. J Clin Gastroenterol. 2002;35:234–239. doi: 10.1097/00004836-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 12.Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol. 1999;94:2373–2379. doi: 10.1111/j.1572-0241.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 13.Danesh J. Helicobacter pylori infection and gastric cancer: systematic review of the epidemiological studies. Aliment Pharmacol Ther. 1999;13:851–856. doi: 10.1046/j.1365-2036.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang WM, Chen CY, Jan CM, Chen LT, Perng DS, Lin SR, Liu CS. Long-term follow-up and serological study after triple therapy of Helicobacter pylori-associated duodenal ulcer. Am J Gastroenterol. 1994;89:1793–1796. [PubMed] [Google Scholar]
- 15.Cutler AF, Prasad VM. Long-term follow-up of Helicobacter pylori serology after successful eradication. Am J Gastroenterol. 1996;91:85–88. [PubMed] [Google Scholar]
- 16.Kato S, Furuyama N, Ozawa K, Ohnuma K, Iinuma K. Long-term follow-up study of serum immunoglobulin G and immunoglobulin A antibodies after Helicobacter pylori eradication. Pediatrics. 1999;104:e22. doi: 10.1542/peds.104.2.e22. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Lu AP, Zhang L, Li YD. Anti-Helicobacter pylori immunoglobulin G (IgG) and IgA antibody responses and the value of clinical presentations in diagnosis of H. pylori infection in patients with precancerous lesions. World J Gastroenterol. 2003;9:755–758. doi: 10.3748/wjg.v9.i4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 19.Maeda S, Yoshida H, Ogura K, Yamaji Y, Ikenoue T, Mitsushima T, Tagawa H, Kawaguchi R, Mori K, Mafune Ki, et al. Assessment of gastric carcinoma risk associated with Helicobacter pylori may vary depending on the antigen used: CagA specific enzyme-linked immunoadsorbent assay (ELISA) versus commercially available H. pylori ELISAs. Cancer. 2000;88:1530–1535. [PubMed] [Google Scholar]
- 20.Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harry XH. Association between Helicobacter pylori and gastric cancer: current knowledge and future research. World J Gastroenterol. 1998;4:93–96. doi: 10.3748/wjg.v4.i2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havlasová J, Bures J, Rejchrt S, Voxová B, Krejsek J. [Helicobacter pylori CagA antigen antibodies] Cas Lek Cesk. 1998;137:404–409. [PubMed] [Google Scholar]
- 23.Everhart JE, Kruszon-Moran D, Perez-Perez G. Reliability of Helicobacter pylori and CagA serological assays. Clin Diagn Lab Immunol. 2002;9:412–416. doi: 10.1128/CDLI.9.2.412-416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekström AM, Held M, Hansson LE, Engstrand L, Nyrén O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121:784–791. doi: 10.1053/gast.2001.27999. [DOI] [PubMed] [Google Scholar]
- 25.Andersen LP, Espersen F, Souckova A, Sedlackova M, Soucek A. Isolation and preliminary evaluation of a low-molecular-mass antigen preparation for improved detection of Helicobacter pylori immunoglobulin G antibodies. Clin Diagn Lab Immunol. 1995;2:156–159. doi: 10.1128/cdli.2.2.156-159.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madico G, Verástegui M. Serodiagnosis of Helicobacter pylori infection by enzyme-linked immunoelectrotransfer blot. Gastrointestinal Physiology Working Group of Cayetano Heredia University. J Diarrhoeal Dis Res. 1995;13:122–126. [PubMed] [Google Scholar]
- 27.Voland P, Weeks DL, Vaira D, Prinz C, Sachs G. Specific identification of three low molecular weight membrane-associated antigens of Helicobacter pylori. Aliment Pharmacol Ther. 2002;16:533–544. doi: 10.1046/j.1365-2036.2002.01221.x. [DOI] [PubMed] [Google Scholar]
- 28.Schistosomes , liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [Google Scholar]
- 29.Xu CD, Chen SN, Jiang SH, Xu JY. Seroepidemiology of Helicobacter pylori infection among asymptomatic Chinese children. World J Gastroenterol. 2000;6:759–761. doi: 10.3748/wjg.v6.i5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha SK, Martin B, Sargent M, McConnell JP, Bernstein CN. Age at acquisition of Helicobacter pylori in a pediatric Canadian First Nations population. Helicobacter. 2002;7:76–85. doi: 10.1046/j.1083-4389.2002.00063.x. [DOI] [PubMed] [Google Scholar]
- 31.Oleastro M, Matos R, Cabral J, Barros R, Lopes AI, Ramalho P, Monteiro L. Evaluation of a Western blot test, Helico blot 2.1, in the diagnosis of Helicobacter pylori infection in a pediatric population. Helicobacter. 2002;7:210–215. doi: 10.1046/j.1523-5378.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 32.Monteiro L, Bergey B, Gras N, Mégraud F. Evaluation of the performance of the Helico Blot 2.1 as a tool to investigate the virulence properties of Helicobacter pylori. Clin Microbiol Infect. 2002;8:676–679. doi: 10.1046/j.1469-0691.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- 33.Fusconi M, Vaira D, Menegatti M, Farinelli S, Figura N, Holton J, Ricci C, Corinaldesi R, Miglioli M. Anti-CagA reactivity in Helicobacter pylori-negative subjects: a comparison of three different methods. Dig Dis Sci. 1999;44:1691–1695. doi: 10.1023/a:1026647918258. [DOI] [PubMed] [Google Scholar]
- 34.Leri O, Teichner A, Sinopoli MT, Abbolito MR, Pustorino R, Nicosia R, Paparo Barbaro S. Heat-shock-proteins-antibodies in patients with Helicobacter pylori associated chronic gastritis. Riv Eur Sci Med Farmacol. 1996;18:45–47. [PubMed] [Google Scholar]
- 35.Vorobjova T, Ananieva O, Maaroos H, Sipponen P, Villako K, Utt M, Nilsson I, Wadström T, Uibo R. Seropositivity to Helicobacter pylori heat shock protein 60 is strongly associated with intensity of chronic inflammation, particularly in antrum mucosa: an extension of an 18-year follow-up study of chronic gastritis in Saaremaa, Estonia. FEMS Immunol Med Microbiol. 2001;30:143–149. doi: 10.1111/j.1574-695X.2001.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 36.Sheu BS, Shiesh SC, Wang JT, Yang HB, Lin ST, Wu JJ. Clinical application of 20 MHz endosonography and anti-Helicobacter pylori immunoblots to predict regression of low-grade gastric MALToma by H. pylori eradication. Helicobacter. 2003;8:36–45. doi: 10.1046/j.1523-5378.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- 37.Delchier JC, Lamarque D, Levy M, Tkoub EM, Copie-Bergman C, Deforges L, Chaumette MT, Haioun C. Helicobacter pylori and gastric lymphoma: high seroprevalence of CagA in diffuse large B-cell lymphoma but not in low-grade lymphoma of mucosa-associated lymphoid tissue type. Am J Gastroenterol. 2001;96:2324–2328. doi: 10.1111/j.1572-0241.2001.04036.x. [DOI] [PubMed] [Google Scholar]
- 38.Veenendaal RA, Peña AS, Meijer JL, Endtz HP, van der Est MM, van Duijn W, Eulderink F, Kreuning J, Lamers CB. Long term serological surveillance after treatment of Helicobacter pylori infection. Gut. 1991;32:1291–1294. doi: 10.1136/gut.32.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hook-Nikanne J, Varjonen E, Harvima RJ, Kosunen TU. Is Helicobacter pylori infection associated with chronic urticaria? Acta Derm Venereol. 2000;80:425–426. doi: 10.1080/000155500300012837. [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, Quyyumi AA, Muhlestein JB, Nieto FJ, Horne BD, Zalles-Ganley A, Anderson JL, Epstein SE. Lack of association of Helicobacter pylori infection with coronary artery disease and frequency of acute myocardial infarction or death. Am J Cardiol. 2002;89:155–158. doi: 10.1016/s0002-9149(01)02192-0. [DOI] [PubMed] [Google Scholar]


