Abstract
Aims
To provide comprehensive data on the diagnosis and treatment of autoimmune pancreatitis (AIP) patients in China.
Design
A systematic review.
Methods
All clinical studies concerning AIP from China published between January 2006 and June 2014 were retrospectively reviewed and analyzed.
Results
A total of 26 original articles involving 706 AIP patients were included with an estimated proportion of type 2 AIP as 4.7%. In the 706 AIP patients, the range of mean/median age was 48.6–67.0 years old and the male to female ratio was 4.47:1. The common presentations included obstructive jaundice (pooled rate: 63.4%, 95%CI: 55.4%–71.0%) and abdominal symptoms (pooled rate: 62.3%, 95%CI: 52.4%–71.7%). Biliary involvement was the most common extrapancreatic manifestations, especially the lower part of the common bile duct (pooled rate: 62.3%, 95%CI: 49.9%–73.9%). According to the imaging examinations, 53.8% and 41.6% patients were classified into focal-type and diffuse-type, respectively. Notably, upstream pancreatic duct dilatation was found in parts of patients (pooled rate: 13.8%, 95%CI: 6.6%–23.1%). The levels of serum IgG4 were elevated in most patients (pooled rate: 86.0%, 95%CI: 74.2%–94.6%). Nearly three tenths AIP patients received surgery (pooled rate: 29.7%, 95%CI: 18.1%–42.8%) due to mimicked malignancy. Steroid treatment was given to 78.4% patients (95%CI: 65.3%–89.1%) with a pooled remission rate of 96.2% (95%CI: 94.0%–97.9%). The pooled relapse rate was 13.8% (95%CI: 7.2%–22.0%) with the mean follow-up time ranging from 12 to 45 months.
Conclusion
Type 1 is the predominant type of Chinese AIP patients and the clinical features, diagnostic modalities and therapeutic regimen were similar with those in other countries. Knowledge of AIP should be more widespread to avoid unnecessary surgery.
Introduction
Since it was first reported in 1961 by Sarles et al [1] and was termed as autoimmune pancreatitis (AIP) in 1995 by Yoshida et al [2], AIP has gradually attracted attention in recent years. Nowadays, two subtypes of AIP are known—type 1 is a multi-organ disease associated with IgG4 while type 2 appears to be a pancreas-specific disorder [3]. In recent years, diagnostic criteria of AIP have been proposed in Japan, United States, Korea and Italy, and an International Consensus Diagnostic Criteria (ICDC) for AIP has been reached [4].
China has a high prevalence of pancreatic disease [5], and if the prevalence rate of AIP in Japan (2.2 per 100,000 populations) was used, the estimated amount of AIP patients would be 29 thousand [6]. However, there have been only a few reports about AIP from China [7–10]. In this study, we collected all well-designed clinical studies of AIP from China, and provided comprehensive data on the clinical presentation, diagnosis and treatment of Chinese AIP patients.
Methods
Strategy, criteria, and procedures for the literature search
We conducted a systematic literature search of PubMed, Web of Science and Chinese literature database including Wanfang Data and CNKI on June 20, 2014, and all publications related to AIP between January 2006 and June 2014 were retrieved. Indexing terms used for searching were autoimmune pancreatitis, IgG4-related disease, lymphoplasmacytic sclerosing pancreatitis, idiopathic duct centric pancreatitis, chronic pancreatitis with autoimmune diseases. Inclusion and exclusion criteria were delineated before commencement of the literature search. Inclusion criteria included: (1) cases were Chinese patients; (2) the diagnostic criteria of AIP were clearly defined and consistent with the current accepted ones, which included ICDC [4] and criteria proposed by Japan [11, 12], Mayo Clinic [13], Korea [14], and Asian (Korea-Japan symposium) [15]; (3) The number of patients in single article is ≥ 10; (4) if multiple articles were from the same institution, the one with most patients were selected. All articles other than original contributions, such as case reports, reviews, meta-analyses, editorials and letters were excluded. This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement checklist (S1 PRISMA Checklist).
Definitions
In order to include all relevant articles without loss of the fundamental meanings of the terms, clinical presentation and extrapancreatic manifestation were defined in a relatively broad way. Abdominal symptoms included abdominal pain or discomfort. Because the diagnostic criteria for acute pancreatitis were not clearly given in most of the literature, the presentation of acute pancreatitis mentioned in some articles was classified as abdominal symptoms. Bile duct involvement means bile duct stricture, divided into lower part of the common bile duct stricture and hilar/intrahepatic bile ducts stricture. Because ICDC clearly defines the subtypes of AIP, the proportion of type 1 and type 2 AIP was estimated according to studies adopting ICDC as criteria.
Data extraction from eligible articles
Data on the clinical presentations, laboratory tests, imaging examinations, pathological examinations, treatment and outcomes of the patients were retrieved. Missing data or indeterminate definitions were resolved by direct contact with authors if possible and were otherwise considered not available. Two authors (Q.Q.M., L.X.) determined the relevant original articles and extracted the data independently, whereas the third author (W.Y.L) checked the results. If a disagreement existed, the relevant programs were repeated until a consensus was achieved among the authors.
Statistical analysis
Descriptive analysis or meta-analysis was conducted to provide the overall data of clinical presentation, laboratory test, imaging examination, pathological examination, treatment and outcome of the patients. In the meta-analysis, Cochran’s Q test was used to test the heterogeneity of results across studies, and I2 was calculated to quantify the heterogeneity among studies. If the p value was less than 0.10 (Cochran’s Q test), the results were considered heterogeneous and random-effects model was used to obtain the pooled estimates. Otherwise, fixed-effects model was applied. The potential presence of publication bias was evaluated statistically by the Begg-Mazumdar’s and Egger’s test. Meta-analysis was performed with StatsDirect Statistical software, version 2.7.8 (StatsDirect Ltd, Altrincham, UK).
Results
A total of 26 articles (5 English-language and 21 Chinese-language articles) were enrolled in the study ([8, 9, 16] and S1 File). The detailed search procedures are outlined in Fig 1. The detailed data related to diagnostic criteria for AIP are outlined in Table 1. In the 5 studies which adopted ICDC as criteria, the proportion of type 2 AIP was 4.7% (8/171). Detailed data and forest plots for all of the variables are listed in S2 File.
Fig 1. Flow chart for literature search on original articles about Chinese AIP patients.
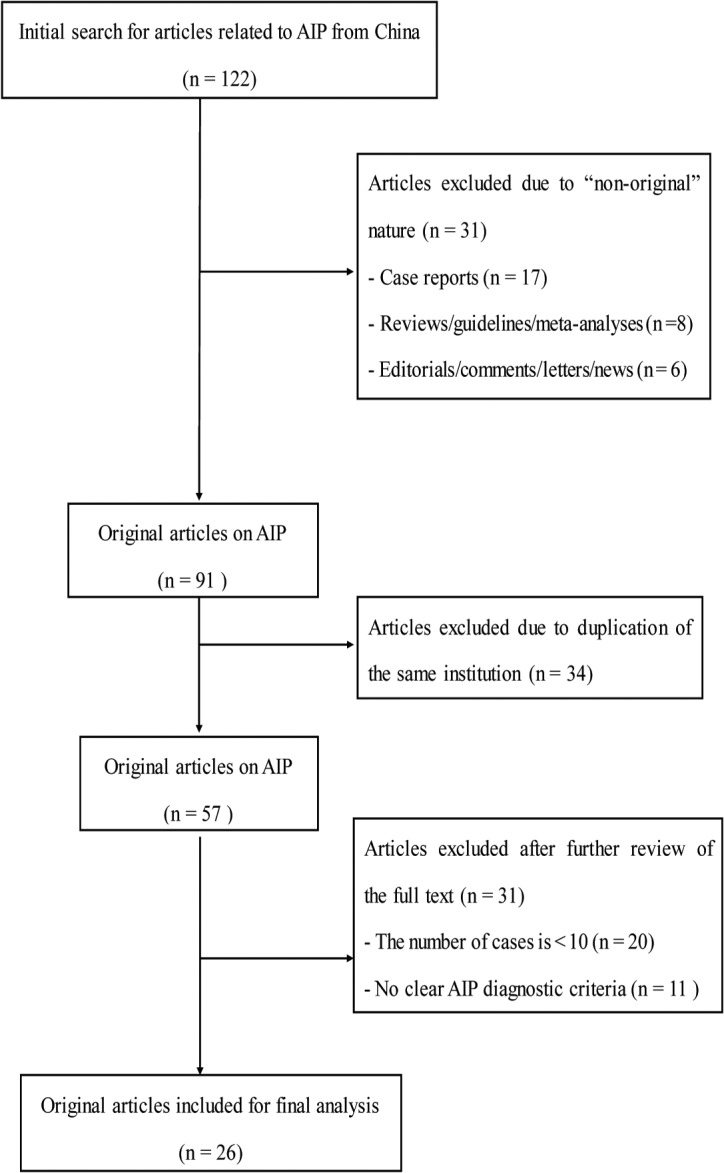
Table 1. The diagnostic criteria for AIP adopted by 26 studies from China (N = 706)a.
| Diagnostic Criteria | No.of studies (%) | No. of cases (%) |
|---|---|---|
| Japanese criteria | 7 (26.9) | 126 (17.8) |
| Mayo Clinic's HISORt criteria | 3 (11.5) | 150 (21.2) |
| Korean criteria | 2 (7.7) | 30 (4.2) |
| Asian diagnostic criteria | 12(46.2) | 416(58.9) |
| ICDC | 5(19.2) | 171(24.2) |
a Different kinds of diagnostic criteria for AIP were adopted by three studies
Demographics and Clinical Presentation
Among the 706 AIP patients, 577 were men (male/female: 4.47:1) and the range of mean/median age of the patients was 48.6–67.0 years old.
Clinical presentation of AIP patients were described in all 26 articles. Obstructive jaundice is the most common presentation (pooled rate: 63.4%, 95%CI: 55.4%–71.0%). Abdominal symptoms was another common presentation (pooled rate: 62.3%, 95%CI: 52.4%–71.7%). The other presentations included weight loss (pooled rate: 45.1%, 95%CI: 34.8%–55.6%) and diabetes mellitus (pooled rate: 26.9%, 95%CI: 18.3%–36.4%). Moreover, pancreatic mass or enlargement was found incidentally by imaging examinations in some asymptomatic patients (pooled rate: 2.9%, 95%CI: 1.6%–4.5%) (Table 2).
Table 2. Pooled positive rate of various manifestation in Chinese AIP patients.
| Tests of Heterogeneity | Tests of Publication Bias | |||||||
|---|---|---|---|---|---|---|---|---|
| Manifestation | Studies No. | Positive Cases / Total Cases | Pooled Positive Rate, % (95%CI) | Q Value | P | I 2 (%) | Begg’s P | Egger’s P |
| Clinical presentation | ||||||||
| Obstructive jaundice | 26 | 432/706 | 63.4(55.4–71.0) | 116.89 | <0.0001 | 78.6 | 0.1932 | 0.0185 |
| Abdominal symptoms | 26 | 382/706 | 62.3(52.4–71.7) | 178.82 | <0.0001 | 86 | 0.3655 | 0.8228 |
| Weight loss | 18 | 206/495 | 45.1(34.8–55.6) | 94.18 | <0.0001 | 81.9 | 0.2107 | 0.0008 |
| Diabetes mellitus | 23 | 157/595 | 26.9(18.3–36.4) | 139.53 | <0.0001 | 84.2 | 0.4918 | <0.0001 |
| Asymptomatic patients | 26 | 22/706 | 2.9(1.6–4.5) | 34.23 | 0.103 | 27 | <0.0001 | 0.1127 |
| Extrapancreatic manifestation | ||||||||
| Lower part of the common bile duct stricture | 19 | 290/473 | 62.3(49.9–73.9) | 131.11 | <0.0001 | 86.3 | 0.7792 | 0.0086 |
| Hilar/intra hepatic bile ducts stricture | 17 | 32/409 | 3.6(1.1–7.3) | 45.66 | 0.0001 | 65 | 0.0063 | 0.7545 |
| Abdominal lymph nodes enlargement | 20 | 99/420 | 19.7(9.2–32.9) | 190.81 | <0.0001 | 90 | 0.0007 | 0.0139 |
| Swelling salivary gland | 18 | 51/466 | 12.0(5.7–20.3) | 98.38 | <0.0001 | 82.7 | <0.0001 | 0.0022 |
| Swelling lacrimal gland | 18 | 12/504 | 2.7(1.0–5.1) | 32.33 | 0.0137 | 47.4 | <0.0001 | 0.0165 |
| Symptom similar with Sjögren syndrome | 12 | 26/274 | 8.9(4.2–15.1) | 29.24 | 0.0021 | 62.4 | 0.0088 | 0.0038 |
| Interstitial nephritis | 18 | 29/515 | 4.6(1.9–8.5) | 52.57 | <0.0001 | 67.7 | 0.0003 | 0.0361 |
| Retroperitoneal fibrosis | 14 | 15/464 | 3.4(2.0–5.2) | 16.84 | 0.2066 | 22.8 | 0.0546 | 0.1481 |
| Interstitial lung disease | 16 | 15/471 | 3.0(1.0–6.0) | 36.92 | 0.0013 | 59.4 | 0.0001 | 0.0844 |
| Ulcerative colitis | 14 | 4/446 | 1.6(0.6–2.9) | 4.19 | 0.989 | 0 | 0.0748 | 0.7726 |
The details of extrapancreatic manifestation were reported in 20 articles. Biliary involvement was the most common extrapancreatic manifestation, which included lower part of the common bile duct stricture (pooled rate: 62.3%, 95%CI: 49.9%–73.9%) and hilar/intra hepatic bile ducts stricture (pooled rate: 3.6%, 95%CI: 1.1%–7.3%). Abdominal lymph nodes enlargement is a relatively common manifestation (pooled rate: 19.7%, 95%CI: 9.2%–32.9%). Fifty-one (pooled rate: 12.0%, 95%CI: 5.7%–20.3%) patients and 12 (pooled rate: 2.7%, 95%CI: 1.0%–5.1%) patients have swelling salivary gland and swelling lacrimal gland, respectively, while 26 (pooled rate: 8.9%, 95%CI: 4.2%–15.1%) patients have symptom similar with Sjögren syndrome. Other extrapancreatic manifestations included interstitial nephritis (pooled rate: 4.6%, 95%CI: 1.9%–8.5%), retroperitoneal fibrosis (pooled rate: 3.4%, 95%CI: 2.0%–5.2%), interstitial lung disease (pooled rate: 3.0%, 95%CI: 1.0%–6.0%) and ulcerative colitis (pooled rate: 1.6%, 95%CI: 0.6%–2.9%) (Table 2).
Imaging examinations
The general manifestation of pre-treatment computed tomography (CT), magnetic resonance imaging (MRI) or ultrasound examination was reported in 627 patients from 25 articles. Among these 627 patients, 337 (53.8%) were classified into diffuse-type, 261 (41.6%) were focal-type and 29 (4.6%) with atypical imaging manifestation could not be clearly classified into either type. Most lesions of focal-type were located in the head (74.4%, 174/234), and the other were located in the body (11.5%, 27/234) and the tail (14.1%, 33/234). Delayed enhancement was the most typical manifestation (272/286, pooled rate: 94.4%, 95%CI: 88.5%–98.2%). Moreover, rim-like enhancement is found in considerable part of patients (226/445, pooled rate: 62.7%, 95%CI: 48.3%–76.0%). Calcification in parenchyma (18/487, pooled rate: 3.9%, 95%CI: 1.8%–6.8%) and pancreatic pseudocysts (14/487, pooled rate: 3.4%, 95%CI: 2.0%–5.1%) were rare manifestations (Table 3).
Table 3. Pooled positive rate of various imaging manifestations in Chinese AIP patients.
| Tests of Heterogeneity | Tests of Publication Bias | |||||||
|---|---|---|---|---|---|---|---|---|
| Imaging Manifestation | Studies No. | Positive Cases/ Total Cases | Pooled Positive Rate, % (95%CI) | Q Value | P | I 2 (%) | Begg’s P | Egger’s P |
| Parenchymal imaging | ||||||||
| Delayed enhancement | 13 | 272/286 | 94.4 (88.5–98.2) | 38.41 | 0.0001 | 68.8 | <0.0001 | 0.0018 |
| Rim-like enhancement | 18 | 226/445 | 62.7 (48.3–76.0) | 162.47 | <0.0001 | 89.5 | 0.3686 | 0.5849 |
| Calcification in parenchyma | 21 | 18/487 | 3.9 (1.8–6.8) | 39.94 | 0.0051 | 49.9 | <0.0001 | 0.0033 |
| Pancreatic pseudocysts | 21 | 14/487 | 3.4 (2.0–5.1) | 17.38 | 0.6281 | 0 | 0.0303 | 0.0394 |
| Ductal imaging | ||||||||
| Stricture of the pancreatic duct | 18 | 231/347 | 68.1 (49.3–84.3) | 232.83 | <0.0001 | 92.7 | 0.0813 | 0.0035 |
| Dilation of pancreatic duct | 19 | 64/367 | 13.8 (6.6–23.1) | 101.00 | <0.0001 | 82.2 | <0.0001 | 0.0007 |
| Pancreatic duct calculi | 20 | 6/460 | 1.9 (0.9–3.3) | 11.97 | 0.8869 | 0 | <0.0001 | 0.2087 |
In the aspect of pancreatic duct, about two-thirds patients showed stricture of pancreatic duct (231/347, pooled rate: 68.1%, 95%CI: 49.3%–84.3%) and dilatation was also found in parts of patients (64/367, pooled rate: 13.8%, 95%CI: 6.6%–23.1%). Pancreatic duct calculi was rare manifestation (6/460, pooled rate: 1.9%, 95%CI: 0.9%–3.3%) (Table 3).
Laboratory tests
Various serum markers were measured in different amount of patients (Table 4). In 9 studies, the level of serum IgG4 exceeds the upper limit of normal in 170 of 195 patients (pooled rate: 86.0%, 95%CI: 74.2%–94.6%). The positive rates of IgG and IgE were 65.7% (257/391, pooled rate: 70.2%, 95%CI: 60.6%–79.1%) and 43.4% (49/113, pooled rate: 61.8%, 95%CI: 23.7%–92.9%), respectively.
Table 4. Pooled positive rate of various serum markers in Chinese AIP patients.
| Tests of Heterogeneity | Tests of Publication Bias | |||||||
|---|---|---|---|---|---|---|---|---|
| Serum markers | Studies No | Positive Cases / Total Cases | Pooled Positive Rate, % (95%CI) | Q Value | P | I 2(%) | Begg’s P | Egger’s P |
| IgG4 a | 9 | 170/195 | 86.0(74.2–94.6) | 31.71 | 0.0001 | 74.8 | 0.0446 | 0.0265 |
| IgG b | 19 | 257/391 | 70.3(60.6–79.1) | 74.87 | <0.0001 | 76 | 0.195 | 0.3595 |
| IgE c | 4 | 49/113 | 61.8(23.7–92.9) | 37.6 | <0.0001 | 91.9 | 0.75 | 0.7564 |
| γ-globulin | 12 | 154/225 | 67.9(56.9–78.0) | 34.67 | 0.0003 | 68.3 | 0.0138 | 0.0019 |
| Rheumatoid factor | 6 | 34/110 | 35.7(20.7–52.2) | 14.75 | 0.0115 | 66.1 | 0.2722 | 0.0517 |
| ANA d | 13 | 83/266 | 33.3(24.8–42.4) | 29.21 | 0.0037 | 58.9 | 0.0573 | 0.074 |
| ESR e | 7 | 65/109 | 59.4(50.4–68.2) | 10.20 | 0.1163 | 41.2 | 0.1668 | 0.0051 |
| CA19-9 | 18 | 178/411 | 41.5(34.9–48.3) | 32.16 | 0.0144 | 47.1 | 0.9697 | 0.3708 |
aImmunoglobulin G4
bImmunoglobulin G
cImmunoglobulin E
A
dAnti-neutrophil antibody
eErythrocyte sedimentation rate
Pathology
In the 706 patients, pancreatic cytology specimens were obtained in 79 (11.2%) by endoscopic ultrasonography (EUS)-guided or CT-guided fine-needle aspiration (FNA) and no malignant or atypical cells were found. Pancreatic histology specimens were obtained from 220 (31.2%) patients by surgery. A total of 212 and 8 histology examinations supported the diagnosis of lymphoplasmacytic sclerosing pancreatitis and idiopathic duct-centric pancreatitis respectively. In the 66 patients (4 studies) receiving pancreatic immunohistochemical examination, marked IgG4 positive cells (>10 cells / high-power fields [HPF]) were found in 58 (pooled rate: 88.7%, 95%CI: 65.2%–99.7%) patients.
Treatment and follow-up
Due to mimicked malignancy, nearly one third AIP patients received surgery (pooled rate: 29.7%, 95%CI: 18.1%–42.8%). Surgical procedures included pancreaticoduodenectomy (43.0%, 52/121), distal pancreatectomy with or without splenectomy (9.1%, 11/121), choledojejunostomy with the resection of hilar mass (1.6%, 2/121), biliary-entericanastomosis (27.3%, 33/121) and exploratory laparotomy (19.0%, 23/121).
Steroid treatment was given to most patients (pooled rate: 78.4%, 95%CI: 65.3%–89.1%). In the 272 patients with detailed regimen, a fixed dose (30mg or 40mg prednisone per day) were given as the initial dose to 206 (75.7%) patients, while a personalized initial dose (0.6mg/kg per day) were given to other 66 (24.3%) patients. After steroid therapy, most patients got remission (pooled rate: 96.2%, 95%CI: 94.0%–97.9%). In 269 patients receiving steroid treatment and having follow-up data, relapse occurred in 37 patients (pooled rate: 13.8%, 95%CI: 7.2%–22.0%) with the mean follow-up time ranging from 12 to 45 months. Repeated steroid treatment was given to most patients with relapse (pooled rate: 95.8%, 95%CI: 89.8%–99.3%) and obtained good remission outcome (pooled rate: 94.8%, 95%CI: 88.2%–98.8%) (Table 5).
Table 5. Pooled positive rate of treatment and prognosis in Chinese AIP patients.
| Tests of Heterogeneity | Tests of Publication Bias | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment and Outcome | Studies No. | Positive Cases / Total Cases | Pooled Positive Rate, % (95%CI) | Q Value | P | I 2(%) | Begg’s P | Egger’s P |
| Surgery treatment | 25 | 236/689 | 29.7(18.1–42.8) | 317.31 | <0.0001 | 92.4 | 0.0082 | 0.3828 |
| Steroid treatment | 21 | 383/603 | 78.4(65.3–89.1) | 262.80 | <0.0001 | 92.4 | 0.013 | 0.0137 |
| Remission after steroid treatment | 20 | 333/346 | 96.2(94.0–97.9) | 23.76 | 0.2057 | 20 | <0.0001 | 0.072 |
| Relapse after steroid treatment | 12 | 37/269 | 13.8(7.2–22.0) | 34.91 | 0.0003 | 68.5 | 0.459 | 0.0015 |
| Repeated steroid treatment after relapse | 8 | 58/59 | 95.8(89.8–99.3) | 2.37 | 0.9365 | 0 | 0.0786 | 0.7643 |
| Remission after repeated steroid treatment | 8 | 56/58 | 94.8(88.2–98.8) | 4.28 | 0.7466 | 0 | 0.0786 | 0.6022 |
Discussion
It has been nearly two decades since Yoshida et al. proposed the term autoimmune pancreatitis in 1995 [2] and AIP has now become a focus in research. Our study provided the comprehensive data of Chinese AIP patients. This study showed that the clinical features of AIP patients in China were consistent with the reports from other countries [6, 13, 17] except for the considerable proportion of pancreatic duct dilation (13.8%). Nearly one-third patients (29.7%) underwent surgeries due to suspected malignancy. The steroid regimen with an initial dose of 30 mg, 40 mg or 0.6mg/kg prednisone per day was effective in most patients with low rates of relapse and complications.
AIP is a rare disease and still a diagnostic challenge despite of increasing study in recent years. As a region with high prevalence of pancreatic disease, China would have a large amount of AIP patients. However, the concept of AIP has just been introduced in this decade and the first clinical report about Chinese AIP patients was published in 2008 [7]. In this study, we could only collect 706 patients from 26 articles, which suggested a large gap in the study of AIP between China and developed countries. Nowadays, several AIP diagnostic criteria including Japanese criteria, Mayo Clinic's HISORt criteria, Korean criteria, Asian diagnostic criteria and ICDC were proposed. Our study showed that the criteria above were adopted by different institutions in China, and 58.9% of AIP patients were diagnosed by Asian diagnostic criteria. Asian diagnostic criteria are simple and have good practicability, and may be more suitable for Chinese AIP patients due to similar ethnic background.
In ICDC, AIP is classified into two subtypes. Type 1 is recognized to be part of IgG4-related disease and type 2 is characterized by intraductal neutrophilic infiltration and no IgG4 elevation [4]. Type 2 only covered a proportion of 1% to 6% AIP patients in East Asia, while this proportion increased to 15% to 38% in Europe and North America [3, 18–20]. The present study revealed that type 2 AIP covered a proportion of 4.7% in China. This low proportion was consistent with the previous studies from Japan and Korea. Notably, type 2 AIP is much more difficult to be diagnosed because there is no specific serum marker and histological examination is always required. However, in clinical practice, an adequate specimen of the pancreas is not frequently available. In this study, 11.2% AIP patients received EUS-guided or CT-guided FNA but most of them only obtained cytology diagnosis. Several recent studies also showed the difficulty of obtaining adequate specimens for histopathological evaluation through FNA [21, 22]. From this perspective, the true prevalence of type 2 AIP in China may be underestimated. Due to the low proportion of type 2, our study mainly showed the features of type 1 AIP.
The clinical presentation of Chinese AIP was similar with previous reports from other countries [6, 13, 17]. The most common presentation were obstructive jaundice (pooled rate: 63.4%, 95%CI: 55.4%–71.0%) and abdominal symptoms (pooled rate: 62.3%, 95%CI: 52.4%–71.7%), both of which are nonspecific symptoms. Extrapancreatic lesion is an important diagnostic clue and evidence for AIP. In this study, we found that the proportions of biliary involvement, abdominal lymph nodes enlargement, swelling salivary gland, swelling lacrimal gland, retroperitoneal fibrosis, interstitial nephritis and interstitial lung disease were 65.9%, 19.7%, 12.0%, 2.0%, 3.4%, 4.6% and 3.0%, respectively. Biliary involvement, namely, IgG4-related sclerosing cholangitis is characterized by lower and upper bile duct stricture. Our study showed that the lower common bile duct stricture and hilar/intra hepatic bile ducts stricture covered 62.3% and 3.6% patients, respectively, which was similar with previous reports [23, 24]. Ulcerative colitis was more common seen in type 2 AIP than in type 1 [4]. In an international survey of AIP, ulcerative colitis was found in 15.6% (10/64) type 2 AIP patients and 1.3% (2/153) type 1 AIP patients [20]. Another study from United States showed that ulcerative colitis was present in 15.8% (3/19) with type 2 patients and 4.0% (2/50) with type 1 patients [25]. Our study also showed a low proportion of ulcerative colitis (pooled rate: 1.6%, 95%CI: 0.6%–2.9%) in Chinese AIP patients.
CT and MRI are main pancreatic parenchymal imaging modalities and play an essential role in the diagnosis of AIP. Our study showed some differences compared with ICDC. In ICDC, diffuse enlargement and segmental/focal enlargement are proposed to be typical and indeterminate findings, respectively [4]. However, in our study, 41.6% patients were classified into focal-type. The relatively high proportion of focal-type may be attributed to the fact that about one third patients obtained diagnosis of AIP after surgery and focal-type patients tends to receive surgery with suspect of malignancy, which led to potential selection bias. Moreover, pancreatic duct imaging was adopted by ICDC as an important diagnostic evidence for AIP and long and multiple strictures were recognized as the main manifestation, while dilation of the pancreatic duct was recognized as a rare manifestation to differentiate AIP from pancreatic cancer. In our study, about two-thirds patients showed stricture of pancreatic duct (pooled rate: 68.1%, 95%CI: 49.3%–84.3%) but dilatation was also found in 13.8% patients. In the previous study from our center [16], marked upstream dilatation (duct size > 5 mm) upon stricture was seen in 16.0% (4/25) patients and in another report from China [9], dilation of pancreatic duct were found in 45.5% (10/22) type 1 AIP patients. Theoretically, the pathogenesis of AIP is different with ordinary chronic pancreatitis and dilated and irregularly shaped medium-sized and large ducts are rarely found in histology examination. Therefore, further study is needed to clarify the pancreatic duct imaging manifestation of Chinese AIP patients.
It was reported that the sensitivity of IgG4 elevation varies between 44%–95% in different settings [17, 26]. In our study, the level of serum IgG4 exceeds the upper limit of normal were observed in 86.0% patients in 9 (34.6%) articles, which indicated the similar diagnostic value for Chinese AIP patients. In the other 17 (65.4%) studies, IgG4 test could not be performed and the diagnosis of AIP depended on other evidences. However, IgG4 was the only recommended serological marker for type 1 AIP in the ICDC, performing IgG4 test would improve the accuracy and simplicity of diagnosing AIP.
Steroid treatment was given to nearly 80% AIP patients in China, including many patients who received surgery for suspected malignancy. AIP is highly responsive to steroid treatment. To date, although the ICDC defines the initial dose of steroid for induction of remission as 0.6 to 1 mg/kg per day, a steroid regimen has not been standardized, and there is no consensus on the duration of induction, the tapering schedule, and the optimal dose and duration of maintenance therapy [4, 27]. In China, an initial of 30mg or 40mg prednisone per day is adopted as the most commonly used regimen and our study showed the pooled remission rate as 96.2%, which proved the excellent response of Chinese AIP patients to steroid. Relapse after steroid treatment is an important issue. According to a multicenter, international survey, the relapse rate was 35.8% (245/684) after a long-term follow-up [28]. A study from Mayo Clinic showed that 44.8% (52/116) AIP patients experienced relapse during a median follow-up of 47 months [29]. In our study, the pooled rate of relapse was only 13.8% (37/269) with the mean follow-up time ranging from 12 to 45 months. A shorter follow-up time may explain the relatively low relapse rate in China. However, a report from Japan also showed the relapse was 12.5% after a mean follow- up of 43.5 months [30]. We think different steroid regimens may be another explanation. Maintenance steroid therapy, which could decrease relapse [31], was not adopted in most reports from Western countries but was always adopted in Eastern countries [32]. From this perspective, maintenance therapy may be a better choice for patients at high risk for relapse.
Limitations
There are several limitations of this study. First, due to the nature of this systematic review, publication bias could not be avoided and significant heterogeneity existed in many aspects. We believed this is a suboptimal and feasible option to provide an overview of AIP in China, when a national study is not available. Second, type 1 and type 2 were clearly defined in ICDC in 2011; however, not all enrolled studies adopted these criteria, so we could not exactly distinguish type 1 and type 2 AIP. However, with low proportion of type 2, our study mainly showed the features of Chinese type 1 AIP patients.
Conclusions
In conclusion, type 1 is the predominant type of Chinese AIP patients and the clinical features, diagnostic modalities and therapeutic regimen were fundamentally similar with those in other countries. Knowledge of AIP should be more widespread to avoid unnecessary surgery.
Supporting Information
(DOC)
(DOC)
(RTF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by National Science Foundation of Shanghai (Grant No. 12ZR1438800, http://www.stcsm.gov.cn/, [LWW]). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas—an autonomous pancreatic disease? Am J Dig Dis. 1961; 6: 688–698. [DOI] [PubMed] [Google Scholar]
- 2. Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995; 40: 1561–1568. [DOI] [PubMed] [Google Scholar]
- 3. Sah RP, Chari ST. Autoimmune pancreatitis: an update on classification, diagnosis, natural history and management. Curr Gastroenterol Rep. 2012; 14: 95–105. 10.1007/s11894-012-0246-8 [DOI] [PubMed] [Google Scholar]
- 4. Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011; 40:352–358. 10.1097/MPA.0b013e3182142fd2 [DOI] [PubMed] [Google Scholar]
- 5. Wang LW, Li ZS, Li SD, Jin ZD, Zou DW, Chen F. Prevalence and clinical features of chronic pancreatitis in China: a retrospective multicenter analysis over 10 years. Pancreas. 2009; 38: 248–254. 10.1097/MPA.0b013e31818f6ac1 [DOI] [PubMed] [Google Scholar]
- 6. Kanno A, Nishimori I, Masamune A, Kikuta K, Hirota M, Kuriyama S, et al. Nationwide epidemiological survey of autoimmune pancreatitis in Japan. Pancreas. 2012; 41: 835–839. 10.1097/MPA.0b013e3182480c99 [DOI] [PubMed] [Google Scholar]
- 7. Song Y, Liu QD, Zhou NX, Zhang WZ, Wang DJ. Diagnosis and management of autoimmune pancreatitis: experience from China. World J Gastroenterol. 2008; 14: 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu B, Li J, Yan LN, Sun HR, Liu T, Zhang ZX. Retrospective study of steroid therapy for patients with autoimmune pancreatitis in a Chinese population. World J Gastroenterol. 2013; 19: 569–574. 10.3748/wjg.v19.i4.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang XG, Zhang XP, Li W, Jiang L, Zhang XL, Gao Y, et al. Clinical analysis of 36 cases of autoimmune pancreatitis in China. PLoS One. 2012; 7: e44808, 10.1371/journal.pone.0044808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang MM, Zou DW, Wang Y, Zheng JM, Yang H, Jin ZD, et al. Contrast enhanced ultrasonography in the diagnosis of IgG4-negative autoimmune pancreatitis: A case report. J Interv Gastroenterol. 2011; 1: 182–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Members of the Criteria Committee for Autoimmune Pancreatitis of the Japan Pancreas Society. Diagnostic criteria for autoimmune pancreatitis by the Japan Pancreas Society. Suizo. 2002; 17:585–587. [Google Scholar]
- 12. Shimosegawa T; Working group members of the Japan Pancreas Society; Research Committee for Intractable Pancreatic Disease by the Ministry of Labor, Health and Welfare of Japan. The amendment of the Clinical Diagnostic Criteria in Japan (JPS2011) in response to the proposal of the International Consensus of Diagnostic criteria (ICDC) for autoimmune pancreatitis. Pancreas. 2012; 41:1341–1342. 10.1097/MPA.0b013e3182706ed5 [DOI] [PubMed] [Google Scholar]
- 13. Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006; 4: 1010–1016. [DOI] [PubMed] [Google Scholar]
- 14. Kim KP, Kim MH, Kim JC, Lee SS, Seo DW, Lee SK. Diagnostic criteria for autoimmune chronic pancreatitis revisited. World J Gastroenterol. 2006; 12:2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008; 43:403–408. 10.1007/s00535-008-2205-6 [DOI] [PubMed] [Google Scholar]
- 16. Xin L, He YX, Zhu XF, Zhang QH, Hu LH, Zou DW, et al. Diagnosis and treatment of autoimmune pancreatitis: experience with 100 patients. Hepatobiliary Pancreat Dis Int. 2014; 13:642–648. [DOI] [PubMed] [Google Scholar]
- 17. Frulloni L, Scattolini C, Falconi M, Zamboni G, Capelli P, Manfredi R, et al. Autoimmune pancreatitis: differences between the focal and diffuse forms in 87 patients. Am J Gastroenterol. 2009; 104: 2288–2294. 10.1038/ajg.2009.327 [DOI] [PubMed] [Google Scholar]
- 18. Song TJ, Kim JH, Kim MH, Jang JW, Park do H, Lee SK, et al. Comparison of clinical findings between histologically confirmed type 1 and type 2 autoimmune pancreatitis. J Gastroenterol Hepatol. 2012; 27:700–708. 10.1111/j.1440-1746.2011.06934.x [DOI] [PubMed] [Google Scholar]
- 19. Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimoseqawa T, et al. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas. 2010; 39: 549–554. 10.1097/MPA.0b013e3181e4d9e5 [DOI] [PubMed] [Google Scholar]
- 20. Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011; 40: 809–814. 10.1097/MPA.0b013e3182258a15 [DOI] [PubMed] [Google Scholar]
- 21. Kanno A, Ishida K, Hamada S, Fujishima F, Unno J, Kume K, et al. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc. 2012; 76:594–602. 10.1016/j.gie.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 22. Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, et al. Endoscopic ultrasound-guided fine needle aspiration in the differentiation of type 1 and type 2 autoimmune pancreatitis. World J Gastroenterol. 2012; 18:3883–3888. 10.3748/wjg.v18.i29.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watanabe T, Maruyama M, Ito T, Maruyama M, Muraki T, Hamano H, et al. Mechanisms of lower bile duct stricture in autoimmune pancreatitis. Pancreas. 2014; 43:255–260. 10.1097/01.mpa.0000437323.52598.20 [DOI] [PubMed] [Google Scholar]
- 24. Hirano K, Tada M, Isayama H, Yamamoto K, Mizuno S, Yaqioka H, et al. Endoscopic evaluation of factors contributing to intrapancreatic biliary stricture in autoimmune pancreatitis. Gastrointest Endosc. 2010; 71:85–90. 10.1016/j.gie.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 25. Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010; 139: 140–148. 10.1053/j.gastro.2010.03.054 [DOI] [PubMed] [Google Scholar]
- 26. Song TJ, Kim MH, Moon SH, Eum JB, Park do H, Lee SS, et al. The combined measurement of total serum IgG and IgG4 may increase diagnostic sensitivity for autoimmune pancreatitis without sacrificing specificity, compared with IgG4 alone. Am J Gastroenterol. 2010; 105: 1655–1660. 10.1038/ajg.2009.689 [DOI] [PubMed] [Google Scholar]
- 27. Kamisawa T, Okazaki K, Kawa S, Ito T, Inui K, Irie H, et al. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 III. Treatment and prognosis of autoimmune pancreatitis. J Gastroenterol. 2014; 49:961–970. 10.1007/s00535-014-0945-z [DOI] [PubMed] [Google Scholar]
- 28. Hart PA, Kamisawa T, Brugge WR, Chung JB, Culver EL, Czakó L, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut.2013; 62:1771–1776. 10.1136/gutjnl-2012-303617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hart PA, Topazian MD, Witzig TE, Clain JE, Gleeson FC, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut. 2013; 62:1607–1615. 10.1136/gutjnl-2012-302886 [DOI] [PubMed] [Google Scholar]
- 30. Kamisawa T, Okamoto A, Wakabayashi T, Watanabe H, Sawabu N. Appropriate steroid therapy for autoimmune pancreatitis based on long-term outcome. Scand J Gastroenterol. 2008; 43:609–613. 10.1080/00365520701731263 [DOI] [PubMed] [Google Scholar]
- 31. Hart PA, Zen Y, Chari ST. Recent Advances in Autoimmune Pancreatitis. Gastroenterology. 2015; in press. [DOI] [PubMed] [Google Scholar]
- 32. Kamisawa T, Shimosegawa T, Okazaki. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009; 58:1504–1507. 10.1136/gut.2008.172908 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(RTF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


