Peripheral T-cell lymphomas remain clinically challenging in the modern era with 3-year progression-free and overall survivals of 32% and 52% in this multicenter analysis. Favorable prognostic factors were stage I-II disease and complete response to upfront therapy. Consolidation with stem-cell transplant offered no clear benefit in first remission. Novel treatment approaches are needed.
Keywords: peripheral T-cell lymphoma, PTCL, anaplastic large-cell lymphoma, angioimmunoblastic T-cell lymphoma, stem-cell transplantation
Abstract
Background
Optimal frontline therapy for peripheral T-cell lymphoma (PTCL) in the modern era remains unclear.
Patients and methods
We examined patient characteristics, treatment, and outcomes among 341 newly diagnosed PTCL patients from 2000 to 2011. Outcome was compared with a matched cohort of diffuse large B-cell lymphoma (DLBCL) patients, and prognostic factors were assessed using univariate and multivariate analyses.
Results
PTCL subtypes included PTCL, not otherwise specified (PTCL-NOS) (31%), anaplastic large T-cell lymphoma (ALCL) (26%), angioimmunoblastic T-cell lymphoma (23%), NK/T-cell lymphoma (7%), acute T-cell leukemia/lymphoma (6%), and other (7%). Median age was 62 years (range 18-95 years), and 74% had stage III-IV disease. Twenty-three (7%) patients received only palliative care whereas 318 received chemotherapy: CHOP-like regimens (70%), hyperCVAD/MA (6%), or other (18%). Thirty-three patients (10%) underwent stem-cell transplantation (SCT) in first remission. The overall response rate was 73% (61% complete); 24% had primary refractory disease. With 39-month median follow-up, 3-year progression-free survival (PFS) and overall survival (OS) were 32% and 52%. PFS and OS for PTCL patients were significantly inferior to matched patients with DLBCL. On multivariate analysis, stage I–II disease was the only significant pretreatment prognostic factor [PFS: hazard ratio (HR) 0.54, 95% confidence interval (CI) 0.34–0.85, P = 0.007; OS: HR 0.42, 95% CI 0.22–0.78, P = 0.006]. ALK positivity in ALCL was prognostic on univariate analysis, but lost significance on multivariate analysis. The most dominant prognostic factor was response to initial therapy (complete response versus other), including adjustment for stage and SCT [PFS: HR 0.19, 95% CI 0.14–0.28, P < 0.0001; OS: HR 0.26, 95% CI 0.17–0.40, P < 0.0001]. No overall survival difference was observed based on choice of upfront regimen or SCT in first remission.
Conclusions
This analysis identifies early-stage disease and initial treatment response as dominant prognostic factors in PTCL. No clear benefit was observed for patients undergoing consolidative SCT. Novel therapeutic approaches for PTCL are critically needed.
introduction
Peripheral T-cell lymphomas (PTCL) are a heterogeneous collection of diseases that account for ∼12% of non-Hodgkin lymphomas diagnosed annually. The most common subtype in North America is PTCL not otherwise specified (PTLC-NOS), followed by anaplastic large T-cell lymphoma (ALCL), angioimmunoblastic T-cell lymphoma (AITL), enteropathy-associated T-cell lymphoma (EATL), NK/T-cell lymphoma (NKTL), and others [1]. Prognosis varies widely across subtypes [1].
Treatment of PTCL has primarily been extrapolated from studies of aggressive lymphomas, which have included few PTCL patients. CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) is the most widely employed regimen based on these data, although this is rarely curative. The addition of etoposide improves event-free survival in younger patients, but without clear impact on OS [2]. Consolidative autologous stem-cell transplantation (SCT) in first remission may improve outcome, though this is largely based on retrospective data and remains controversial [3, 4].
We report a large multicenter analysis of PTCLs treated during the modern era, including examination of frontline therapy and impact of SCT.
methods
patients
We conducted a multicenter retrospective analysis of 401 newly diagnosed PTCL patients from nine US academic centers diagnosed from January 2000 to December 2010. All consecutive patients were identified within databases from participating institutions. The number of patients enrolled per center is included as supplementary Table S1, available at Annals of Oncology online . Thirty-one patients were excluded due to lack of treatment data, 16 due to incomplete follow-up, and 13 due to lack of confirmed diagnosis. This study was approved by the Institutional Review Boards at each center. Baseline data collected at diagnosis included age, gender, histological subtype, stage, performance status, selected comorbid diseases, ‘B’ symptoms, prognostic indices, initial chemotherapy, and SCT. Diagnoses were confirmed by hematopathologists at each institution per the World Health Organization (WHO) classification [5], but pathology was not centrally re-reviewed. Staging and treatment were carried out at the discretion of treating physicians.
statistics
Differences in patient characteristics were compared with two-tailed χ 2 tests for categorical variables and Student's t-test for continuous variables. The distribution of progression-free survival (PFS) and overall survival (OS) was estimated using the Kaplan–Meier method and difference was calculated using the log-rank test. PFS was defined as date of diagnosis until progression or death from any cause. Overall survival was defined as date of diagnosis until death from any cause. Univariate (UVA) and multivariate analyses (MVA) were carried out using the Cox proportional hazard model. Factors with a P value of ≤0.10 on UVA were included in MVA. Hazard ratios (HR) and 95% confidence intervals (CIs) are reported. A level of significance (α) of 0.05 was defined as significant.
A matched cohort survival analysis using propensity scores was carried out comparing our PTCL cohort with a matched cohort of diffuse large B-cell lymphoma (DLBCL) patients treated with rituximab–CHOP over the same time period at two institutions [6]. PTCL patients were exactly matched (without replacement) to DLBCL patients to the tenths decimal place of the propensity score on a 1 : 1 basis (on age, stage, and international prognostic indices), and was blinded to outcome. Cox proportional hazards regression was carried out with stratification on the propensity score (rounded to the tenth place) to account for the matching nature of the data [7]. All statistical analyses were carried out using SAS version 9.2 (SAS Institute, Cary, NC).
results
patient characteristics
The median age was 62 years (range 18–95 years), with one-third of patients ≥70 years. The male-to-female ratio was 1.5. The most common subtypes were PTCL-NOS (31%), ALCL (26%), and AITL (23%). NK/TCL, adult T-cell leukemia/lymphoma (ATLL), EATCL, subcutaneous panniculitis-like T-cell lymphoma (SPTCL), hepatosplenic T-cell lymphoma (HTCL), and transformed cutaneous T-cell lymphoma (t-CTCL) each constituted <10% of cases (Table 1).
Table 1.
Patient characteristics
| Characteristic | Number (%) |
|---|---|
| All patients | 341 (100) |
| Median age, years (range) | 62 years (18–95) |
| Gender | |
| Female | 141 (41) |
| Male | 200 (59) |
| Histology | |
| PTCL-NOS | 107 (31) |
| ALCL, ALK + | 23 (7) |
| ALCL, ALK− | 43 (13) |
| ALCL, ALK unknown | 22 (6) |
| AITL | 77 (23) |
| NK/TCL | 23 (7) |
| ATLL | 20 (6) |
| EATCL | 10 (3) |
| SPTCL | 7 (2) |
| t-CTCL | 4 (1) |
| HSTCL | 5 (1) |
| History of CAD | 42(13) |
| History of DM | 45 (13) |
| History of prior malignancy | 11 (3) |
| Median BMI (range) | 26.2 (13.6–55.2) |
| B symptoms | 154 (47) |
| Performance status | |
| 0 | 117 (34) |
| 1 | 102 (30) |
| 2 | 40 (12) |
| 3 | 25 (7) |
| 4 | 5 (2) |
| NA | 52 (15) |
| Bone marrow involvement | 99 (29) |
| Nonmarrow extranodal diseasea | 168 (49) |
| Extranodal involvement >1 site | 61 (18) |
| Ann Arbor stage | |
| I | 47 (14) |
| II | 39 (11) |
| III | 77 (23) |
| IV | 165 (48) |
| NA | 13 (4) |
| Elevated LDH | 141 (41) |
| Anemia <11.0 g/dl | 170 (50) |
| Platelets <150 × 109/l | 92 (27) |
| Renal insufficiency | 42 (12) |
| Hypoalbuminemia | 119 (35) |
| Bulk >7 cm | 26 (9) |
| IPI score (n = 254) | |
| 0–1 | 82 (32) |
| 2 | 71 (28) |
| 3–5 | 101 (40) |
| PIT score (n = 233) | |
| 0–1 | 115 (49) |
| 2 | 67 (29) |
| 3–4 | 51 (22) |
| IPTCLP score (n = 249) | |
| 0 | 83 (33) |
| 1 | 87 (35) |
| 2–3 | 79 (32) |
| Initial systemic treatment (n = 341) | |
| CHOP-like | 237 (70) |
| HyperCVAD/MA | 20 (6) |
| Other regimenb | 61 (18) |
| Palliative care only | 23 (7) |
| SCT in first remission (n = 318) | |
| Yes | 33 (10) |
| No | 285 (90) |
| Radiation in first remission (n = 318) | |
| Yes | 68 (21) |
| No | 250 (79) |
aMost common sites: skin 26%, liver 13%, lung 12%, nasal/sinus 10%, GI tract 9%, subcutaneous tissue 8%, bone 7%, CNS 4%, and breast 3%.
bOther regimens were administered to fewer than 10 patients each.
PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; ALCL, anaplastic large-cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; NK/TCL, NK/T-cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; EATCL, enteropathy-associated T-cell lymphoma; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; HTCL, hepatosplenic T-cell lymphoma; t-CTCL, transformed cutaneous T-cell lymphoma; NA, not available; CAD, coronary heart disease; DM, diabetes mellitus; BMI, body mass index; LDH, lactate dehydrogenase; IPI, International Prognostic Index; PIT, Prognostic Index for T-cell lymphoma; IPTCLP, International peripheral T-cell lymphoma Project score; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; hyperCVAD/MA, hyperfractionated cyclophosphamide, vincristine, dexamethasone, doxorubicin/ methotrexate, cytarabine; SCT, stem-cell transplantation.
The majority of patients (71%) presented with advanced stage disease (Ann Arbor stage III–IV). Nearly half of patients had B symptoms at diagnosis and 21% had ECOG performance status ≥2. Extranodal disease was common with 29% bone marrow involvement and 49% nonmarrow extranodal disease. The most common nonmarrow extranodal sites were skin (26%), liver (13%), and lung (12%). Half of patients presented with anemia and 27% had thrombocytopenia. Forty percent of patients had high-risk disease by the International Prognostic Index (IPI), 22% by the Prognostic Index for T-cell lymphoma, and 32% by the International PTCL Project score. Data regarding medical comorbidities included renal insufficiency, coronary artery disease (CAD) or diabetes mellitus in 12%, 13%, and 13% of patients, respectively.
treatment
Frontline treatment regimens are detailed in Table 1. Twenty-three (7%) patients received only palliative therapy (no cytotoxic therapy), all of whom survived <3 months. Compared with patients treated with chemotherapy, palliative patients were older and likelier to present with anemia, hypoalbuminemia, and high-risk prognostic scores. (supplementary Table S2, available at Annals of Oncology online).
Among 318 patients treated with at least one cycle of chemotherapy, CHOP-like therapy was the most common (70%), followed by hyperCVAD/MA (hyperfractionated cyclophosphamide, vincristine, adriamycin, dexamethasone/methotrexate, cytarabine) (6%). Sixty-one (18%) patients received an alternative regimen, each of which was administered to <10 patients and are grouped together as ‘other’. These included EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin), CMED (cyclophosphamide, methotrexate, etoposide, dexamethasone), gemcitabine-based, ifosfamide-based, and others. Of 237 patients who received CHOP-like therapy, 13 (5%) included etoposide. Sixty-eight patients (21%) received consolidative radiation therapy (XRT) as part of initial treatment. The most common histologies receiving XRT were ALCL (24), NK/TCL (16), and PTCL-NOS (15). Among irradiated patients, 62% had limited-stage disease, including 100% of limited-stage NK/TCL patients. Thirty-three (10%) patients underwent SCT in first remission (26 autologous, 7 allogeneic). Among transplanted patients, the majority had PTCL-NOS (11), followed by ALCL (6 ALK−, 1 ALK+), AITL (6) and ATLL (3), and one each in NK/TCL, EATL, HSL, and SPTCL. XRT or transplant carried out as salvage therapy was not considered upfront consolidation.
response
Overall response rate (ORR) among 318 patients treated with chemotherapy was 73%, with 61% achieving complete response (CR). Twenty-four percent had primary refractory disease. The ORR and complete response rate (CRR) with CHOP-like therapy were 69% and 58%, respectively, with 20% progressive disease. The ORR and CRR among patients who received hyperCVAD/MA were 85% and 80% (P = 0.0006 and P = 0.002, respectively, versus CHOP-like therapy).
survival
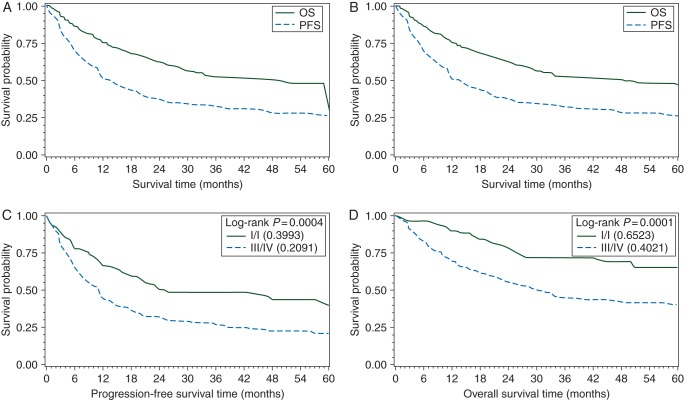
With median follow-up of 39 months (range 6–109 months), 3-year PFS and OS for all patients were 29% and 49%, respectively (Figure 1A). Among 318 patients who received chemotherapy, 3-year PFS and OS were 32% and 52%, respectively (Figure 1B). We compared 3-year outcomes of the entire PTCL cohort with a matched population of DLBCL patients, and found an inferior PFS (30% versus 61%, P < 0.0001) and OS (49% versus 73%, P = 0.002) in PTCL (Figure 2). Three-year PFS with a CHOP-like regimen, hyperCVAD/MA, and other were 32%, 53%, and 25%, respectively (P = 0.050). Three-year OS showed no significant difference among frontline regimens (CHOP-like 55%, hyperCVAD/MA 49%, and other 43%, P = 0.098). Among CHOP-like treated patients, there was no benefit observed for the inclusion of etoposide (P = 0.80), but the number of patients was small.
Figure 1.
PFS and OS for all patients (A) and for only patients treated with chemotherapy (B). Among chemotherapy-treated patients, PFS (C) and OS (D) by Ann Arbor stage.
Figure 2.
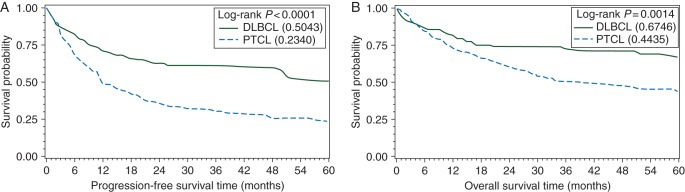
PFS (A) and OS (B) of peripheral T-cell lymphoma patient population compared with matched DLBCL patients.
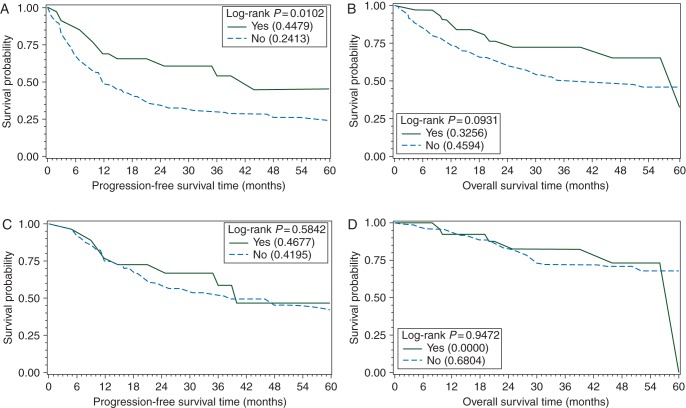
Patients who received CHOP-like therapy and consolidative SCT (n = 26) had 3-year PFS and OS of 58% and 74%, compared with 30% and 53% for patients who received CHOP-like therapy without SCT (n = 211) (PFS P = 0.02 and OS P = 0.07).
prognostic factors
In comparing all subtypes with PTCL-NOS on UVA, superior PFS was observed for ALK+ ALCL and NKTL, and inferior for t-CTCL (Table 2). For OS, ALK+ ALCL, ALK− ALCL, and AITL were superior to PTCL-NOS; NKTL demonstrated borderline improved survival (Figure 3). Among clinical factors, stage was the most dominant factor predictive of PFS and OS (Figure 1C and D). Hypoalbuminemia and elevated LDH predicted inferior PFS and OS. Female gender and absence of prior CAD predicted superior OS. On MVA of pretreatment factors, only limited-stage disease remained significant for improved PFS and OS (PFS: HR 0.59, 95% CI 0.38–0.93, P = 0.023; OS: HR 0.46, 95% CI 0.25–0.85, P = 0.014), while history of CAD portended inferior OS (HR 1.90, 95% CI 1.13–3.24, P = 0.016).
Table 2.
Prognostic Analyses (Univariate)
| PFS |
OS |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Subtypes (versus PTCL-NOS) | ||||||
| ATLL | 1.03 | 0.56–1.91 | 0.91 | 1.21 | 0.63–2.32 | 0.56 |
| NK/TCL | 0.50 | 0.25–1.00 | 0.05 | 0.47 | 0.20–1.09 | 0.07 |
| EATCL | 0.97 | 0.42–2.23 | 0.93 | 0.84 | 0.30–2.31 | 0.73 |
| HSL | 1.60 | 0.58–4.40 | 0.36 | 1.43 | 0.45–4.56 | 0.55 |
| SPTCL | 0.33 | 0.08–1.34 | 0.12 | 0.51 | 0.12–2.08 | 0.35 |
| t-CTCL | 3.32 | 1.21–9.15 | 0.02 | 0.85 | 0.21–3.48 | 0.82 |
| ALK+ ALCL | 0.44 | 0.22–0.88 | 0.02 | 0.30 | 0.12–0.74 | 0.009 |
| ALK− ALCL | 0.99 | 0.55–1.79 | 0.69 | 0.47 | 0.26–0.85 | 0.012 |
| AITL | 0.92 | 0.60–1.41 | 0.35 | 0.52 | 0.32–0.82 | 0.005 |
| Prognostic scores | ||||||
| IPI | 1.28 | 1.13–1.45 | 0.0001 | 1.59 | 1.35–1.86 | <.0001 |
| PIT | 1.25 | 1.08–1.45 | 0.004 | 1.47 | 1.22–1.77 | <.0001 |
| IPTCLP | 1.26 | 1.06–1.50 | 0.002 | 1.51 | 1.23–1.86 | <.0001 |
| Clinical/treatment factors | ||||||
| Gender (Female versus male) | 0.86 | 0.65–1.14 | 0.29 | 0.70 | 0.49–1.00 | 0.047 |
| History of CAD | 1.44 | 0.97–2.16 | 0.07 | 2.06 | 1.32–3.21 | 0.001 |
| Hypoalbuminemia | 1.50 | 1.08–2.08 | 0.016 | 1.86 | 1.24–2.78 | 0.003 |
| Elevated LDH | 1.50 | 1.08–2.09 | 0.02 | 1.90 | 1.26–2.87 | 0.002 |
| Stage (1/2 versus 3/4)a | 0.55 | 0.39–0.78 | 0.0008 | 0.40 | 0.25–0.65 | 0.0002 |
| HyperCVAD/MA frontline | 0.53 | 0.29–0.72 | 0.05 | 0.49 | 0.24–0.70 | 0.098 |
| Consolidative RT | 0.68 | 0.47–0.97 | 0.03 | 0.55 | 0.34–0.89 | 0.015 |
| Consolidative SCT | 0.48 | 0.27–0.84 | 0.01 | 0.48 | 0.24–0.98 | 0.044 |
aRemained significant on multivariate analysis.
PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; ALCL, anaplastic large-cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; NK/TCL, NK/T-cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; EATCL, enteropathy-associated T-cell lymphoma; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; HTCL, hepatosplenic T-cell lymphoma; t-CTCL, transformed cutaneous T-cell lymphoma; PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; IPI, International Prognostic Index; PIT, Prognostic Index for T-cell lymphoma; IPTCLP, International peripheral T-cell lymphoma Project; LDH, lactate dehydrogenase; SCT, stem-cell transplantation; RT, radiotherapy.
Figure 3.
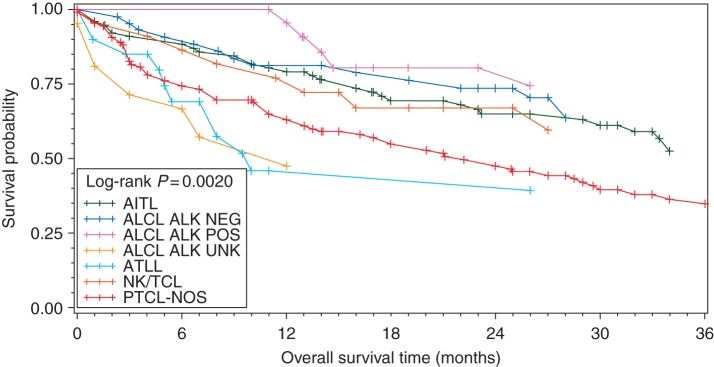
OS for all patients by histology. Only histologies with >10 subjects are included in this analysis.
Among treatment-related factors, response to initial therapy (CR/PR) was strongly predictive of improved survival (PFS: HR 0.15, 95% CI 0.11–0.21, P < 0.0001; OS: HR 0.19, 95% CI 0.13–0.28, P < 0.0001). Despite benefit for PFS favoring hyperCVAD/MA as initial therapy in UVA, no benefit was seen on MVA adjusting for age, stage, and LDH (HR 0.50, 95% CI 0.22–1.17, P = 0.11). On UVA, both consolidative XRT and SCT were associated with improved PFS and OS; however, no benefit was seen on MVA for consolidative SCT when controlling for CR to initial chemotherapy as well as stage, LDH, and hypoalbuminemia (PFS: HR 0.71, 95% CI 0.52–1.37, P = 0.49; OS: HR 0.71, 95% CI 0.52–1.37, P = 0.49) (Figure 4A–D). Similarly, the benefit for XRT was negated after controlling for stage and LDH (PFS: HR 0.84, 95% CI 0.40–1.26, P = 0.24; OS: HR 1.01, 95% CI 0.54–1.87, P = 0.98).
Figure 4.
PFS (A) and OS (B) by consolidative SCT in all patients, and PFS (C) and OS (D) by SCT limited to patients in CR following induction chemotherapy.
discussion
We present a multicenter retrospective analysis of PTCL in the modern era. We confirm that PTCL patients continue to have an inferior prognosis compared with DLBCL. Patients with PTCL present with high-risk features including advanced stage, extranodal disease, elevated LDH, and B symptoms. Use of consolidative XRT and SCT were predictive of improved survival on UVA but lost significance on MVA. Collectively, the most dominant prognostic factor was response to initial therapy.
It is not clear if survival has improved for PTCL over the past several decades [8]. The 3-year OS in our contemporary population of 49% demonstrates that PTCL continues to pose significant challenges. We found no significant differences in OS between PTCL histologies on MVA, including both ALK+ and ALK− ALCL, which lost prognostic significance after adjusting for pretreatment risk factors. ALK+ ALCL has long been observed to have a favorable prognosis relative to other PTCLs [9, 10], but this may reflect the presentation of these patients with lower risk features including younger age, limited-stage, and lower risk IPI scores. Limited-stage disease was the only pretreatment risk factor to maintain significance for OS on MVA, emphasizing the importance of counseling patients in view of their overall risk profile rather than histology alone.
We evaluated the role of select medical comorbidities and found that a diagnosis of CAD independently conferred an inferior prognosis. The impact of comorbidity has not been well explored in lymphoma patients, but reports have suggested adverse impact on prognosis [11, 12]. Patients with comorbidities often receive less aggressive therapy and are less likely to complete a full course of cancer therapy.
A Dutch cancer registry analysis found medical comorbidities to confer twice the risk of death in lymphoma patients, which was independent of IPI risk [13]. Most studies evaluating the impact of comorbidities have employed the Charlson Comorbidity Index, which we could not calculate in our cohort. Though this is a weakness in our analysis, our data highlight the need to consider medical conditions in the prognostication for our patients, and the need to evaluate comorbidities in prospective clinical trials.
Optimal chemotherapy for PTCL remains undefined, though the majority of patients receive CHOP-like regimens. Our analysis suggests that hyper CVAD/MA may be superior to CHOP in terms of response rate and PFS, however the PFS benefit was lost on MVA and there was no difference in OS. Given the small number of patients who received this regimen, this finding is hypothesis generating as the difference may reflect patient selection rather than differential efficacy. A retrospective analysis of 135 patients with PTCL treated with either CHOP or intensified induction chemotherapy also found no difference in OS [14].
Retrospective analyses have suggested improved outcomes favoring consolidation with autologous SCT, though such analyses are biased by the preferential inclusion of younger, healthier patients with chemosensitive disease [15–17]. Our analysis also demonstrated a benefit favoring autologous SCT, but this disappeared in MVA after adjusting for initial treatment response. These data must be tempered by the small numbers in our cohort who proceeded to transplant. These data suggest, however, that the most dominant factor predictive of a favorable OS is achievement of CR to initial chemotherapy. The true impact of SCT for PTCL patients who achieve a response to initial chemotherapy remains unknown and should be studied in a randomized clinical trial.
In summary, our large multicenter analysis provides further insights into the contemporary management, outcomes, and prognostication of PTCL. We identify early-stage disease and initial treatment response as significant prognostic factors. Survival rates continue to be poor for PTCL, even at academic centers. While we were unable to distinguish an induction regimen associated with improved outcome, intensified initial therapy and consolidative SCT warrant ongoing evaluation. Moreover, this analysis highlights the need to incorporate novel therapeutic approaches for the first-line treatment of PTCL.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Trumper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418–3425. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez J, Conde E, Gutierrez A, et al. The results of consolidation with autologous stem-cell transplantation in patients with peripheral T-cell lymphoma (PTCL) in first complete remission: the Spanish Lymphoma and Autologous Transplantation Group experience. Ann Oncol. 2007;18:652–657. doi: 10.1093/annonc/mdl466. [DOI] [PubMed] [Google Scholar]
- 4.Feyler S, Prince HM, Pearce R, et al. The role of high-dose therapy and stem cell rescue in the management of T-cell malignant lymphomas: a BSBMT and ABMTRR study. Bone Marrow Transplant. 2007;40:443–450. doi: 10.1038/sj.bmt.1705752. [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. [Google Scholar]
- 6.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 7.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27:2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 8.Morgensztern D, Walker GR, Koniaris LG, Lossos IS. Lack of survival improvement in patients with peripheral T-cell lymphoma: a Surveillance, Epidemiology, and End Results analysis. Leuk Lymphoma. 2011;52:194–204. doi: 10.3109/10428194.2010.542596. [DOI] [PubMed] [Google Scholar]
- 9.Gascoyne RD, Aoun P, Wu D, et al. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood. 1999;93:3913–3921. [PubMed] [Google Scholar]
- 10.Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 11.van Spronsen DJ, Janssen-Heijnen ML, Lemmens VE, et al. Independent prognostic effect of co-morbidity in lymphoma patients: results of the population-based Eindhoven Cancer Registry. Eur J Cancer. 2005;41:1051–1057. doi: 10.1016/j.ejca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Nabhan C, Smith SM, Helenowski I, et al. Analysis of very elderly (>/=80 years) non-Hodgkin lymphoma: impact of functional status and co-morbidities on outcome. Br J Haematol. 2012;156:196–204. doi: 10.1111/j.1365-2141.2011.08934.x. [DOI] [PubMed] [Google Scholar]
- 13.Janssen-Heijnen ML, van Spronsen DJ, Lemmens VE, et al. A population-based study of severity of comorbidity among patients with non-Hodgkin's lymphoma: prognostic impact independent of International Prognostic Index. Br J Haematol. 2005;129:597–606. doi: 10.1111/j.1365-2141.2005.05508.x. [DOI] [PubMed] [Google Scholar]
- 14.Escalon MP, Liu NS, Yang Y, et al. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: the M. D. Anderson Cancer Center experience. Cancer. 2005;103:2091–2098. doi: 10.1002/cncr.20999. [DOI] [PubMed] [Google Scholar]
- 15.Gui L, Shi YK, He XH, et al. High-dose therapy and autologous stem cell transplantation in peripheral T-cell lymphoma: treatment outcome and prognostic factor analysis. Int J Hematol. 2014;99:69–78. doi: 10.1007/s12185-013-1465-y. [DOI] [PubMed] [Google Scholar]
- 16.Jantunen E, Wiklund T, Juvonen E, et al. Autologous stem cell transplantation in adult patients with peripheral T-cell lymphoma: a nation-wide survey. Bone Marrow Transplant. 2004;33:405–410. doi: 10.1038/sj.bmt.1704367. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez J, Caballero MD, Gutierrez A, et al. High-dose chemotherapy and autologous stem cell transplantation in peripheral T-cell lymphoma: the GEL-TAMO experience. Ann Oncol. 2003;14:1768–1775. doi: 10.1093/annonc/mdg459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.