Abstract
Background
Environmental enrichment can enhance expression of species-specific behaviour. While foraging enrichment is encouraged in laboratory animals, its impact on novelty induced behaviour remain largely unknown.
Purpose
Here, we studied behavioural response of mice to acute and subchronic oral monosodium glutamate (MSG) in an open field with /without foraging enrichment.
Methods
Adult male mice, assigned to five groups were administered vehicle (distilled water), or one of four selected doses of MSG (10, 20, 40 and 80 mg/kg) for 21 days. Open field novelty induced behaviours i.e. horizontal locomotion, rearing and grooming were assessed after the first and last doses of MSG. Results were analysed using MANOVA followed by Tukey HSD multiple comparison test and expressed as mean ± S.E.M.
Results
Following acute MSG administration without enrichment, locomotor activity reduced, grooming increased, while rearing activity reduced at lower doses and increased at higher doses. Subchronic administration without enrichment was associated with increased locomotor activity and reduction in grooming, rearing activity however still showed a biphasic response. Addition of enrichment with acute administration resulted in sustained reduction in locomotor and rearing activities with a biphasic grooming response. Subchronically, there was reduction in horizontal locomotion, biphasic rearing response and sustained increase in grooming activity.
Conclusion
Behavioural response to varying doses of MSG as observed in the open field is affected by modifications such as foraging enrichment, which can reverse or dampen the central effects seen irrespective of duration of administration.
Keywords: Foraging enrichment, Monosodium glutamate, Novelty-induced behaviours, Open field, Behavioural Neuroscience, Rodents
 Introduction
Introduction
Environmental enrichment in laboratory animals involves modifications to their physical and social environment to mimic a natural habitat. Such modifications improve well- being, enhance expression of specie-specific behaviour1 and reduce the incidence or severity of stereotypy.2
Researches on environmental enrichment focus on physical enrichment, which entails provision of complex inanimate and social stimulation to animals within their home cage,3 addition of toys, exercise wheels, perches and climbing frames. Enrichment protocols are known to induce behavioural modifications and enhance neuroplasticity by increasing physical activity, learning experiences, visual inputs and social interactions.4
Environmental enrichment can be classified into physical, social, sensory, nutritional and occupational enrichment. Nutritional/foraging enrichment involves modification to food, such as introduction of novel foods and changes in food delivery method,5 e.g. making the animals forage rather than feed ad libitum from a food cup within the housing. This is close to the natural environment, where rodents spend a great part of their time foraging for food. Food items scattered in the floor or bedding also encourages ambulation. Small treats of different types can be used depending on the experimental conditions and desired endpoints.
Rodents exhibit a strong motivation to forage and rats readily work for the retrieval of food in the presence of freely accessible identical food.6 Carder and Berkowitz7 found that rats preferred earned food to free food provided the work entailed was minimal. Nutritional enrichment has been studied and validated in other farm animals such as horses,8,9 cattle10 and pigs,11,12 although the modalities and endpoints differ. Enrichment studies in mice have shown alteration in gene expression and variability of behaviour regardless of time of exposure13 as well as alteration in genes coding for new synapses, reorganization of existing synapses and neurotransmitter release.14 Although a lot of research has been done on some types of environmental enrichment, nutritional/foraging enrichment in particular has not been adequately explored as evidenced by a dearth of literature.
Monosodium glutamate (MSG) is a naturally occurring sodium salt of glutamic acid; a commonly used flavour enhancer which dissociates into sodium cations and glutamate anions.15 Glutamate is one of the most abundant amino acids in the brain and a primary excitatory neurotransmitter in the brain. Studies have shown MSG altering neurobehaviour and even orally administered low dose of MSG caused central inhibition in the open field test.16 This study intends to assess the neurobehavioural response to low doses of MSG in mice exposed in an enriched open field paradigm, with an intention of bringing to light possible interactions between a known driver of central inhibition and a behavioural model that encourages ambulation.
Methods
Reagents and Drugs
Monosodium glutamate (99% purity) (Ajinomoto®) purchased from the open market, was weighed and dissolved in distilled water to get desired concentrations. MSG at the varying doses (10, 20, 40 and 80 mg/kg) was administered orally using a cannula.
Animals
Adult Swiss mice (Empire Breeders, Osogbo, Osun State, Nigeria) weighing 22.5 ± 2.5g were used. Mice were housed in plastic cages measuring 16 x 12 x 10 inches (6 mice in each cage). Housing was a temperature-controlled (22.5°C ± 2.5°C) quarters with 12 hours of light. Mice had free access to food and water except during the behavioural tests. All rules related to animal safety and care were observed.
Experimental Method
The behavioural model used was the open field. Mice were randomly assigned to 5 groups of 6 animals each. Respective groups received vehicle (distilled water) or one of four doses of monosodium glutamate (10, 20, 40 and 80 mg/kg/day) for a period of 21 days. Tests were carried out after the first and last doses of MSG. At the beginning of the behavioural test, each animal was placed in the apparatus and its behaviour was videotaped for subsequent analysis.
Behavioural testing
Thirty minutes period of the following behavioural states; locomotion, rearing and grooming were observed and scored at 10 minutes interval. This was used to characterize behavioural changes in the mice when placed in the open field. Animals were tested with or without enrichment. Foraging enrichment was achieved by incorporation of a tasty treat in the open field. This was a simple home baked snack consisting of fine flour and sugar to which the mice were already accustomed. About twenty grams of the treat was ground into fine powder and spread evenly on the floor of the field. The open field is a rectangular arena composed of a hard board floor measuring 36 x 36 x 26 cm and made of white painted wood. The floor was divided by permanent red markings into 16 equal squares at the bottom. Generally, spontaneous motor activity was monitored for 30 minutes in the open field as described by Ajayi and Ukponmwan.17 After treatment as earlier explained, each mouse was introduced into the field and the total locomotion (number of floor units entered with all paws), rearing frequency (number of times the animal stood on its hind legs or with its fore arms against the walls of the observation cage or free in the air) and frequency of grooming (no of body cleaning with paws, picking of the body and pubis with mouth and face washing actions) within each 10 minute interval were recorded. The arena was cleaned with 5% alcohol to eliminate olfactory bias. A fresh animal was then introduced after the arena is dry. Behavioural testing was done between 7.00 a.m. and 3.00 p.m.
Statistical Analysis
Data was analysed using Chris Rorden’s ezANOVA statistical package for windows, version 0.98. Hypothesis testing was performed using multifactorial analysis of variance (MANOVA). MANOVA models were used to test effects of dose, enrichment and time on horizontal locomotor activity, rearing and grooming behaviours. Tukey HSD test was used for within and between group comparisons. Results are expressed as mean ± S.E.M, p values less than 0.05 were considered statistically significant.
Results
Horizontal locomotion
Monosodium glutamate’s effect on horizontal locomotor activity in mice was evaluated following 30 minutes of open field exposure. A very significant main effect of MSG dosage (F = 14.5, p = 0.001), nutritional enrichment (F = 65.5, p = 0.001) and time (acute or chronic) (F = 9.90, p = 0.002) was seen; there were also significant interactions between MSG dosage and the presence or absence of enrichment (F = 23.2, p = 0.001), MSG dosage and time (F = 36.2, p = 0.001), enrichment and time (F = 137, p = 0.001) and MSG dosage, enrichment and time (F = 55.6, p = 0.001).
Figure 1 compares locomotor response of mice to corresponding vehicle (distilled water) and acute and subchronic administration of varying doses of MSG with and without enrichment. Following acute administration there was a reduction in locomotor activity at all doses of MSG, both with and without enrichment, although statistically significant effects were only seen at 10 (F = 4.31, p = 0.002), 20 (F = 3.41, p = 0.007) and 40 (F = 3.99, p = 0.003) in animals tested without enrichment and at 20 (F = 13.02, p = 0.001), 40 (F = 3.07, p = 0.012) and 80 mg/Kg (F = 5.09, p = 0.001) in animals tested with introduction of enrichment. With subchronic administration of MSG, locomotor activity increased significantly at all doses of MSG {10 (F = 2.40, p = 0.037); 20 (F = 2.89, p = 0.016); 40 (F = 8.28, p = 0.001) and 80mg/Kg (F = 2.26, p = 0.047)} without enrichment, with enrichment introduced, locomotor activity decreased significantly at 10 (F = 13.45, p = 0.001), 40 (F = 18.82, p = 0.001) and 80mg/Kg (F = 12.22, p = 0.000). At 20 mg/kg however locomotor activity increased.
Fig. 1:
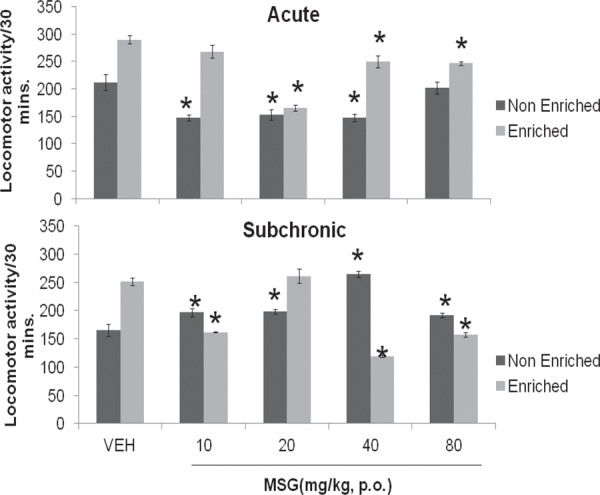
Effect of Monosodium glutamate on horizontal locomotion in enriched and non enriched mice. Each bar represents Mean ± S.E.M, *p<0.05 compared to vehicle. n = 6; VEH: Vehicle.
Effects of enrichment on locomotor activity following vehicle or MSG administration was assessed. Following acute (F = 4.71, p = 0.000) and subchronic (F = 6.80, p = 0.001) administration of vehicle, locomotor activity increased significantly with enrichment compared to without enrichment Following acute administration of MSG locomotor activity increased at {10 (F = 5.24, p = 0.001); 20 (F = 1.14, p = 0.2824), 40 (F = 8.05, p = 0.001) and 80 (F = 3.87, p = 0.003) with enrichment compared to without enrichment with subchronic administration of MSG, locomotor activity increased at 20 (F = 4.71, p = 0.001) mg/kg and reduced at 10 (F = 4.67, p = 0.001), 40 (F = 25.77, p = 0.001) and 80 (F = 5.87, p = 0.001) mg/kg with enrichment compared to without enrichment as shown in Table 1.
Table 1: Effects of enrichment on horizontal locomotion.
| Dose | Acute Non Enriched | Acute enriched | F | Tukey HSD | P value |
|---|---|---|---|---|---|
| Mean ± S.E.M | Mean ± S.E.M | ||||
| Mean ± S.E.M, VEH: Vehicle. * p<0.05 Comparison of means between enriched and non enriched mice, n = 6. | |||||
| VEH | 212.33 ± 14.6 | 289.83 ± 7.61 | 65.5 | 4.71 | 0.001* |
| 10 | 147.33 ± 5.86 | 268.17 ± 11.32 | ” | 5.24 | 0.001* |
| 20 | 153.17 ± 9.38 | 165.67 ± 5.75 | ” | 1.14 | 0.282 |
| 40 | 147.33 ± 7.21 | 250 ± 10.52 | ” | 8.05 | 0.001* |
| 80 | 202 ± 11 | 246.83 ± 3.67 | ” | 3.87 | 0.003* |
| Dose | Subchronic Non En-riched | Subchronic Enriched | F | Tukey HSD | P value |
| Mean ± S.E.M | Mean ± S.E.M | ||||
| VEH | 165.67 ± 10.72 | 251.33 ± 6.6 | 65.5 | 6.8 | 0.001* |
| 10 | 197 ± 7.46 | 162 ± 0.73 | ” | 4.67 | 0.001® |
| 20 | 198.67 ± 3.94 | 261.33 ± 12.7 | ” | 4.71 | 0.001* |
| 40 | 264.8 ± 5.1 | 119.67 ± 2.32 | ” | 25.77 | 0.001® |
| 80 | 191.83 ± 4.35 | 157.33 ± 3.95 | ” | 5.87 | 0.001® |
The effects of time (acute vs. subchronic) on administration of vehicle or MSG was also analysed. With enrichment (F = 3.82, p = 0.003) and without enrichment (F = 2.58, p = 0.079) locomotor activity decreased significantly with subchronic compared to acute administration of vehicle. With MSG; locomotor activity increased significantly with subchronic compared to acute administration at 10 (F = 5.42, p = 0.001), 20 (F = 4.47, p = 0.001) and 40 (F = 13.21, p = 0.001) mg/kg; while at 80 no significant difference was seen, in animals tested without enrichment, with enrichment a decrease in locomotor activity was seen at 10 (F = 9.36, p = 0.001), 40 (F = 12.10, p = 0.001) and 80 (F = 16.60, p = 0.001) mg/kg of MSG and an increase at 20 (F = 6.86, p = 0.000) mg/kg following subchronic compared to acute administration as shown in Table 2.
Table 2: Effects of duration of administration (Time) on horizontal locomotion.
| Dose | Acute Non Enriched | Subchronic Non En-riched | F | Tukey HSD | P value |
|---|---|---|---|---|---|
| Mean ± S.E.M | Mean ± S.E.M | ||||
| Mean ± S.E.M, VEH: Vehicle. * p<0.05 Comparison of means between acute and subchronic administration of Monosodium glutamate, n = 6. | |||||
| VEH | 212.33 ± 14.6 | 165.67 ± 10.72 | 9.90 | 2.58 | 0.078 |
| 10 | 147.33 ± 5.86 | 197 ± 7.46 | “ | 5.42 | 0.001* |
| 20 | 153.17 ± 9.38 | 198.67 ± 3.94 | “ | 4.47 | 0.001* |
| 40 | 147.33 ± 7.21 | 264.8 ± 5.1 | “ | 13.21 | 0.001* |
| 80 | 202 ± 11 | 191.83 ± 4.35 | “ | 0.86 | 0.410 |
| Dose | Acute Enriched | Subchronic Enriched | F | Tukey HSD | P value |
| Mean ± S.E.M | Mean ± S.E.M | ||||
| VEH | 289.83 ± 7.61 | 251.33 ± 6.6 | 9.90 | 3.82 | 0.001® |
| 10 | 268.17 ± 11.32 | 162 ± 0.73 | “ | 9.36 | 0.001® |
| 20 | 165.67 ± 5.75 | 261.33 ± 12.7 | “ | 6.86 | 0.001* |
| 40 | 250 ± 10.52 | 119.67 ± 2.32 | “ | 12.1 | 0.001® |
| 80 | 246.83 ± 3.67 | 157.33 ± 3.95 | “ | 16.6 | 0.001® |
Rearing activity
Secondly, we assessed the effect of monosodium glutamate on rearing activity in mice. A very strong main effect of MSG dosage (F = 2.48, p = 0.001), enrichment (F = 13.7, p = 0.001) and time (acute or subchronic) (F = 4.70, p = 0.033) was seen; we also found significant interactions between MSG dosage and the presence or not of enrichment (F = 29.6, p = 0.001), MSG dosage and time (F = 4.69, p = 0.002) and between MSG dosage, enrichment and time (F = 48.6, P = 0.001), there was however no significant interaction between the presence or not of enrichment and time.
Figure 2 compares the rearing response of mice following acute and subchronic administration of varying doses of MSG with or without enrichment respectively to corresponding vehicle (distilled water). Following acute administration without enrichment, rearing activity decreased at 10 (F = 3.69, p = 0.004) and 20 (F = 0.47, p = 0.648) mg/kg of MSG and increased at 40 (F = 2.20, p = 0.053) and 80 (F = 2.29, p = 0.045) mg/kg though, significant effects occurred only at 10 and 80 mg/kg, with introduction of enrichment, rearing activity reduced at all doses of MSG, {10 (F = 0.29, p = 0.777), 20 (F = 11.52, p = 0.001), 40 (F = 1.62, p = 0.137) and 80 (F = 3.66,p = 0.004)}, with significant effects at 20 and 80. Following subchronic administration of MSG without enrichment, rearing activity decreased significantly at 10 (F = 7.96, p = 0.001); 20 (F = 9.82,p = 0.001) and 80 (F = 5.80, p = 0.001) mg/kg MSG, with enrichment introduced, increased significantly at 20 (F = 6.30, p = 0.001) mg/kg, at 10 and 40 mg/kg a reduction in rearing occurred while at 80 mg/kg an increase is seen although this effect was only visual.
Fig. 2:
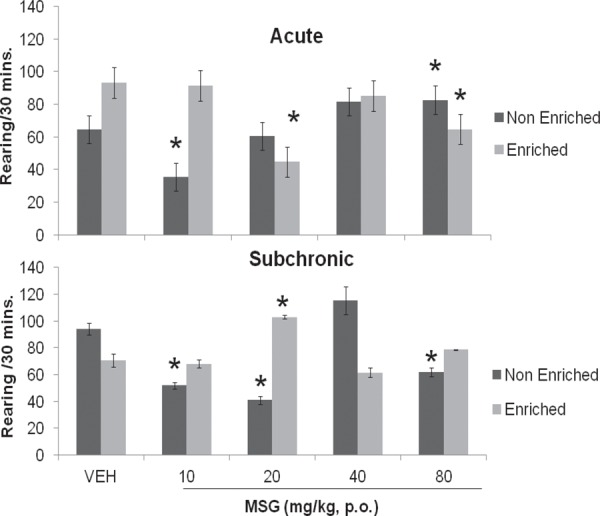
Effect of Monosodium glutamate on rearing activity in enriched and non enriched mice. Each bar represents Mean ± S.E.M, *p<0.05 compared to vehicle. n = 6; VEH: Vehicle.
The effect of enrichment (non enriched vs. enriched) on rearing activity following either vehicle or MSG was assessed. Following acute (F = 4.71,p = 0.001) administration of vehicle rearing increased with enrichment compared to e without, while with subchronic (F = 3.20, p = 0.006) administration enrichment resulted in a reduction in rearing; with acute administration of MSG, introduction of enrichment resulted in a significant increase in rearing at 10 (F = 3.31, p = 0.008) mg/kg, and reduction at 20 (F = 4.13, p = 0.001) and 80 (F = 5.27, P = 0.001) mg/kg compared to without, following subchronic administration however, rearing increased significantly with introduction of enrichment at 10 (F = 4.81, p = 0.006) and 20 (F = 18.58, p = 0.001) and reduced at 40 (F = 4.80, p = 0.001) and 80 (F = 5.14, p = 0.001) mg/kg compared to without as shown in Table 3.
Table 3: Effects of enrichment on rearing activity.
| Dose | Acute Non Enriched | Acute Enriched | F | Tukey HSD | P value |
|---|---|---|---|---|---|
| Mean ± S.E.M | Mean ± S.E.M | ||||
| Mean ± S.E.M, VEH: Vehicle. *® p<0.05 Comparison of means between enriched and non enriched mice, n = 6. | |||||
| VEH | 64.60 ± 7.60 | 93.17 ± 4.17 | 13.7 | 3.31 | 0.008* |
| 10 | 35.50 ± 1.98 | 91.50 ± 3.91 | “ | 12.77 | 0.001* |
| 20 | 60.50 ± 9.3.19 | 44.67 ± 0.56 | “ | 4.31 | 0.002® |
| 40 | 81.50 ± 1.41 | 85.17 ± 2.65 | “ | 1.22 | 0.251 |
| 80 | 82.26 ± 2.226 | 64.67 ± 6.56 | “ | 5.27 | 0.001® |
| Dose | Subchronic Non En-riched | Subchronic En-riched | F | Tukey HSD | P value |
| Mean ± S.E.M | Mean ± S.E.M | ||||
| VEH | 94.00 ± 4.49 | 70.50 ± 4.95 | 13.7 | 3.52 | 0.006® |
| 10 | 51.83 ± 2.82 | 68..00 ± 2.92 | “ | 4.81 | 0.001* |
| 20 | 41.00 ± 3.00 | 103.00 ± 1.46 | “ | 18.58 | 0.001* |
| 40 | 115.50 ± 10.73 | 61.50 ± 3.35 | “ | 4.8 | 0.001® |
| 80 | 191.83 ± 4.35 | 78.50 ± 0.22 | “ | 5.41 | 0.001® |
The effects of time (acute vs. subchronic) was also compared. Following administration of vehicle without enrichment (F = 3.34, p = 0.008) rearing increased significantly following subchronic compared to acute administration, in the enriched (F = 3.50, p = 0.006) paradigm decreased significantly. Following MSG without enrichment rearing increased at 10 (F = 4.47, p = 0.001), 40 (F = 3.41,p = 0.011) and 80 (F = 5.27, p = 0.001) mg/kg and decreased it at 20 (F = 4.03, p = 0.002) mg/kg with subchronic compared to acute administration, with enrichment rearing decreased significantly at 10 (F = 3.98, p = 0.003) and 40 (F = 5.54, p = 0.000) mg/kg of MSG and increased at 20 mg/kg (F = 3.98, p = 0.001) with subchronic compared to acute administration as shown in Table 4.
Table 4: Effects of duration of administration (Time) on rearing activity.
| Dose | Acute non Enriched | Subchronic Non En-riched | F | Tukey HSD | P value |
|---|---|---|---|---|---|
| Mean ± S.E.M | Mean ± S.E.M | ||||
| Mean ± S.E.M, VEH: Vehicle. *®p<0.05 Comparison of means between acute and subchronic administration of Monosodium glutamate, n = 6. | |||||
| VEH | 64.60 ± 7.60 | 94.00 ± 4.49 | 4.70 | 3.34 | 0.008* |
| 10 | 35.50 ± 1.98 | 51.83 ± 2.82 | “ | 4.74 | 0.001* |
| 20 | 60.50 ± 3.19 | 41.00 ± 3.00 | “ | 4.03 | 0.002® |
| 40 | 81.50 ± 1.41 | 115.50 ± 10.73 | “ | 3.41 | 0.011* |
| 80 | 82.26 ± 2.226 | 191.83 ± 4.35 | “ | 5.27 | 0.001* |
| Dose | Acute Enriched | Subchronic Enriched | F | Tukey HSD | P value |
| Mean ± S.E.M | Mean ± S.E.M | ||||
| VEH | 93.17 ± 4.17 | 70.50 ± 4.95 | 4.70 | 3.5 | 0.006® |
| 10 | 91.50 ± 3.91 | 68.00 ± 2.92 | “ | 3.98 | 0.003® |
| 20 | 44.67 ± 0.56 | 103.00 ± 1.46 | “ | 37.31 | 0.001* |
| 40 | 85.17 ± 2.65 | 61.50 ± 3.35 | “ | 5.54 | 0.001® |
| 80 | 64.67 ± 6.56 | 78.50 ± 0.22 | “ | 2.11 | 0.061 |
Grooming behaviour
Lastly we assessed the effect of monosodium glutamate on grooming behaviour in mice. Multifactorial ANOVA analysis revealed very strong main effect of MSG dose (F = 12.6, p = 0.001) and time (acute or subchronic) (F = 45.0, p = 0.001) and the lack of a main effect of the enrichment (F = 0.04, p = 0.841); we however found significant interactions between MSG dosage and enrichment (F = 21.4, p = 0.001), drug dose and time (F = 47.3, p = 0.001), enrichment and time (F = 84.6, p = 0,001) and between MSG dose, enrichment and time (F = 45.1, p = 0.001).
Figure 3 compares the grooming response of mice following acute and subchronic administration of varying doses of MSG with or without enrichment respectively to corresponding vehicle (distilled water). Following acute administration without enrichment there was a significant increase in grooming at 10 (F = 12.67, p = 0.001), 20 (F = 8.87, p = 0.001) and 40 (F = 5.51, p = 0.001) mg/kg of MSG, with introduction of enrichment, there was a significant reduction in grooming at 10 (F = 5.42,p = 0.001) and a significant increase at 20 (F = 3.98, p = 0.003), increments seen at 40 and 80 were only visual. Following subchronic administration of MSG without enrichment, grooming decreased significantly at 10 (F = 7.84, P = 0.001); 20 (F = 10.92,p = 0.001) and 40 (F = 8.58, p = 0.001) mg/kg, with enrichment introduced, grooming increased significantly at all doses of MSG {10 (F = 7.16, p = 0.001); 20 (F = 7.76, p = 0.001); 40 (F = 10.13, p = 0.001) and 80 (F = 9.28, p = 0.001)} mg/kg.
Fig. 3:
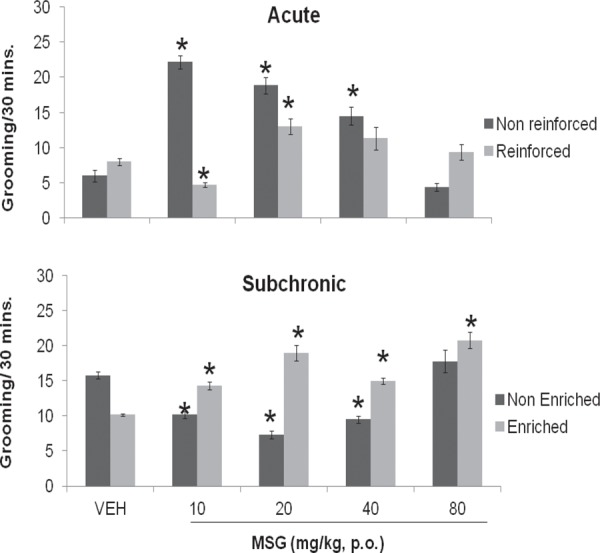
Effect of Monosodium glutamate on grooming in enriched and non enriched mice. Each bar represents Mean ± S.E.M, *p<0.05 compared to vehicle. n = 6; VEH: Vehicle.
The effect of enrichment (non enriched vs. enriched) on grooming behaviour was compared. Following acute (F = 2.00, p = 0.073) administration of vehicle grooming increased with enrichment compared to without, with subchronic (F = 9.98, p = 0.001) administration grooming decreased with enrichment introduced compared to without. Following acute administration of MSG, grooming reduced significantly at 10 (F = 17.45, p = 0.001) and 20 (F = 3.55, p = 0.005) mg/kg, and decreased significantly at 80 (F = 4.01, p = 0.003) mg/kg with enrichment compared to without enrichment. Following subchronic administration of MSG there was a significant increase in grooming at 10 (F = 5.68, p = 0.001), 20 (F = 9.29, p = 0.001) and 40 (F = 8.20, p = 0.001) mg/kg with enrichment compared to effects seen without enrichment as shown in Table 5.
Table 5: Effects of enrichment on grooming.
| Dose | Acute non Enriched | Acute Non Enriched | F | Tukey HSD | P value |
|---|---|---|---|---|---|
| Mean ± S.E.M | Mean ± S.E.M | ||||
| Mean ± S.E.M, VEH: Vehicle. *® p<0.05 Comparison of means between enriched and non enriched mice, n = 6. | |||||
| VEH | 6.00 ± 0.86 | 8.00 ± 0.52 | 0.04 | 2 | 0.0734 |
| 10 | 22.17 ± 0.95 | 4.67 ± 0.33 | “ | 17.45 | 0.0001® |
| 20 | 18.83 ± 1.17 | 13.00 ± 1.15 | “ | 3.65 | 0.01® |
| 40 | 14.50 ± 1.28 | 11.33 ± 1.61 | “ | 1.54 | 0.155 |
| 80 | 4.33 ± 0.56 | 9.337 ± 1.12 | “ | 4.01 | 0.003* |
| Dose | Subchronic non Enriched | Subchronic Enriched | F | Tukey HSD | P value |
| Mean ± S.E.M | Mean ± S.E.M | ||||
| VEH | 15.83 ± 0.54 | 10.17 ± 0.17 | 0.04 | 9.98 | 0.0001® |
| 10 | 10.17 ± 0.48 | 14.33 ± 0.56 | “ | 5.68 | 0.0002* |
| 20 | 7.33 ± 0.56 | 19.00 ± 1.13 | “ | 9.29 | 0.0001* |
| 40 | 9.50 ± 0.50 | 15.00 ± 0.45 | “ | 8.2 | 0.0001* |
| 80 | 17.83 ± 1.60 | 20.83 ± 1.14 | “ | 1.53 | 0.157 |
The effects of time (acute vs. subchronic) was compared. Following administration of vehicle with (F = 3.99, p = 0.03) and without (F = 9.70, p = 0.001) enrichment grooming increased significantly with subchronic compared to acute administration., following MSG without enrichment grooming decreased at 10 (F = 11.33, p = 0.001), 20 (F = 8.89, p = 0.001) and 40 (F = 3.63,P = 0.01) mg/kg and an increased at 80 (F = 7.97, P = 0.001) mg/kg with subchronic compared acute administration, with enrichment grooming increased at 10 (F = 14.88, p = 0.001) 20 (F = 3.72, p = 0.004) and 80 (F = 7.12, p = 0.001) mg/kg with subchronic compared to acute administration as shown in Table 6.
Table 6: Effect of duration of administration (Time) on grooming.
| Dose | Acute non Enriched | Subchronic Non Enriched | F | Tukey HSD | P value |
|---|---|---|---|---|---|
| Mean ± S.E.M | Mean ± S.E.M | ||||
| Mean ± S.E.M, VEH: Vehicle. * p<0.05. Comparison of means between acute and subchronic administration of Monosodium glutamate, n = 6. | |||||
| VEH | 6.00 ± 0.86 | 15.83 ± 0.54 | 45.0 | 9.7 | 0.0001* |
| 10 | 22.17 ± 0.95 | 10.17 ± 0.48 | “ | 11.33 | 0.0001® |
| 20 | 18.83 ± 1.17 | 7.33 ± 0.56 | “ | 8.89 | 0.001® |
| 40 | 14.50 ± 1.28 | 9.50 ± 0.50 | “ | 3.63 | 0.005® |
| 80 | 4.33 ± 0.56 | 17.83 ± 1.60 | “ | 5.27 | 0.003* |
| Dose | Acute Enriched | Subchronic Enriched | F | Tukey HSD | P value |
| Mean ± S.E.M | Mean ± S.E.M | ||||
| VEH | 8.00 ± 0.52 | 10.17 ± 0.17 | 45.0 | 3.99 | 0.003* |
| 10 | 4.67 ± 0.33 | 14.33 ± 0.56 | “ | 14.88 | 0.0001* |
| 20 | 13.00 ± 1.15 | 19.00 ± 1.13 | “ | 3.72 | 0.004* |
| 40 | 11.33 ± 1.61 | 15.00 ± 0.45 | “ | 2.2 | 0.052 |
| 80 | 9.337 ± 1.12 | 20.83 ± 1.14 | “ | 7.22 | 0.0001* |
Discussion
This study set out to test the interactions that exist among three main factors (MSG dose, enrichment and time i.e. repeated dosing) in mice. The results showed the existence of very strong association and interactions among these factors, with each capable of acting as a positive or negative reinforcement. The presentation of a reinforcer contingent on its effect on behaviour resulting in increases or reduction in the rate of this behaviour is called positive or negative reinforcement8.
Acute administration of monosodium glutamate without foraging enrichment caused a reduction in locomotor activity and an increase in grooming, rearing activity also reduced at lower doses but increased at higher doses. With subchronic administration however, locomotor activity increased, grooming decreased while rearing still showed a biphasic response.
In the enriched open field, we saw a sustained reduction in locomotor activity both acutely and sub chronically, although acutely, higher locomotor activity was seen with the effect lost with subchronic administration; in the case of rearing activity, a decrease was noticed with acute administration and following subchronic administration a biphasic effect is seen, grooming behaviour resulted in a biphasic response acutely and a sustained increase sub chronically.
The results of this study cannot be compared against any of the other studies on enrichment because the methodology applied is unique, that being said, earlier studies on environmental enrichment have shown that mice from enriched environments explored the open field more compared to controls19 and this is similar to results from our study, although some other researchers have reported a reduction in locomotor activity in rodents exposed to enriched environment.20 Ryan Scauzillo21 also studied the impact of foraging enrichment on behaviour and he reported that foraging did not significantly affect behaviour, although in the open field there was a trend towards reduction in locomotor activity.21
Reduction in open field exploration has been reported following administration of certain chemicals such as monosodium glutamate (MSG) in rodents. Acutely administered MSG at low doses (0.5, 1.0 and 1.5 mg/kg) caused a reduction in horizontal locomotor activity and rearing16 similar to some of the acute effects seen in the present study. MSG dissociates to yield glutamate after ingestion and its behavioural effects are attributable to glutamate’s effect on inhibitory neurotransmitters like gamma amino butyric acid (GABA)22 and its interaction with dopamine receptors.23
After administering MSG in the open field without enrichment; in the acute behavioural studies, locomotor activity was reduced, grooming activity increased, while rearing activity reduced at lower doses and increased at higher doses. Response seen with horizontal locomotion was similar to that seen in earlier studies with MSG as said above; even though in this study, MSG is being given using a set of doses that differ from many of such studies. This probably establishes the fact that acute MSG has a central inhibitory effect in the open field across a wide range of doses. Same can be said regarding rearing effects, albeit only at the lower doses, as inhibitory effect appears to be lost at higher doses.
Subchronic administration without enrichment was associated with increased horizontal locomotor activity and reduction in grooming, rearing activity however still showed a biphasic response. Behavioural tests after subchronic administration showed a reversal of behavioural effects seen after acute dosing, especially regarding horizontal locomotion and grooming, also rearing response was increasing significantly at higher doses. This trend suggests that at least the behavioural responses seen with MSG are not cumulative following continuous administration. What remains to be understood is the exact mechanism that may underlie this apparent reversal of behavioural response.
Foraging enrichment of the behavioural area with acute MSG administration resulted in reduction in locomotor and rearing activities with a biphasic grooming response. However, the total horizontal locomotion and rearing (for some doses) were significantly higher compared to those seen in the non-enriched arena. With grooming, this effect was seen only with controls and at the highest dose. On exposure to an enriched arena, we see the central inhibitory effects of MSG being dulled possibly by a strong exploratory override induced by foraging. Subchronically, there was reduction in horizontal locomotion, biphasic rearing response and sustained increase in grooming activity. Subchronically in an enriched field, mice continue to exhibit the reduced response to MSG in the presence of enrichment especially for grooming behaviour but less so for horizontal locomotion and rearing. Food is essential for survival, so it is imperative that multiple pathways and physiological systems exist to ensure that adequate energy intake is achieved, also food has both central and peripheral influences in the brain.18 Dopamine is a known mediator of locomotory response to food.24 In this context, food in the arena is a psychostimulant that induces locomotion through stimulation of the dopaminergic system. Dopamine has been shown to act on both presynaptic and postsynaptic signalling25 and thus can influence downstream behavioural rhythms.26 Dopamine is also known to affect glutamatergic signalling through ionotropic glutamate receptors. Finally, dopamine influence on grooming behaviour and how experimental conditions may influence this is also seen. Dopamine is particularly involved in grooming and it is known that lesions in brain regions rich in dopaminergic inputs significantly influence the implementation of grooming syntax.27 In the study, by comparing non enriched to enriched groups, the increased dopaminergic signalling that is believed to have occurred due to presence of food was seen to translate to increased grooming at some doses acutely and across all doses subchronically.
The responses observed in the current study represent the endpoint of two influences leading towards opposite behavioural outcomes. Food-induced, locomotion/grooming-stimulating dopaminergic influence versus locomotion-inhibiting and long-term-grooming inhibiting MSG influence.
Conclusions
Behavioural response to MSG in the open field can be significantly influenced by environmental enrichment as shown in the study.
Authorship contributions
Olakunle J Onaolapo: Conceived and designed the work as a part of his Phd thesis, Olakunle J Onaolapo and Adejoke Y Onaolapo: Responsible for the care of animals, collection, collation and analysis of data, and drafting of manuscript, Moses A Akanmu and Gbola Olayiwola: Interpretation of data, supervision of the entire study and drafting of manuscript.
Footnotes
This article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Conflict of interest: None; Funding: None.
References
- 1.Gottlieb DH, Ghirardo S, Minier DE et al. Efficacy of three types of foraging enrichment for rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2011;50(6):888–94. [PMC free article] [PubMed] [Google Scholar]
- 2.Young RJ. UFAW animal welfare series. Blackwell publishers; UK: 2003. Environmental enrichment for captive animals. [Google Scholar]
- 3.Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behaviour. Behav Brain Res. 1996;78(1):57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 4.Viola GG, Botton PH, Moreira JD et al. Influence of environmental enrichment on an object recognition task in CF1 mice Physiol Behav. 2010;99(1):17–21. doi: 10.1016/j.physbeh.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Bloomsmith MA, Brent LY, Schapiro SJ. Guidelines for developing and managing an environmental enrichment program for nonhuman primates. Lab Anim Sci. 1991;41:372–77. [PubMed] [Google Scholar]
- 6.Neuringer AJ. Animals respond for food in the presence of free food. Science. 1969;166:399–401. doi: 10.1126/science.166.3903.399. [DOI] [PubMed] [Google Scholar]
- 7.Carder B, Berkowitz K. Rats’ preference for earned food in comparison with free food. Science. 1970;169:1273–74. doi: 10.1126/science.167.3922.1273. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin DH, Davidson PB, Harris P. Foraging enrichment for stabled horses: Effects on behaviour and selection. Equine Vet J. 2002;34:686–91. doi: 10.2746/042516402776250450. [DOI] [PubMed] [Google Scholar]
- 9.Cooper JJ, Mcall N, Johnson S et al. The short-term effects of increasing meal frequency on stereotypic behaviour of stabled horses. Appl Anim Behav Sci. 2005;90:351–64. [Google Scholar]
- 10.Redbo I, Nordblad A. Stereotypies in heifers are affected by feeding regime. Appl Anim Behav Sci. 1997;53:193–204. [Google Scholar]
- 11.Van der Peet-Schwering CMC, Spoolder HAM, Kemp B et al. Development of stereotypic behaviour in sows fed a starch diet or a non-starch polysaccharide diet during gestation and lactation over two parities. Appl Anim Behav Sci. 2003;83:81–97. [Google Scholar]
- 12.De Leeuw JA, Zonderland JJ, Altena H et al. Effects of levels and sources of dietary fermentable non-starch polysaccharides on blood glucose stability and behaviour of group-housed pregnant gilts. Appl Anim Behav Sci. 2005;94:15–29. [Google Scholar]
- 13.Henderson ND. Short exposures to enriched environments can increase genetic variability of behavior in mice. Developmental Psychobiol. 1976;9(6):549–53. doi: 10.1002/dev.420090608. [DOI] [PubMed] [Google Scholar]
- 14.Rampon C, Jiang CH, Dong H et al. Effects of environmental enrichment on gene expression in the brain. Proceedings of the National Academy of Sciences. 2000;97(23):12880–84. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sano C. “History of glutamate production”. Am. J. Clin. Nutr. 2009;90 doi: 10.3945/ajcn.2009.27462F. [DOI] [PubMed] [Google Scholar]
- 16.Onaolapo OJ, Onaolapo AY. Acute low dose monosodium glutamate retards novelty induced behaviours in male Swiss albino mice J. Neurosc Behav Health. 2011;3(4):1–56. [Google Scholar]
- 17.Ajayi AA, Ukponmwan OE. Evidence of angiotensin II and endogenous opiod modulation of novelty induced rearing in the rat. Afr J Med Sci. 1994;23:287–90. [PubMed] [Google Scholar]
- 18.Epstein LH, Leddy JJ, Temple JL et al. Food reinforcement and eating: A multilevel analysis, Psychol Bull. 2007;133(5):884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manosevitz M, Joel U. Behavioral effects of environmental enrichment in randomlybred mice. J Comparative Physiol Psych. 1973;85(2):373. doi: 10.1037/h0035041. [DOI] [PubMed] [Google Scholar]
- 20.Amaral OB, Vargas RS, Hansel G et al. Duration of environmental enrichment influences the magnitude and persistence of its behavioral effects on mice. Physiol Behav. 2008;93(1):388–394. doi: 10.1016/j.physbeh.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Ryan S. Novel foods as a form of environmental enrichment for mice (mus musculus): Effects on behavior, physiology and reproduction. Texas A&M University-Commerce. M.Sc. Thesis. 2014 [Google Scholar]
- 22.Cortese BM, Phan LK. The role of glutamate in anxiety and related disorders CNS. Spectr. 2005;10(10):820–830. doi: 10.1017/s1092852900010427. [DOI] [PubMed] [Google Scholar]
- 23.Dalia A, Uretsky NJ, Wallace LJ. Induction of locomotor activity by the glutamate antagonist DNQX injected into the ventral tegmental area. Brain Res. 1996;728:209–214. doi: 10.1016/0006-8993(96)00399-x. [DOI] [PubMed] [Google Scholar]
- 24.Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 25.Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Ann Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 26.Harris-Warrick RM, Coniglio LM, Barazangi N et al. Dopamine modulation of transient potassium current evokes phase shifts in a central pattern generator network. J Neurosci. 1995;15:342–358. doi: 10.1523/JNEUROSCI.15-01-00342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J Neurosci. 1996;16:3444–58. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


