Abstract
Background
Human embryonic stem cells (hESCs) are pluripotent cells that have the potential to self-renew and differentiate into all types of human cells.
Purpose
The present study was aimed at establishing the safety of hESC therapy in patients with terminal/incurable conditions.
Methods
This was a single cohort study conducted at Nutech Mediworld, New Delhi. The patients suffering from various degenerative diseases were included in the study from year 2002 to 2004. hESCs (0.25 mL) were injected under skin in the abdominal wall. The safety of hESC therapy was evaluated by assessing the AEs experienced by patients during the study. Any disabling symptom/ sign, teratoma or antigen-antibody reaction that a patient suffered post transplantation of hESCs was considered as an AE.
Results
A total of four, six and twenty three patients received hESC therapy in the year 2002, 2003 and 2004 respectively. Pain and fever were the most common AEs observed during the study. Other AEs included headache, mild pain in the abdomen, swelling of legs (edema), urinary tract infection (UTI), rash/erythema, pain at the lower back and limbs and body ache. All the AEs reported were mild in nature and resolved within one or two days with symptomatic medication and rest. No serious AEs were reported. The improvement in specific parameters of the patients was observed after the therapy.
Conclusion
hESCs used in the present study are safe for use in humans afflicted with incurable/terminal conditions. Future, prospective controlled studies to substantiate the present study are ongoing.
Keywords: Human Embryonic Stem Cells, Transplantation, Stem cell therapy
 Introduction
Introduction
Human embryonic stem cells (hESCs) are self-renewing cells with a potential to differentiate into all types of human cells.1 These cells have the potential for cell replacement and regeneration therapies for human diseases. hESCs were derived and characterized as early as 1982 from fresh or frozen cleavage stage donated human embryos produced by in vitro fertilization (IVF).2 The viable cell lines were obtained from the inner cell mass or blastocyst. hESCs have also been derived and established from single blastomeres of the 4 or 8 celled embryo and 16 celled morula.3–7 Since then, a plethora of research has been done using hESCs for various diseases like diabetes, liver disorders, auto-immune disorders, immune disorders, Parkinson’s disease, Alzheimer’s disease, age related macular degeneration and spinal cord injury.8–14
Despite huge potential in curing chronic and terminal conditions, hESCs have not been used extensively in humans. This is largely due to the ethical consideration in procuring the hESC lines and also lack of knowledge for the use of hESCs. Further, hESC cell lines have shown chromosomal and genomic instability, with acquisition of loss of heterozygosity or copy-number variation in cancer-related genes.15,16 hESCs have also been associated with teratoma formation and fear of being immunologically rejected.17 These challenges have hindered the use of hESCs to their full potential.
A Phase 1 human clinical trial using hESCs was approved by FDA in 2009 popularly referred to as the Geron trial. Though the initial results of the trial were promising, it was left mid-way due to financial constraints.18 Further, there have been safety concerns and challenges in the use of hESCs. Most of the hESCs being used have been exposed to xeno-products during isolation and propagation. As a result, these could carry a risk of xenogenetic pathogen cross transfer and other unknown substances capable of eliciting a detrimental immune response in transplanted hosts. The cells used in Geron trial also contained animal components such as B27 supplement or Matrigel.18 Recently, Asterias Biotechnology Inc. of Menlo Park has bought the rights of Geron to conduct clinical trial with hESC in humans; that has been approved by FDA.19 Advanced Cell technology (ACT), Inc. is also focusing on developing hESC based therapies for various disorders and has got promising initial results in patients with macular degeneration.20
We used an in-house developed patented technology to culture and maintain hESCs in our GMP, GLP and GTP certified laboratory. The hESCs were obtained from a one-time harvest made at the pre-blastocyst stage. The cell line thus developed is created from a single expendable fertilized ovum 24–48 hours after fertilization when the conceptus is assumed to have reached 4–16 celled stage. Further, we have not used any animal product or exposed our cell lines to any animal product. We have developed a simplified cell culture system free of exogenous cells and supplements of animal origin for expansion of hESCs in a substantially undifferentiated state. In this article, we present the safety and efficacy data of our cell lines.
Methods
Study Characteristics
This was a single cohort study to establish safety and efficacy of hESCs in terminally ill patients carried out at Nutech Mediworld, New Delhi. The study included the patients enrolled in different cohorts in 2002 and 2004. The first patient was admitted on 31 March 2002. The patients were included in the study after an informed consent. The consent process involved a detailed discussion about the hESC therapy with the patient accompanied by a family member or caretaker. All the patients were informed that the treatment protocol being followed is being developed and is not yet finalized. The patients were also made aware of the adverse events (AEs) that might occur due to hESC therapy. All the information regarding the patients, a detailed report of the therapy and outcomes on patients and commencement of therapy was given to Government of India. We followed the guidelines of biomedical research (year 2000) on human participants in India.20 During the entire procedure, an anesthetist was present and safety and sterile measures for hESC transplantation were followed. The transplantation was done at a medical centre registered under Delhi Government.
Study Population
Patients suffering from various degenerative diseases including Parkinson’s disease, spinal cord injuries, autosomal recessive disorders, motor sensory neuropathy with diplopia (vasculitis), mild diffuse cerebral atrophy, Huntington’s chorea, liver metastasis, diabetic foot (amputation), diabetes mellitus, psoriasis, chronic renal failure secondary to lupus nephritis, systemic lupus erythematosus (SLE), Duchene’s muscular dystrophy (DMD), cirrhosis, mental retardation with microcephaly, hypothalamic astrocytoma, post traumatic paraplegia, developmental delay, colitis, and acute cauda equine lesions received hESC therapy. Patients who were pregnant, lactating or confirmed to have received other forms of cell therapy within 12 months of the treatment were not included.
Cell Culture and Differentiation
hESCs were obtained from a single, spare, expendable, pre implantation stage fertilized ovum taken during natural in vitro fertilization (IVF) process with due consent. These cells are cultured and maintained as per our proprietary in-house technology in a GMP, GLP and GTP certified laboratory. The technology has now been patented (United States Granted Patent No US 8592, 208, 52). The detailed cell culture and differentiation techniques have been elaborated elsewhere. The cell lines have been cultured and maintained in animal products free conditions making them suitable for clinical cell therapy. A detailed composition of hESCs used in this study their derivatives, methods of use, and methods of preparation are available at http://patentscope.wipo.int/search/en/WO2007141657).
A quality check was performed on the stored cell batches which included integrity, viability and microbial contamination. The cells were characterized and the transplanted cells were octamer-binding transcription factor 4 positive (OCT4 +ve); Stage-specific embryonic antigen 3 (SSEA3)+ve; NANOG +ve; SOX +ve; i actin +ve; -human chorionic gonadotropin (o –HCG) +ve; alkaline phosphatase +ve; CD 34 +ve; Nestin +ve, GATA +ve; GAF +ve; NeuN +ve; and transfer gene (TRA) –ve. The characterization was done by fluorescence-activated cell sorting (FACS), polymerase chain reaction (PCR) and immunoflourescence (Nikkon Ellipse E200; BD Acuri, Biorad T 100 Thermal cycle) (Unpublished data, Paper under submission).
Study Procedure
The cells were transplanted through simple injections of hESC suspension under skin in the abdominal wall. A single injection contained 0.25 mL of hESC suspension (1 mL contains approximately 4 million cells).
Variables for Analysis
Safety Evaluation: The safety of hESC therapy was evaluated by assessing the AEs experienced by patients during the study. Any disabling symptom/sign that a patient suffered after the test dose was given was considered as an AE. The medical staff of Nutech Mediworld carefully examined the patients for any AEs keeping the ones related to hESCs in mind. These AEs included teratomas, antigen-antibody reactions and any other sign and symptom.
Efficacy Evaluation: The efficacy of hESC therapy was evaluated based on the improvement seen in the patients.
Data Analysis
No formal sample size was calculated for this study. Each case was assessed at admission or soon after admission to determine the pre-therapy status of the case. The safety analyses were performed on safety population (patients who took at least one dose of hESC). The efficacy was assessed based on the improvement seen in the patients. No statistical assumptions were made.
Results
Study Patients
In the year 2002, 4 patients started hESC therapy. The first injection of hESCs was given to a patient with cortico basal degeneration on 31 Mar 2002. In year 2003, six patients received hESC therapy. The details of these patients are shown in Table 1. In the year 2004, 23 patients with different chronic or terminal conditions were administered hESC therapy. Of these 23 patients, 4 had SCI, 3 had Duchenne’s muscular dystrophy, 2 had Huntington’s Chorea, 1 had Parkinsonism, 1 had Psoriasis, 1 had cauda equina Syndrome, 1 had postastrocytoma brain damage with seizures and slow learning, 1 had systemic lupus erythematosus, 1 had developmental delay, 1 had dementia, 1 had Alzheimer’s disease, 1 had alcoholic cirrhosis with portal hypertension, 1 had genetic disorder, 2 had post cerebro vascular accident (CVA), 1 had uncontrolled diabetes with right bundle branch block and 1 had multiple sclerosis.
Table 1: Characteristics of Patients, hESC Therapy Schedule and Condition Before and After the Treatment (Year 2000 and 2003).
| Code | Age (yr)/ Gender | Diagnosis | Presenting Condition | hESC Therapy Schedule | Condition after Therapy |
|---|---|---|---|---|---|
| Year 2002 | |||||
| 80001 | 56/M | Cortico Basal degeneration | Rigidity | 2 injections | Rigidity decreased |
| Stammering and slurring | 31 Mar 2002 | Speech improved | |||
| Tightly clenched fists | 1 Dec 2002 | No clenched fists | |||
| Inability to blink Inability to swallow Inability to walk | Could swallow and chew food | ||||
| Could walk a few steps | |||||
| 80002 | 64/M | Parkinsonism | Pain in lower back and lower limbs | 3 injections | Lesser pain in lower back and lower limbs |
| Pain before micturition | 26 Apr 2002 | Micturition normal | |||
| Tremors in the upper limbs | 8 Sep 2002 | No tremors | |||
| Inability to speak properly | 26 Oct 2002 | Speech improved | |||
| Inability to walk properly | Ability to walk improved | ||||
| Medication decreased | |||||
| 80003 | 53/M | Becker’s Muscular Dystrophy | Slurring of speech | 2 injections | Speech improved |
| Inability to walk | 29 Sep 2002 | Able to walk without support | |||
| Inability to lift arms | 17 Nov 2002 | Weakness in limbs decreased | |||
| Difficulty in breathing | Breathing pattern improved | ||||
| Hand grip improved | |||||
| 80004 | 37/F | Post traumatic encephalomalacia and gliosis with quadriplegia (Right>Left) | Poor gait | Single injection | Better gait |
| Inability to walk without support | 16 Nov 2002 | Able to walk without support | |||
| Scoliosis | Scoliosis improved | ||||
| Poor ability to perform everyday activities Poor speech | More independent in performing everyday activities | ||||
| Poor finger movements | Slight improvement in finger movements | ||||
| Rigid neck | Better neck movements | ||||
| Impaired cognition | Improved cognition | ||||
| Year 2003 | |||||
| 80005 | 65/M | Diabetes Mellitus with renal and cardiac involvement | Chest pain | Single injection | Decreased chest pain |
| Breathlessness | 31 Jan 2003 | Less pain in right shoulder | |||
| Pain in the right shoulder | Serum creatinine- 1.8 mg | ||||
| Serum creatinine-2.4 mg | LVEF-57.3% | ||||
| LVEF-48% | |||||
| 80006 | 72/F | Non-healing burn wound | Non-healing ulcer after a burn | Single injection | Skin covered the ulcer |
| Chest pain | 12 Feb 2003 | Decreased chest pain | |||
| Breathlessness | |||||
| 80007 | 66/M | Type II Diabetes Mellitus with Hypertension and Parkinsonism | High blood sugar levels | Single injection | Lower blood sugar levels, insulin dosage reduced to half |
| Abnormal kidney function | 5 May 2003 | Normal kidney function | |||
| Tremors | Decreased tremors | ||||
| 80008 | 35/F | Primary ovarian failure | No ovulation despite medication | Single injection | Delivered a normal healthy baby |
| 11 Jun 2003 | |||||
| 80009 | 28/F | Becker’s muscular dystrophy with cerebral atrophy | Drooling | Single injection | Mental condition improved |
| Low IQ-35 | 15 Nov 2003 | IQ-100 | |||
| 80010 | 65/F | Atrophy with spino cerebellar ataxia | Catheterization for micturition | Single injection 30 Dec 2003 | No catheterization |
| Difficulty in swallowing | Able to swallow food | ||||
| Tremors and nodding of head | Decreased tremors and nodding of head | ||||
| Inability to walk | Able to walk with walker | ||||
| Slurring of speech | Speech improved | ||||
| Poor balance | Better balance | ||||
| Hypertension | Better balance | ||||
Safety Evaluation
No serious AEs were reported during the study period. All the AEs were mild in nature and resolved within one or two days with symptomatic medication and rest. Pain and fever were the most common AEs observed during the study. Headache, mild pain in the abdomen, swelling of legs (edema), urinary tract infection (UTI), rash/erythema, pain at the lower back and limbs and body ache were the other common AEs observed during the study. Table 2 lists the AEs observed during the study period in our patients.
Table 2: Adverse Events (AEs) Observed in Patients during the Study Period.
| Sr. No. | Adverse Event (AE) | n (%) |
|---|---|---|
| Year 2002 and 2003 (N = 10) | ||
| 1 | Pain (Legs, Abdomen, Chest) | 5 (50) |
| 2 | Fever | 4 (40) |
| 2 | Urinary Tract Infection | 3 (30) |
| 3 | Headache | 2 (20) |
| 4 | Loose motion | 2 (20) |
| 5 | Constipation | 2 (20) |
| 7 | Cough | 2 (20) |
| 8 | Dysponea | 1 (10) |
| 9 | Breathlessness | 1 (10) |
| Year 2004 (N = 23) | ||
| 1 | Fever | 4 (17.4) |
| 2 | Cough | 3 (13) |
| 2 | Lack of sleep | 3 (13) |
| 3 | Headache | 2 (8.7) |
| 5 | Pain in the legs | 2 (8.7) |
| 6 | Loose motions | 2 (8.7) |
| 7 | Vomiting | 2 (8.7) |
| 8 | Backache | 2 (8.7) |
| 9 | Restlessness | 1 (4.4) |
| 10 | Burning pain in the area of ulceration (in psoriatic cases) | 1 (4.4) |
| 11 | Pain in the lower abdomen | 1 (4.4) |
| 12 | Breathlessness | 1 (4.4) |
| 13 | Constipation | 1 (4.4) |
| 14 | Swelling | 1 (4.4) |
| 15 | Knee pain | 1 (4.4) |
| 16 | Loss of apetite | 1 (4.4) |
Efficacy Evaluation
The improvement in patients after the therapy is tabulated in Table 1. Since this was not an efficacy study, we did not analyze the efficacy data. However, we observed an improvement in specific parameters of all our patients during the study, Figure 1.
Fig. 1:
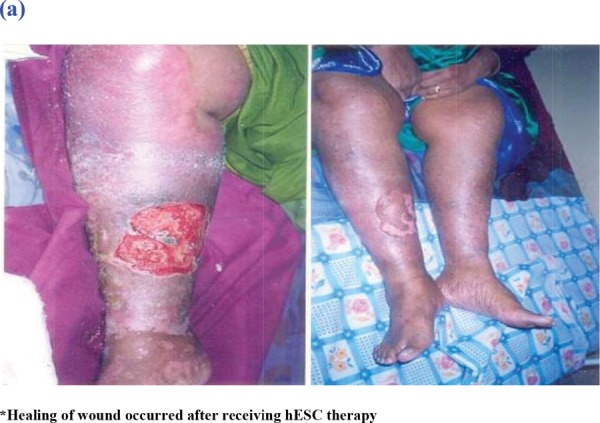
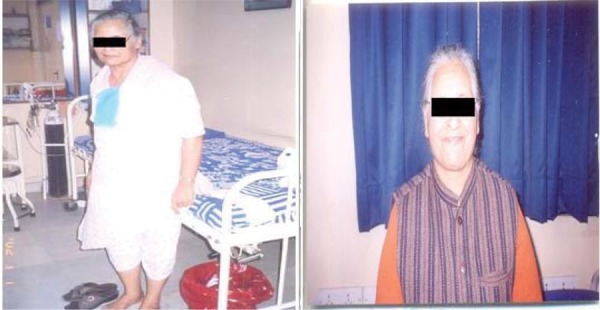
Images of Two Patients Before and After Receiving hESC Therapy.
Discussion
hESCs have an inexhaustible potential to differentiate into different cell types making it a promising treatment option for many debilitating conditions. The first embryonic stem cells (ESCs) were derived from mice (mESCs) in the year 1981.21 Thomson et al isolated the first hESCs derived from human blastocysts about two decades later.2 Since then, studies have been ongoing to prove and utilize their therapeutic prowess. However, ethical considerations and safety regarding the use of hESCs have limited their widespread use.
Adult stem cells are ethically preferable but are lineage restricted and have a limited capacity of self-renewal, which is the essence of stem cell therapy. Further, the sources of human adult stem cells are limited and their isolation is a challenge and can be painful for the patient. Human induced pluripotent stem cells (iPSCs) derived from various somatic cells have generated a tremendous interest for their use in stem cell therapy and regenerative medicine. Though effective, iPSCs might cause genetic and epigenetic abnormalities that could take place during reprogramming or maintenance of iPSCs in subsequent cell culture.22–24 The potential tumorigenicity and immunogenicity associated with iPSC-based cell therapy is of significant concern.25–27 hESCs have an edge over adult stem cells and iPSCs as they display low immunogenicity and could be transplanted with minimal immunosuppression.28–29
Maintaining an undifferentiated stem cell state during large scale expansion (without spontaneous differentiation) is an uphill task that has hindered their widespread use. Another issue is direct or indirect exposure of cells to animal products while culturing that results in high risk of graft rejection and transfer of non-human pathogens to the recipient. Some researchers have been able to maintain and expand undifferentiated hESC on human feeder layers or feeder free matrices but the scale of expansion is low.30,31 Further, majority of the methods adopted for culturing hESCs could result in genetically unstable and aberrant cell lines.32
Most of the hESCs used till date have been derived from inner cell mass (ICM) of blastocyst embryos before implantation.2,33,34 hESC lines are obtained by enzymatic dispersion of the ICM and culturing under particular conditions. This blastocyst is the 256-celled stage of human embryo that has developed from 2-celled, 4-celled, 8-celled stage and so on. The blastocyst continues to mature for an additional 24 hours and is ready to implant into the uterine wall. These preimplantation embryos are able to develop in synthetic culture media for several days and are highly adaptable.35 Though pluripotency at this blastocyst stage has been studied extensively using various marker and characterization studies, there is a paucity of studies about cells at the initial stages of developments. In our study, we used hESCs that are generated in a culture from a one-time harvest made at the pre-blastocyst stage. Expendable, fertilized ovum was taken after a natural IVF cycle and informed consent was sought from the donor. The cell line thus developed is created from a single fertilized ovum 24–48 hr after fertilization when the conceptus is assumed to have reached the 4–16 cell stage. All the media used in the culture are free from animal contaminants and cells of animal origin. The composition of the present therapy is simple to prepare and cost effective. The ready to inject form is easily transportable, scalable and has a good shelf life. The evidence for the use of hESCs at our facility has been gathered over a number of years and was accepted as a written evidence to House of Lords, Regenerative Medicine, Science and Technology Committee report.36
Stem cells have a unique functioning when transplanted. Previous studies suggest that various factors like chemokines, cytokines, and other growth factors released from the site of injury attractant the transplanted stem cells. These cells then migrate to the damage site due to up regulation of selectins and integrins on their surface, a process called “homing”.37–40 After homing at the injured site, the stem cells help in “rescue” and “replacement” of the injured cells. We assume that the hESC in our study also acted in a similar way and reached the site of injury after transplantation. Once there, a trigger of factors led to their differentiation into the cell type of injured area and helped in recovery/regeneration.
When we tested our cell line in patients with differing chronic and life threatening conditions, we got promising results. The studies from 2002 to 2004 were done to establish the safety of these cell lines and develop a protocol for using the therapy. During these years, we did not observe any serious AE associated with the use of out cell line. Fever and generalized pain were the most common AEs that we observed after hESC injections. All the AEs were mild in nature and were observed majorly not due to hESC transplant per se, but were a direct consequence of the patient’s illness and were part of the normal course of the disease. Though not the primary objective, efficacy of the hESC therapy was promising and we observed a continued benefit in our patients. Our cell line is a mixture of “neuronal” and “non-neuronal” cells. The non-neuronal cell lines include progenitor cells for hematopoietic stem cells progenitors, insulin producing stem cells, mesenchymal stem cell, epithelial stem cells, hepatocyte stem cell, and cardiac stem cells. The presence of these two directed cell lines makes them appropriate for usage in a wide variety of conditions. In this study, our patients suffered from different non-curable diseases like cortico-basal degeneration, SCI, cerebral palsy, corticovisual impairment, Parkinsonism etc. but the hESCs seemed to have beneficial effects for all of them. Further, all our patients had come to us after trying all the conventional treatments without any improvement.
Now, the protocol of using hESC therapy in patients with different chronic and life threatening conditions has been fully developed and we have used our therapy in over 1300 patients over last decade. Till date, we have not observed any serious AE in our patients. As largely concerned about the usage of hESCs, we have not observed any teratoma formation in our patients till date. We did not give steroids or immunosuppressant to our patients. None of the patient had an immune response. Our staff is trained to observe any antigenic/anaphylactic response in the patients.
In conclusion, our hESC cell line is safe for use in humans afflicted with incurable conditions. We did not observe any serious AE in our patients. No teratoma formation or immune response was observed. We also observed clinical benefits of these cell lines in all our patients. Future, prospective controlled studies to substantiate the present study are ongoing.
Authorship Contributions
Geeta Shroff: Drafted the manuscript, carried out the experiments, conceived of the study, and participated in its design and coordination and helped to draft the manuscript, J.K Barthakur: participated in the design of the study and performed the statistical analysis, Geeta Shroff and J.K Barthakur: Read and approved the final manuscript.
Acknowledgements
The authors acknowledge Staff of Nutech Mediworld and the patients of this study. The authors also acknowledge Knowledge Isotopes Pvt. Ltd (http://www.knowledgeisotopes.com) for writing support.
Competing Interests
The authors declare that they have no competing interests.
Disclaimer
This is a case/isolated study coducted by Nutech Mediworld to test the efficacy of ESC on humans.
| Serial no. | Comment | Response |
|---|---|---|
| Disclaimer: This is a pure experimental study and the study does not come under the purview of accepted Medical Treatment for the patient as stipulated by regulatory agencies world wide who control the use of Stem Cells on Human subjects. | ||
| Review Round 1 | ||
| 1. | That patients were not charged of any fees. | No fee was charged for the therapy to the patients treated between the years 2002-2004. |
| 2. | Please mention if pregnant or lactating patients received any thera-pies. | No, pregnant or lactating patients were not given any therapy |
| 3. | Embryonic Stem cell usage is under the restricted category of research and cannot be used in injections in human subjects. Hence authors are requested to provide Stem Cell Research committee approval. | We are providing the approval letter from the Institutional Ethics Committee (IEC) with the revised draft. |
| 4. | Any usage of cells in human subjects has to be conducted as a trial which involves an animal study before permission is given to conduct in humans but this stage has to be allowed by regulations and un-til date regulations are slow to react. Big companies like Geron etc (which has been mentioned in the article) have gone into a trial after a rigorous study by FDA. | At the time (in year 2000), when we started hESC research in our facility; stem cell research in India was in its infancy. We recruited our first patient in 2002 and established safety of hESC therapy from 2002 to 2004. Till this time also, no guidelines were available for stem cell research. The only guidelines that were available are from Indian Council of Medical Research (ICMR) on their website and are issued every year (available at http://icmr.nic.in/ethical_guidelines.pdf).Few points to consider here are: |
| For every patient that we recruited, we informed and reported to regulatory authority. Detailed reports of outcome of every patient after the therapy were also submitted . | ||
| The institute has IEC/ICSCRT and has applied for registration to NAC-SC etc. | ||
| The guidelines state that tissues for transplantation can be obtained from embryos after sponta-neous or induced abortion. | ||
| Further, informed consent should be obtained from the mother whose embryo is sought. This was done. | ||
| Lastly, an IEC is in place since 2003. The Institutional Committee for Stem Cell Research and Therapy (ICSCRT) sent annual report to the National Apex Body (ICMR). | ||
| 5. | For any research, there has to be a quantitative value of improve-ment. The 2 tables that were shown here were of qualitative nature. They were of no significant value. | We agree that we have not quantified the improvement. Please note that, all the patients who visited us had chronic level of injury. The patients came after undergoing several other treatments that did not show any benefits. However, after undergoing hESC therapy, all patients suffering from different medical conditions showed improvement in their health. These are medical obser-vations by qualified doctors. |
| The objective of this was only to assess the safety of hESC therapy. Hence, we did not assess the quantitative improvement. | ||
| Review Round 2 | ||
| 6. | The informed consent is written very very badly and there are glar-ing mistakes and misinterpretations. This has to be changed by the author. | The consent is very old and we agree that it is not up to the mark. We have changed our consent form. Further, we are also taking video consents for our patients. |
| 7. | The pictures are sent separately and have to be a part of the paper. This needs to be looked at by the author. | We would modify the paper and send the pictures embedded in the paper. |
| 8. | As this involves a contentious issue, the committees have been very careful to grant permission to such a study unless it goes through a full blown trial such as the Phase I through IV. This involves millions of dollars and not just going to the patient with no backup data. | The backup data is present. All the data from these patients and others also was validated by independent CROs. These included Moody’s International, QSA and GVK biosciences. |
| 9. | This study is not under the scope of DCGI in India so it cannot be a drug and ICMR does not take responsibility for these cells on human subjects. | We agree to this. However, we would like to bring it to your notice that we started this study, we made a project proposal and approached DST. DST guided us to ICMR and then we submitted our project to them. Since then, we have been submitting all our reports to ICMR. Recently, in Dec 2014, we presented our work and submitted report to the expert committee meeting. |
| 10. | This is under the restrictive category of research. Restrictive and pro-hibited categories are banned in India. | Restrictive research with minimal manipulation is allowed under 2012 guidelines (Section 7.1, page 14). |
Footnotes
This article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Conflict of Interests: None: Source of funding: None.
References
- 1.Heins N, Englund MC, Sjöblom C et al. Derivation, characterization, and differentiation of human embryonic stem cells. Stem Cells. 2004;22(3):367–76. doi: 10.1634/stemcells.22-3-367. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JA, Joseph IE, Sander SS et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Chung Y, Irina K, Sandy B et al. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell. 2008;2(2):113–7. doi: 10.1016/j.stem.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Geens M, Mateizel I, Sermon K et al. Human embryonic stem cell lines derived from single blastomeres of two 4-cell stage embryos. Hum Reprod. 2009;24(11):2709–17. doi: 10.1093/humrep/dep262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klimanskaya I, Young C, Sandy B et al. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444(7118):481–5. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- 6.Strelchenko N, Verlinsky O, Kukharenko V et al. Morula-derived human embryonic stem cells. Reprod Biomed Online. 2004;9(6):623–9. doi: 10.1016/s1472-6483(10)61772-5. [DOI] [PubMed] [Google Scholar]
- 7.Strelchenko N, Verlinsky Y. Embryonic stem cells from morula. Methods Enzymol. 2006;418:93–108. doi: 10.1016/S0076-6879(06)18006-4. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26(5):1117–27. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- 9.Cai J, Zhao Y, Liu Y et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45(5):1229–39. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- 10.Cloutier F, Siegenthaler MM, Nistor G et al. Transplantation of human embryonic stem cell-derived oligodendrocyte progenitors into rat spinal cord injuries does not cause harm. Regen Med. 2006;1(4):469–79. doi: 10.2217/17460751.1.4.469. [DOI] [PubMed] [Google Scholar]
- 11.Idelson M, Alper R, Obolensky A et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5(4):396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Kroon E, Martinson LA, Kadoya K et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 13.Senju S1,, Hirata S, Motomura Y et al. Pluripotent stem cells as source of dendritic cells for immune therapy. Int J Hematol. 2010;91(3):392–400. doi: 10.1007/s12185-010-0520-1. [DOI] [PubMed] [Google Scholar]
- 14.Wong SS, Bernstein HS. Cardiac regeneration using human embryonic stem cells: producing cells for future therapy. Regen Med. 2010;5(5):763–75. doi: 10.2217/rme.10.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefort N, Feyeux M, Bas C et al. Human embryonic stem cells reveal recurrent genomic instability at 20q11.21. Nat Biotechnol. 2008;26(12):1364–6. doi: 10.1038/nbt.1509. [DOI] [PubMed] [Google Scholar]
- 16.Närvä E, Autio R, Rahkonen N et al. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nat Biotechnol. 2010;28(4):371–7. doi: 10.1038/nbt.1615. [DOI] [PubMed] [Google Scholar]
- 17.Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2002;2(11):859–71. doi: 10.1038/nri934. [DOI] [PubMed] [Google Scholar]
- 18.Lukovic D, Stojkovic M, Moreno-Manzano V et al. Perspectives and future directions of human pluripotent stem cell-based therapies: lessons from Geron's clinical trial for spinal cord injury. Stem Cells Dev. 2014;23(1):1–4. doi: 10.1089/scd.2013.0266. [DOI] [PubMed] [Google Scholar]
- 19.R L. Available from: http://www.bizjournals.com/sanfrancisco/blog/biotech/2014/08/embryonic-stem-cells-asterias-geron-spinal-cord.html ; Stem cell trial for spinal cord injuries cleared by FDA. [accessed on Sep 17th;2014 ].
- 20.Available at: http://icmr.nic.in/ethical_guidelines.pdf ; Ethical guidelines for biomedical research on human participants. [assessed on Nov 7;2014 ].
- 21.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 22.Hussein SM, Batada NN, Vuoristo S et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471(7336):58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 23.Lister R, Pelizzola M, Kida YS et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471(7336):68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mummery C. Induced pluripotent stem cells--a cautionary note. N Engl J Med, 2011;364(22):p. 2160–2. doi: 10.1056/NEJMcibr1103052. [DOI] [PubMed] [Google Scholar]
- 25.Miura K, Okada Y, Aoi T et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27(8):743–5. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 26.Tsujia O, Miuraa K, Okada Y et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A, 2010;107(28):12704–9. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao T, Zhang ZN, Rong Z et al. Immunogenicity of induced pluripotent stem cells. Nature, 2011;474(7350):212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 28.Drukker M, Katz G, Urbach A et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99(15):9864–9. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Baroja ML, Majumdar A et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22(4):448–56. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Powell S, Brunette E et al. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng. 2005;91(6):688–98. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- 31.Yoo SJ, Yoon BS, Kim JM et al. Efficient culture system for human embryonic stem cells using autologous human embryonic stem cell-derived feeder cells. Exp Mol Med. 2005;37(5):399–407. doi: 10.1038/emm.2005.50. [DOI] [PubMed] [Google Scholar]
- 32.Catalina P, Montes R, Ligero G et al. Human ESCs predisposition to karyotypic instability: Is a matter of culture adaptation or differential vulnerability among hESC lines due to inherent properties? Mol Cancer. 2008;7(76) doi: 10.1186/1476-4598-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reubinoff BE, Pera MF, Fong CY et al. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18(4):399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 34.Cowan CA, Klimanskaya I, McMahon J et al. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med. 2004;350(13):1353–6. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- 35.Cockburn K, Rossant J. Making the blastocyst: lessons from the mouse. J Clin Invest. 2010;120(4):995–1003. doi: 10.1172/JCI41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Available from http://www.parliament.uk/documents/lords-committees/science-technology/RegenerativeMedicine/RegenMed.pdf . Regenerative Medicine. [accessed on Nov 3;2014 2012 ].
- 37.Borlongan CV, Glover LE, Tajiri N et al. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95(2):213–28. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ezzat T, Dhar DK, Malago M et al. Dynamic tracking of stem cells in an acute liver failure model. World J Gastroenterol. 2012;18(6):507–16. doi: 10.3748/wjg.v18.i6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang SK, Shin IS, Ko MS et al. Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. 342968. Stem Cells Int. 2012;2012 doi: 10.1155/2012/342968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. 130763. Stem Cells Int. 2013;2013 doi: 10.1155/2013/130763. [DOI] [PMC free article] [PubMed] [Google Scholar]


