Ann Oncol 2014; 25: 2230–2236 (doi: 10.1093/annonc/mdu367)
In the original manuscript, there was an error in Figure 1 panel E and F.
The orange line should indicate TG/GG-placebo and the pink line should indicate TG/GG-cetuximab in the survival plots. The figure has been corrected below.
Figure 1.
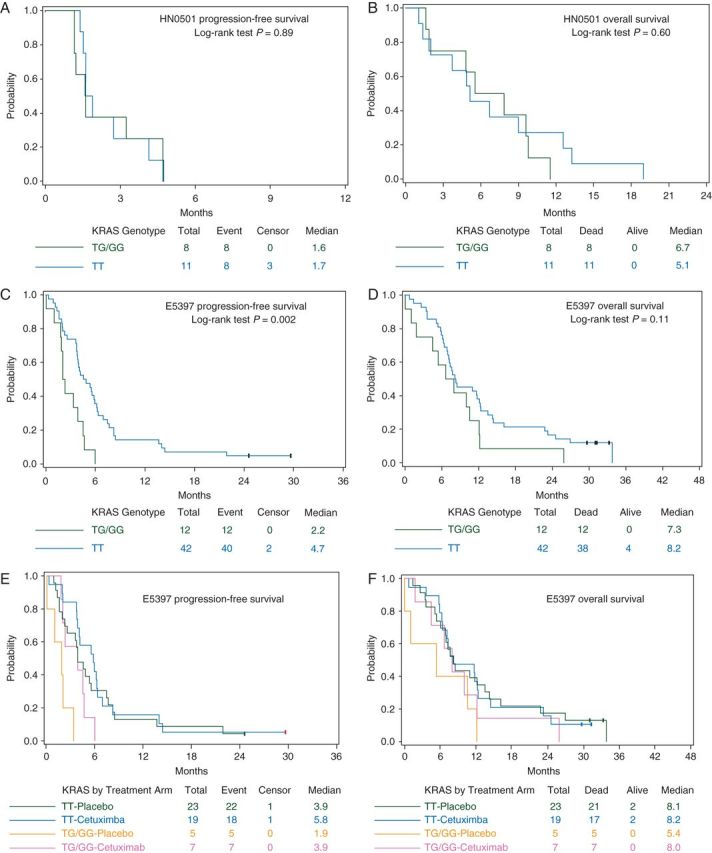
Kaplan–Meier survival plots by KRAS-variant status (variant TG/GG versus non-variant TT). (A) Progression-free survival from HN0501 (phase II trial of docetaxel + bortezomib in R/M HNSCC patients); (B) overall survival from HN0501; (C) progression-free survival from E5397 (randomized phase III trial of cisplatin + placebo versus cisplatin + cetuximab in R/M HNSCC patients); (D) overall survival from E5397; (E) progression-free survival by KRAS-variant status (variant TG/GG versus non-variant TT) and E5397 treatment arms (cisplatin + placebo versus cisplatin + cetuximab); and (F) overall survival by KRAS-variant status (variant TG/GG versus non-variant TT) and E5397 treatment arms (cisplatin + placebo versus cisplatin + cetuximab).


