Transmission of tuberculosis in communities is driven by heterogeneities in human behavior, mycobacterial activity, and host susceptibility. The only realistic way to achieve ambitious targets for tuberculosis elimination is to tailor local interventions to these catalysts of tuberculosis transmission.
Keywords: communicable disease control, epidemiology, transmission, tuberculosis
Abstract
The global tuberculosis control community has committed itself to ambitious 10-year targets. To meet these targets, biomedical advances alone will be insufficient; a more targeted public health tuberculosis strategy is also needed. We highlight the role of “tuberculosis transmission catalysts,” defined as variabilities in human behavior, bacillary properties, and host physiology that fuel the propagation of active tuberculosis at the local level. These catalysts can be categorized as factors that increase contact rates, infectiousness, or host susceptibility. Different catalysts predominate in different epidemiological and sociopolitical settings, and public health approaches are likely to succeed only if they are tailored to target the major catalysts driving transmission in the corresponding community. We argue that global tuberculosis policy should move from a country-level focus to a strategy that prioritizes collection of data on key transmission catalysts at the local level followed by deployment of “catalyst-targeted” interventions, supported by strengthened health systems.
More people die of tuberculosis than from any other infectious agent except human immunodeficiency virus (HIV) [1]. However, on a population level, tuberculosis remains a “rare” disease, with an incidence 5% that of malaria [2] and prevalence 5% that of hepatitis B [3]. As a result, population-based approaches to tuberculosis control are difficult to implement and sustain. It is estimated that a staggering 3 million people currently have active tuberculosis that will never be detected (of whom nearly half will die) [4], but a global search to “reach the 3 million” would require screening >2000 people for every case found. In May 2014, the World Health Assembly announced new targets for global control of tuberculosis that include a 50% reduction in incidence between 2015 and 2025 [5]. Current approaches—including a system that diagnoses tuberculosis on average nearly a year after the onset of infectiousness [4], preventive therapy that is delivered to <1% of all tuberculosis-infected individuals per year [4], and drugs that are too toxic and slow-acting to allow mass empiric treatment [6]—have hardly budged the annual number of people who develop active tuberculosis. The suite of new interventions likely to become available within the next 10 years may not yield more than incremental benefit. For example, diagnostic tests that improve sensitivity may not increase the number of diagnoses made [7], vaccines will likely require multiple doses in adolescence or adulthood [8], and drug regimens are unlikely to shorten therapy to less than 4 months [9]. Thus, if we are to rise to the 2025 challenge of halving tuberculosis incidence, we cannot rely on biomedical advances alone. A more targeted public health strategy for tuberculosis control is needed.
Since 2006, the global public health approach to tuberculosis has been guided by the Stop TB Strategy, the 6 components of which include the pursuit of high-quality expansion and enhancement of tuberculosis diagnosis and treatment; contribution to health system strengthening; and engagement of all care providers [10]. Taken at face value, this strategy recommends the same interventions in all settings. At the community level, public health professionals implement this and other disease control strategies in locally tailored fashion—but in tuberculosis, they often lack both high-level guidance and fine-grained data to inform these efforts. For example, continuous isoniazid preventive therapy might halve the risk of tuberculosis among HIV-uninfected individuals who work in settings of very high transmission (eg, mines [11]), but in very few high-burden settings is there an effort to measure tuberculosis incidence at the local level, nor guidance from global policy bodies as to what level of incidence would be sufficiently high as to trigger consideration of such a strategy. Rather, broader guidance is given—namely, to provide isoniazid to all individuals living with HIV and close contacts (including children) [12]. But where dramatic and rapid declines in tuberculosis incidence have been achieved—from Alaska in the 1950s [13], New York City in the 1990s [14], and Tomsk Oblast (Russian Federation) in the 2000s [15]—interventions were not applied in such broad fashion, but rather by strengthening health systems to identify and interrupt key hotspots and drivers of tuberculosis transmission, from homelessness and HIV to prisons and alcoholism, at the community level.
A locally targeted approach to disease control has been critical for successes with other diseases, from malaria [16] to measles [17]. For example, malaria control efforts target high-risk areas, defined at the district level as having >1 case per 1000 population [2], and the Joint United Programme on HIV/AIDS 2011–2015 strategy prioritizes key populations with an emphasis on “the principle of ‘know your epidemic, know your response,’ which is based on understanding and responding to the local specifics of an epidemic” [18]. In other words, for both of these diseases, global strategy is driven by a focus on high-risk populations that are clearly defined, and at the level of disease transmission. By contrast, tuberculosis control efforts center on 22 high-burden countries [3]; within those countries, data on high-incidence districts or key populations are often unavailable. The post-2015 Global TB Strategy [4] likewise supports adaptation at the country level as a key principle, and while it also mentions such interventions as poverty alleviation, it does not clearly define any high-risk target population (other than people living with HIV). Whereas tuberculosis control professionals “on the ground” undoubtedly focus their efforts on populations at highest risk, there is a need for a coordinated public health approach to tuberculosis data collection and control that is not adapted at the country level, but rather empowers health systems to target interventions on the level at which tuberculosis is transmitted: that of the community.
We highlight here the importance of developing a public health approach to tuberculosis that targets “tuberculosis transmission catalysts,” which we define as variabilities in human behavior, bacillary properties, and host physiology that increase the rate at which active tuberculosis propagates on the local level beyond that which might be expected from “average” calculations at the mass population (including country) level. We illustrate how, by targeting these catalysts, tuberculosis incidence might feasibly be halved, without relying on interventions that must be scaled up across entire countries.
TUBERCULOSIS TRANSMISSION CATALYSTS: A CONCEPTUAL FRAMEWORK
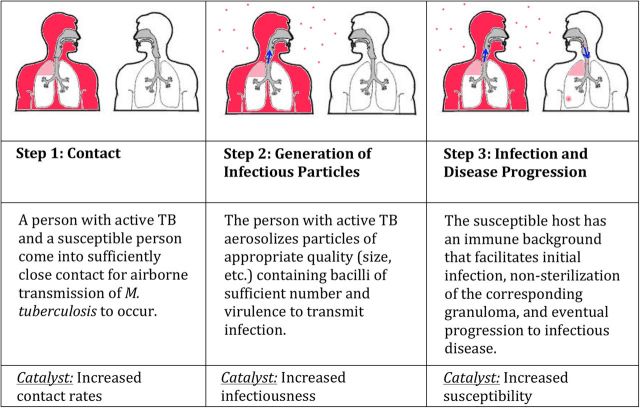
The critical cascade of events in tuberculosis dynamics is the transmission of Mycobacterium tuberculosis from an infectious person to a susceptible person who develops active (infectious) tuberculosis disease as a result. This cascade requires contact, generation of infectious particles from an infectious source, and infection followed by progression to disease in a recipient host (Figure 1). Strategies that target any of these 3 events can break the cascade of transmission; conversely, factors (“catalysts”) that facilitate these events will fuel tuberculosis transmission. Such catalysts generally come in 3 types (Table 1): factors that increase contact rates, infectiousness, and susceptibility.
Figure 1.
The cascade of tuberculosis (TB) transmission and disease.
Table 1.
Catalysts of Tuberculosis Transmission: Classification and Examples
| 1. Increased contact rates | ||
| Clustering (in Space) | Movement (Over Time) | |
| Small-scale | Households | Occupational: healthcare, mining, etc |
| Prisons, shelters | Public transportation/commuting | |
| Mines | Social: prisoners, homeless, etc | |
| Transit vehicles | ||
| Large-scale | Urban slums | Seasonal work (eg, floating populations) |
| Age-dependent mixing | Internal displacement | |
| City/regional hotspots | Urbanization | |
| 2. Increased infectiousness | ||
| Bacillus | Host | |
| Infectivity | Outbreak-causing strains | High-risk occupations (eg, singers) |
| Specific strain properties (eg, W-Beijing strain) | Highly symptomatic individuals | |
| Phylogenetic lineage (eg, geographic) | High bacillary load/smear-positive | |
| Disease duration | Drug-resistant strains | “Chronic” tuberculosis |
| Strain-induced pathology | Losses to follow-up | |
| Rural/indigenous/poor populations | ||
| Poor access to healthcare | ||
| 3. Increased susceptibility | ||
| Pulmonary Risk | Immunologic Risk | |
| Tuberculosis-specific factors | Prior tuberculosis | Markers of the immune response to tuberculosis (eg, propensity to develop a positive tuberculin skin test after exposure) |
| Fibrotic lung lesions | ||
| Macrophage response | ||
| Extrinsic factors | Smoking | HIV |
| Silicosis | Diabetes | |
| Indoor air pollution | TNF-α blockade | |
| Neonates, elderly | ||
Abbreviations: HIV, human immunodeficiency virus; TNF, tumor necrosis factor.
Tuberculosis control policies that are enacted at the country level implicitly ignore these catalysts by assuming homogeneous behavior within the country (Figure 2A). Under this assumption, transmission events are random, unpredictable occurrences, and the only way to reduce tuberculosis transmission by 50% is to halve the “infectious pool,” or the population prevalence of infectious tuberculosis. This could be accomplished, for example, by immediately detecting and treating half of cases, or halving the duration of infectiousness among all cases. However, tuberculosis transmission is not uniform: contact rates are higher among certain subpopulations (eg, crowded slums, household contacts, working-age individuals), certain individuals are more infectious (eg, due to more pathogenic bacteria or more efficient aerosol generation), and other people are more susceptible (eg, due to immune dysfunction or malnutrition). As a result, tuberculosis incidence is heterogeneous. For example, a reported country-wide incidence of multidrug-resistant tuberculosis in Moldova of 21 per 100 000 per year from 2007 to 2010 hides the fact that, within the penitentiary system, the incidence was 1300 per 100 000 per year [19]. Without high-quality data at the community level and high-level guidance as to how to use that data, local tuberculosis control officials may not operate in a fashion that achieves optimal results. Ultimately, the populations in which tuberculosis cases occur are largely predictable, if one can identify and collect data on the mix of catalysts most responsible for transmission in a given epidemiological setting (Figure 2B). This goal can only be accomplished through collaboration with, and improvement of, existing health systems at all levels, down to that of the community or district. Catalyst-targeted disease control strategies can therefore halve the infectious pool while covering far less than half of the entire population. Deploying existing tools in such an efficient and focused fashion may have much greater impact on tuberculosis transmission than developing new tools and implementing them in the populations (eg, those with better existing access to care) who need them least [20].
Figure 2.
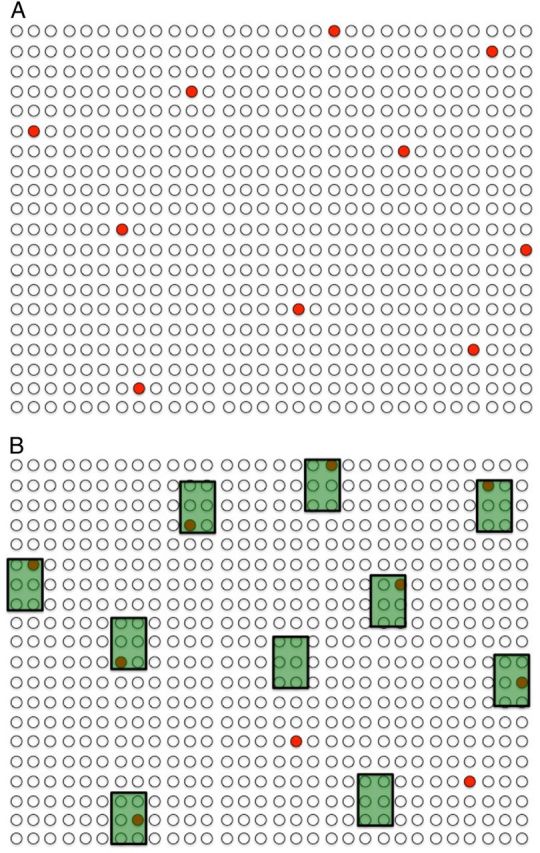
Catalysts of tuberculosis transmission. Closed (or black) dots represent 10 cases of prevalent infectious tuberculosis in a community of 600 individuals (ie, prevalence of >1600 per 100 000—note that tuberculosis is a rare disease even at a prevalence >10 times the global average). These individuals represent the “tuberculosis infectious pool” responsible for ongoing transmission in the community. A, A naive evaluation considers these tuberculosis cases to be randomly distributed, suggesting that 60 people without tuberculosis would need to be screened to find and treat 1 case of active tuberculosis (10% of the infectious pool). B, In reality, tuberculosis cases in a community often cluster according to transmission catalysts—shown as shaded boxes—that increase contact rates, infectiousness, and susceptibility. These catalysts may not be immediately recognizable to local disease control officials without additional data. However, if data can be collected and the key catalysts identified, screening (or other tuberculosis control efforts) can be targeted accordingly. In this hypothetical example, screening the 10% of the population that was associated with specific catalysts (eg, sites of high transmission [eg, prison, public transit], increased susceptibility profiles [eg, low body mass index, human immunodeficiency virus], or long infectious periods [eg, long distance from clinic, poor access to care]) would identify 80% of infectious tuberculosis cases.
TRANSMISSION CATALYST 1: INCREASED CONTACT RATES
Exposure to infectious tuberculosis is not uniform. A retired individual in a suburban neighborhood with little social activity may have few effective respiratory contacts, even on a monthly basis. By contrast, a working-age person living in and commuting from an urban slum to a crowded workplace may have hundreds of effective contacts per day. Should these individuals both develop active tuberculosis, the transmission impact of identifying and treating the urban worker would be many-fold greater than that of treating the retiree. Contact rates reflect specific locations (eg, prisons [21], slums [20], mines [22]), demography (eg, age [23], household structure [24]), and social structures (eg, occupation [25], transportation [26], homelessness [27], migration [28]) that vary widely across settings. These diverse catalysts of contact can generally be classified as patterns of clustering (the distribution of people across space) or patterns of movement (changes in those spatial distributions over time), across small or large geographic scales (Table 1). Furthermore, the predominant catalysts of contact can be identified at the local level by empowering district-level health systems to collect and analyze programmatic data. For example, systematically recording the locations of residence, work, and migration for all individuals presenting with active tuberculosis in a community could help identify that community's main contributors to increased contact rates. With higher-level guidance, those fine-grained programmatic data could then be used to prioritize public health interventions (eg, preventive therapy, environmental controls, systematic screening, contact tracing) most likely to impact tuberculosis transmission at the community level.
TRANSMISSION CATALYST 2: INCREASED INFECTIOUSNESS
Whether in outbreaks [29] or at the population level [30], a small number of individuals (“superspreaders”) are likely responsible for the majority of tuberculosis transmission events. Such “superspreaders” are not just those with increased contact rates, as above. Rather, this phenomenon can also reflect increased transmissibility or pathogenicity of different M. tuberculosis strains (eg, W-Beijing strain [31]), the propensity of specific individuals to generate infectious aerosols [32], differences in bacillary burden (eg, as represented by smear status [33]) over time, or increased duration of infectiousness (eg, drug-resistant isolates initially treated with first-line drugs, individuals with poor access to care). These catalysts of infectiousness can be categorized as characteristics of bacillus or host, and may reflect increases in infectivity (infectiousness per unit time) and/or duration of infectiousness. If individuals with these characteristics can be identified at the local level—either by innovative public health methods (eg, venue-based strategies that reach or incentivize people least likely to access care) or novel biomedical techniques (eg, cough aerosol chambers)—resources can be optimized for rapid diagnosis, treatment, and contact tracing of those likely responsible for the greatest infectious burden.
TRANSMISSION CATALYST 3: INCREASED SUSCEPTIBILITY
The impact of the HIV epidemic as a catalyst of tuberculosis transmission in sub-Saharan Africa is well documented [34]. However, on a global scale, other drivers of susceptibility, including undernutrition, indoor air pollution, smoking, diabetes mellitus, and poverty, are likely responsible for a greater population attributable fraction of tuberculosis than HIV [35]. These heterogeneities in tuberculosis susceptibility— that is, given the same level of exposure to infectious particles, some individuals are far more likely to develop active tuberculosis than others—classify broadly as either pulmonary or immunological in nature, and specific to the host response to tuberculosis vs reflective of extrinsic disease. These heterogeneities in susceptibility highlight that what has traditionally been considered a binary division between “latent” and “active” tuberculosis is more appropriately characterized as a spectrum of disease [36], with different individuals more likely to develop different manifestations, at different times. While we await better tests to identify the individuals at highest risk of progressing to infectious disease, many high-risk individuals can be detected through simple tests such as body mass index, blood glucose testing, and smoking surveys. These individuals could then be targeted for preventive interventions, with disproportionate impact on tuberculosis transmission in areas (eg, South Asia) where progression of disease in such high-risk individuals within a massive pool of infected individuals may represent the primary contribution to ongoing tuberculosis incidence [37]. Systematic approaches to collecting data on the prevalence and strength of susceptibility-based catalysts of transmission could facilitate the identification of geographic or sociodemographic “pockets of susceptibility” (eg, age groups, occupations, countries of origin, or residential areas with disproportionate levels of undernutrition, diabetes, smoking, or HIV) in each community. Such data collection and deployment of data-driven local approaches can only be accomplished by enhancing health systems at that level. Combining district/community-level health systems strengthening with high-level guidance on prioritization of tuberculosis case finding, diagnosis, treatment, and prevention based on community risk profiles would not only encourage collection of fine-grained data but also empower local professionals to target the catalysts of tuberculosis transmission in their communities without spreading scarce resources too thin.
CONCLUSIONS
If we are to meet the aggressive tuberculosis control targets set for 2025, we must start improving tuberculosis control today. Although disruptive technologies may be essential for tuberculosis elimination, we cannot use the search for such tools as an excuse to delay implementation of the interventions we already have at our fingertips, including active case finding, improved diagnosis, high-quality treatment (of drug-susceptible and drug-resistant tuberculosis), and preventive therapy. Naive observational studies, mathematical models, and even randomized trials [38, 39] may predict only marginal impact from “blind” implementation of such interventions at the population level, especially when scale-up is incomplete. However, appropriately targeted interventions based on a better understanding of local catalysts of tuberculosis transmission—factors that increase contact rates, infectiousness, and susceptibility at the community level—may augment these interventions' impact by an order of magnitude. Virtually every example of rapid progress in tuberculosis control has targeted 1 or more such catalysts, from prisons in the former Soviet Union [15] to HIV, multidrug-resistant tuberculosis, and homelessness in New York City [14]. Even settings widely cited as successes due to “universal” approaches (eg, Peru) incorporated strategies (eg, food packages and employment support for the poor, health promotion agents for people with poor access to care) that targeted local catalysts of transmission [40]. To replicate such successes, we must first understand which catalysts drive tuberculosis transmission at each local level. This could be accomplished by strengthening district-level health systems to collect additional survey-based data from individuals presenting with incident tuberculosis on a finer scale; such data have been utilized to help target interventions in settings from Cape Town [41] to Seattle [42], but have not found wide utility in high-burden, lowest-resource settings. We must therefore develop a high-level framework for deciding how to target the right set of interventions to the right populations, depending on the relative contributions of each catalyst and the locally available capacity. For example, simple nomograms could be developed to estimate the tuberculosis disease burden in a community as a function of programmatic data on tuberculosis cases and population size, coupled with susceptibility profiles. As data on infectiousness (eg, molecular epidemiology and duration of disease) become available, such data could be incorporated as well. Different levels of tuberculosis control intensity could then be recommended based on risk profiles developed at the community level, with the most intensive efforts aggressively targeted toward populations or settings of highest risk. High-level guidance explaining how to create and use such risk profiles could encourage data collection at a finer scale, empower local tuberculosis control officials to adopt a more data-driven approach to their efforts, and serve as a useful monitoring tool to track performance. The time for a “one size fits all” approach to global tuberculosis control has come to an end. To achieve global targets in tuberculosis control, we must embrace heterogeneity in tuberculosis epidemics at the local level, strengthen district-level health systems to collect data on the catalysts driving tuberculosis transmission at a finer scale, and use that knowledge to deploy targeted interventions, one community at a time.
Notes
Disclaimer. The funding source had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the B. Frank and Kathleen Polk Assistant Professorship in Epidemiology at the Johns Hopkins Bloomberg School of Public Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality for 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Geneva, Switzerland: WHO; 2013. World malaria report 2013. [Google Scholar]
- 3.Averhoff F. Centers for Disease Control and Prevention. CDC health information for international travel 2014. New York: Oxford University Press; 2014. Hepatitis B. [Google Scholar]
- 4.World Health Organization. Geneva, Switzerland: WHO; 2013. Global tuberculosis report 2013. ix, 9, 29. [Google Scholar]
- 5.67th World Health Assembly. Global strategy and targets for tuberculosis prevention, care and control after 2015. Available at: http://www.who.int/tb/post2015_tbstrategy.pdf?ua=1. Accessed 12 June 2014.
- 6.Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Global tuberculosis drug development pipeline: the need and the reality. Lancet. 2010;375:2100–9. doi: 10.1016/S0140-6736(10)60359-9. [DOI] [PubMed] [Google Scholar]
- 7.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383:424–35. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 8.Brennan MJ, Stone MR, Evans T. A rational vaccine pipeline for tuberculosis. Int J Tuberc Lung Dis. 2012;16:1566–73. doi: 10.5588/ijtld.12.0569. [DOI] [PubMed] [Google Scholar]
- 9.Dooley KE, Nuermberger EL, Diacon AH. Pipeline of drugs for related diseases: tuberculosis. Curr Opin HIV AIDS. 2013;8:579–85. doi: 10.1097/COH.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stop TB Partnership. The Stop TB Strategy. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 11.Churchyard GJ, Fielding KL, Lewis JJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370:301–10. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Geneva, Switzerland: WHO; 2011. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. [Google Scholar]
- 13.Comstock GW, Philip RN. Decline of the tuberculosis epidemic in Alaska. Public Health Rep. 1961;76:19–24. [PMC free article] [PubMed] [Google Scholar]
- 14.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York City—turning the tide. N Engl J Med. 1995;333:229–33. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 15.Partners in Health. PIH/Russia recognized for TB achievements. Available at: http://www.pih.org/blog/pih-russia-recognized-for-tb-achievements. Accessed 14 March 2014.
- 16.Bousema T, Griffin JT, Sauerwein RW, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharti N, Djibo A, Ferrari MJ, et al. Measles hotspots and epidemiological connectivity. Epidemiol Infect. 2010;138:1308–16. doi: 10.1017/S0950268809991385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joint United Nations Programme on HIV/AIDS (UNAIDS) Getting to zero: UNAIDS 2011–2015 strategy. Geneva, Switzerland: UNAIDS; 2010. p. 16. [Google Scholar]
- 19.Jenkins HE, Plesca V, Ciobanu A, et al. Assessing spatial heterogeneity of multidrug-resistant tuberculosis in a high-burden country. Eur Respir J. 2013;42:1291–301. doi: 10.1183/09031936.00111812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowdy DW, Golub JE, Chaisson RE, Saraceni V. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proc Natl Acad Sci U S A. 2012;109:9557–62. doi: 10.1073/pnas.1203517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coninx R, Maher D, Reyes H, Grzemska M. Tuberculosis in prisons in countries with high prevalence. BMJ. 2000;320:440–2. doi: 10.1136/bmj.320.7232.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanifa Y, Grant AD, Lewis J, Corbett EL, Fielding K, Churchyard G. Prevalence of latent tuberculosis infection among gold miners in South Africa. Int J Tuberc Lung Dis. 2009;13:39–46. [PubMed] [Google Scholar]
- 23.Wood R, Liang H, Wu H, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;14:406–12. [PMC free article] [PubMed] [Google Scholar]
- 24.Chamie G, Wandera B, Luetkemeyer A, et al. Household ventilation and tuberculosis transmission in Kampala, Uganda. Int J Tuberc Lung Dis. 2013;17:764–70. doi: 10.5588/ijtld.12.0681. [DOI] [PubMed] [Google Scholar]
- 25.Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis. 2007;11:593–605. [PubMed] [Google Scholar]
- 26.Andrews JR, Morrow C, Wood R. Modeling the role of public transportation in sustaining tuberculosis transmission in South Africa. Am J Epidemiol. 2013;177:556–61. doi: 10.1093/aje/kws331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes PF, el-Hajj H, Preston-Martin S, et al. Transmission of tuberculosis among the urban homeless. JAMA. 1996;275:305–7. [PubMed] [Google Scholar]
- 28.Liu L, Wu J, Zhao XQ. The impact of migrant workers on the tuberculosis transmission: general models and a case study for China. Math Biosci Eng. 2012;9:785–807. doi: 10.3934/mbe.2012.9.785. [DOI] [PubMed] [Google Scholar]
- 29.Gardy JL, Johnston JC, Ho Sui SJ, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011;364:730–9. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]
- 30.Ypma RJ, Altes HK, van Soolingen D, Wallinga J, van Ballegooijen WM. A sign of superspreading in tuberculosis: highly skewed distribution of genotypic cluster sizes. Epidemiology. 2013;24:395–400. doi: 10.1097/EDE.0b013e3182878e19. [DOI] [PubMed] [Google Scholar]
- 31.Yang C, Luo T, Sun G, et al. Mycobacterium tuberculosis Beijing strains favor transmission but not drug resistance in China. Clin Infect Dis. 2012;55:1179–87. doi: 10.1093/cid/cis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fennelly KP, Jones-Lopez EC, Ayakaka I, et al. Variability of infectious aerosols produced during coughing by patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 2012;186:450–7. doi: 10.1164/rccm.201203-0444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–9. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 34.Chaisson RE, Martinson NA. Tuberculosis in Africa—combating an HIV-driven crisis. N Engl J Med. 2008;358:1089–92. doi: 10.1056/NEJMp0800809. [DOI] [PubMed] [Google Scholar]
- 35.Lonnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–29. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 36.Barry CE, III, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–55. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271–86. doi: 10.1146/annurev-publhealth-031912-114431. [DOI] [PubMed] [Google Scholar]
- 38.Ayles H, Muyoyeta M, Du Toit E, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;382:1183–94. doi: 10.1016/S0140-6736(13)61131-9. [DOI] [PubMed] [Google Scholar]
- 39.Durovni B, Saraceni V, Moulton LH, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster-randomised trial. Lancet Infect Dis. 2013;13:852–8. doi: 10.1016/S1473-3099(13)70187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suarez PG, Watt CJ, Alarcon E, et al. The dynamics of tuberculosis in response to 10 years of intensive control effort in Peru. J Infect Dis. 2001;184:473–8. doi: 10.1086/322777. [DOI] [PubMed] [Google Scholar]
- 41.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42:1040–7. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 42.Buskin SE, Gale JL, Weiss NS, Nolan CM. Tuberculosis risk factors in adults in King County, Washington, 1988 through 1990. Am J Public Health. 1994;84:1750–6. doi: 10.2105/ajph.84.11.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]