Abstract
In higher eukaryotic cells, long non-protein-coding RNAs (lncRNAs) have been implicated in a wide array of cellular functions. Cell- or tissue-specific expression of lncRNA genes encoded in the mammalian genome is thought to contribute to the complex gene networks needed to regulate cellular function. Here, we have identified a novel species of polypurine triplet repeat-rich lncRNAs, designated as GAA repeat-containing RNAs (GRC-RNAs), that localize to numerous punctate foci in the mammalian interphase nuclei. GRC-RNAs consist of a heterogeneous population of RNAs, ranging in size from ~1.5 kb to ~4 kb and localize to subnuclear domains, several of which associate with GAA.TTC-repeat-containing genomic regions. GRC-RNAs are components of the nuclear matrix and interact with various nuclear matrix-associated proteins. In mitotic cells, GRC-RNAs form distinct cytoplasmic foci and, in telophase and G1 cells, localize to the midbody, a structure involved in accurate cell division. Differentiation of tissue culture cells leads to a decrease in the number of GRC-RNA nuclear foci, albeit with an increase in size as compared with proliferating cells. Conversely, the number of GRC-RNA foci increases during cellular transformation. We propose that nuclear GRC-RNAs represent a novel family of mammalian lncRNAs that might play crucial roles in the cell nucleus.
Key words: Non-coding RNAs, Nuclear domains, Nuclear matrix, Triplex repeats, Midbody
Introduction
Large-scale genomic studies have estimated that ~50% of the mammalian genome is transcribed into RNA on one or both strands, whereas all of the predicted protein-coding part of transcripts together constitute only ~2–5% of the genome (Cheng et al., 2005; Mattick and Makunin, 2006; van Bakel et al., 2010). This suggests that a large proportion of genome-encoded transcripts consist of non-protein coding RNAs (ncRNAs) (Mattick, 2009; Mattick and Makunin, 2006; Prasanth and Spector, 2007). Such ncRNAs include housekeeping ncRNAs (examples include ribosomal RNA, transfer RNA, small nuclear and nucleolar RNAs) as well as regulatory ncRNAs, whose expression is modulated in a cell-specific or developmental stage-specific manner (Prasanth and Spector, 2007).
Regulatory ncRNAs are generally categorized into small [18–200 nucleotides (nts)] and long (200 nts to 100 kb) ncRNAs (Prasanth and Spector, 2007). MicroRNAs, an example of small ncRNAs, serve regulatory roles in several crucial biological processes such as cellular proliferation, differentiation and developmental timing (Mattick, 2005). Genome-wide transcriptome analyses have now shown that a major fraction of the regulatory ncRNAs in mammals consists of long ncRNAs (lncRNAs) (Dinger et al., 2009; Mercer et al., 2009; Wilusz et al., 2009). LncRNAs were initially thought to be an artifact of chromatin remodeling or ‘transcriptional noise’ in the cell (Struhl, 2007), but recent data have indicated that a large number of mammalian lncRNAs are highly conserved and are involved in diverse cellular functions (Dinger et al., 2009; Guttman et al., 2009; Khalil et al., 2009). Some of the well-characterized mechanisms involving lncRNAs include dosage compensation and genome imprinting, programmed whole-genome rearrangements in ciliate differentiation, human brain evolution, T-cell receptor recombination, maintenance of telomeres, stress-response and cellular differentiation (Amaral et al., 2008; Wilusz et al., 2009).
Nuclear-retained RNAs (nrRNAs) constitute a subclass of eukaryotic regulatory RNAs that are predominantly enriched in the cell nucleus and are suggested to play structural roles or act as riboregulators (Pederson, 2009; Prasanth and Spector, 2007). Well-characterized examples of mammalian nrRNAs include Xist and Tsix that are involved in dosage compensation (Brockdorff et al., 1992; Lee et al., 1999), Air and Kcnq1ot1 RNAs involved in genome imprinting (Braidotti et al., 2004; Pandey et al., 2008) and MEN ε/β (Neat1) involved in the structural organization of paraspeckles (Chen and Carmichael, 2009; Clemson et al., 2009; Sasaki et al., 2009; Sunwoo et al., 2009). In Schizosaccharomyces pombe, repeat-containing nrRNAs are involved in heterochromatin organization (Grewal and Elgin, 2007). Recent studies in mammalian cells also indicated crucial roles for repeat-containing nrRNAs in chromatin-specific functions including heterochromatin organization, neocentromere formation and X-chromosome inactivation (Chow et al., 2010; Chueh et al., 2009; Maison et al., 2002; Wong et al., 2007). Interestingly, a significant number of novel nrRNAs were recently identified in mammalian cells that show tissue- or cell-type specific expression (Mercer et al., 2008; Sone et al., 2007). Understanding how novel nrRNAs regulate gene expression will provide insights into how a higher degree of cellular complexity is achieved in eukaryotes.
In the present study, we have identified a novel species of nrRNAs in mammalian cells: GAA-repeat-containing RNAs (GRC-RNAs) that are distributed throughout the nucleus in a micropunctate pattern. GRC-RNAs primarily consist of polypurine repeats and are heterogeneous in size, ranging from 1.5–4 kb. GRC-RNAs associate with the nuclear matrix and interact with several bona fide nuclear matrix proteins. Our studies further indicate that the nuclear GRC-RNA foci is altered in cells that either are highly proliferating or are cell-cycle-arrested owing to serum deprivation. We propose that GRC-RNAs are novel members of the nrRNA family of RNAs that interact with components of the nuclear matrix and play crucial structural roles in the maintenance of the nuclear matrix and/or regulate gene expression.
Results
GRC-RNAs are members of the nrRNA family of lncRNAs
RNA in situ hybridization studies have previously cataloged ~850 lncRNAs that are expressed in specific cell types or in specific subcellular compartments within the adult mouse brain (Lein et al., 2007; Mercer et al., 2008). To gain further insight into the cellular function of these novel ncRNAs, we analyzed their distribution in various mammalian cell lines by RNA-FISH (fluorescent in situ hybridization). One of these ncRNAs (EST clone AK082015; ~2.2 kb) showed a punctate distribution in the nucleus of mouse NIH-3T3 cells (Fig. 1A). In most of the cells, apart from numerous nuclear foci, 2–5 distinct brightly labeled GRC-RNA-positive foci were also observed in the cytoplasm (see arrows in Fig. 1A; supplementary material Fig. S1B,C). To identify the minimal region of the cDNA probe that hybridized to the nuclear foci-associated RNA, we subcloned AK082015 cDNA into multiple smaller regions and used them individually as probes in RNA-FISH analysis (supplementary material Fig. S1). RNA-FISH using probes containing the fragment-2 region (170 bp) displayed similar micropunctate nuclear staining, whereas the flanking regions of the cDNA failed to show any specific staining (supplementary material Fig. S1B,C). These results indicate that the AK082015 ncRNA by itself does not localize to these nuclear foci but the fragment-2 in AK082015 cDNA hybridizes to a class of RNA(s). Sequencing of the fragment-2 probe revealed that it contains a characteristic GAA.TTC tandem triplet repeat sequence (20 continuous GAA.TTC repeats; supplementary material Fig. S1A). A recent study using a fluorescently labeled GAA.TTC probe or (GAA)20 oligonucleotide probe and performing FISH in non-denatured human cells demonstrated the presence of similar subnuclear foci (Ohno et al., 2002). Based on their results, the authors claimed that the GAA-repeat probe hybridized to the TTC-repeat DNA single strand that was displaced owing to the formation of GAA-GAA-TTC triple-helix DNA structure (Ohno et al., 2002). Our studies using RNA-FISH in mouse NIH-3T3 cells using fluorescent-labeled single-stranded DNA oligonucleotide probes consisting of (GAA)15, (TTC)15, (CCT)15 or (CT)20 revealed that only the (TTC)15 probe displayed the micropunctate nuclear signal (Fig. 1B), indicating the presence of GAA-repeat-containing nuclear RNA. Based on our results (described below), we named this novel species of RNA as GAA-repeat-containing RNA (GRC-RNA). Our results are strikingly in contrast to the previous study that claimed that nuclear foci are apparent only when hybridized with the (GAA)20 probe (Ohno et al., 2002).
Fig. 1.
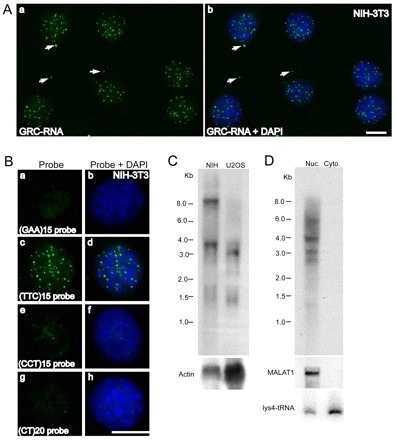
GRC-RNA is a member of the nrRNA family of lncRNAs. (A) RNA-FISH using an AK082015 probe (green) in NIH-3T3 cells revealed the punctate nuclear localization of GRC-RNA. In addition to nuclear foci, GRC-RNA also localized to a few cytoplasmic foci (arrows). Scale bar: 10 μm. (B) RNA-FISH using FITC-labeled (GAA)15 (a,b), (TTC)15 (c,d), (CCT)15 (e,f) and (CT)20 (g,h) single-stranded oligonucleotide probes in NIH-3T3 cells revealed that only the (TTC)15 probe hybridized to nuclear-enriched GRC-RNA (c,d). DNA was counterstained with DAPI (blue). Scale bar: 10 μm. (C) Northern blot using a 32P-labeled (TTC)15 probe from total RNA revealed that GRC-RNAs represent heterogeneous transcripts. Actin RNA was used as a loading control. (D) Northern blot using a 32P-labeled (TTC)15 probe in nuclear and cytoplasmic RNA fractions from EpH4 cells revealed nuclear enrichment of GRC-RNA. MALAT1 and lysine tRNA were used as markers for nuclear and cytoplasmic RNA fractionation, respectively.
Northern hybridization using a 32P-labeled (TTC)15 oligonucleotide probe revealed that GRC-RNAs were heterogeneous in length (Fig. 1C). However, the high-molecular-weight (~8 kb) RNA was present only in the mouse cell line. Northern hybridization on nuclear and cytoplasmic RNA from mouse mammary cells using the (TTC)15 oligonucleotide probe reconfirmed the RNA-FISH observations that GRC-RNAs constitute a heterogeneous population of RNAs that are predominantly enriched in the nuclear fraction (Fig. 1D). Surprisingly, the (TTC)15 probe detected the ~8 kb RNA only in the total RNA fraction and failed to detect it in the fractionated RNA samples from multiple cell lines (Fig. 1D; Fig. 2C).
Fig. 2.
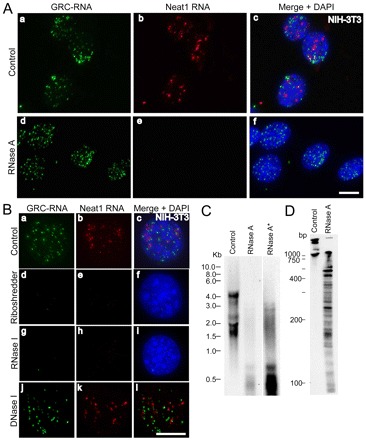
GRC-RNA nuclear foci are sensitive to RNase but not DNase I treatment. (A) Co-RNA-FISH in NIH-3T3 cells for GRC-RNA (green; a,d) and Neat1 RNA (red; b,e) revealed that, unlike Neat1 RNA (e), GRC-RNA foci were insensitive to RNase A treatment (d). Merged images are shown in c and f, with chromatin stained with DAPI in blue. Scale bar: 10 μm. (B) Co-RNA-FISH in NIH-3T3 cells for GRC-RNA (green; a,d,g,j) and Neat1 RNA (red; b,e,h,k) revealed that both GRC-RNA and Neat1 RNA were degraded by Riboshredder (d–f) and RNase I (g–i) treatments, but not by DNase I (j–l) treatment. Chromatin was stained with DAPI in blue. Merged images are shown in c, f, i and l. Scale bar: 10 μm. (C) Northern blot using a 32P-labeled (TTC)15 probe from nuclear RNA of NIH-3T3 cells revealed that RNase A treatment resulted in the cleavage of GRC-RNA into smaller fragments. Longer exposure of the RNase-A-treated RNA blot (RNase A*) revealed the presence of RNase-A-resistant GRC-RNA. (D) Control and RNase-A-treated nuclear RNA were run on an acrylamide gel and hybridized with a 32P-labeled (TTC)15 probe.
GRC-RNA foci are sensitive to RNase but not DNase I treatment
To further confirm the identity of the RNA-FISH signal observed with the fluorescently labeled (TTC)15 probe, we treated NIH-3T3 cells with RNase A prior to RNA-FISH. RNase A pretreatment failed to eliminate the nuclear-foci-like distribution of GRC-RNA (Fig. 2A), whereas the localization of Neat1 lncRNA at paraspeckles was clearly sensitive to RNase A treatment. Moreover, double-strand RNA-specific ribonucleases, RNase VI (supplementary material Fig. S2a-c) and RNase III (supplementary material Fig. S2d–f) failed to remove GRC-RNA foci when they were incubated with the cells prior to RNA-FISH. However, pretreatment of cells with Riboshredder, a cocktail of multiple RNases (Epicenter Inc.) or a single-strand-specific ribonuclease, RNase I, completely abolished GRC-RNA foci (Fig. 2Bd–i). Systematic analysis with various RNase treatments showed that RNase H treatment eliminated the GRC-RNA foci only when the RNase H treatment was performed after the RNA-FISH (but not prior to RNA-FISH) using the oligonucleotide (TTC)15 probe (supplementary material Fig. S2g–l). Because the (TTC)15 probe consists of single-strand oligonucleotide DNA, the sensitivity of GRC-RNA foci to RNase H treatment after hybridization demonstrates the presence of single-stranded RNA in these subnuclear foci. Finally, transfection of NIH-3T3 cells with a modified antisense DNA oligonucleotide that specifically targets GAA-repeat-containing RNA resulted in the complete disappearance of GRC-RNA foci, further confirming the presence of RNA in these subnuclear foci (supplementary material Fig. S3). RNase A preferentially cleaves the phosphodiester bonds at 3′ end of pyrimidines in single-strand RNA, whereas RNase I cleaves the phosphodiester bonds at 3′ end of both purines and pyrimidines (D'Alessio and Riordan, 1997; Meador et al., 1990). Sensitivity of GRC-RNA to RNase I and not RNase A indicated that GRC-RNA is predominantly enriched in homopurine nucleotide repeats. Furthermore, DNase I treatment of cells prior to RNA-FISH failed to remove the GRC-RNA nuclear signal (Fig. 2Bj-l), indicating that GRC-RNA foci do not comprise DNA.
To understand the nature of homopurine stretches in GRC-RNA, northern hybridization using a (TTC)15 probe was conducted in untreated and RNase-A-treated nuclear RNA (Fig. 2C). The untreated nuclear extracts from NIH-3T3 cells displayed several bands of GRC-RNA ranging between 1.5–4 kb. The northern profile in NIH-3T3 cells looked different from that of mouse mammary cells (EpH4; Fig. 1D) and could be attributed to the cell-type-specific changes in the size of GRC-RNAs. RNase A treatment resulted in the disappearence of most of the bands but a smear of <1 kb in size was observed in the gel. Interestingly, when the blot was exposed for a longer duration (48 hours; Fig. 2C, RNase A*), specific higher-molecular-weight bands (~3 kb) were also observed in the RNAse-A-treated extracts. To understand the nature of the low-molecular-weight RNAs (<1 kb) produced upon RNase A treatment, untreated and RNase-A-treated total nuclear RNA was resolved in an acrylamide gel and hybridized using the (TTC)15 probe (Fig. 2D). Northern hybridization using a (TTC)15 probe in RNAs isolated from untreated cells failed to detect any specific signal below 1 kb (Fig. 2D, Control). However, RNase-A-treated samples produced several distinct bands of GRC-RNA ranging in size from 0.1–1 kb. These results indicate that the long GRC-RNAs comprise several stretches of homopurine repeats (100 bp to 1 kb) that are interspersed with pyrimidine nucleotides that are sensitive to RNase-A-mediated cleavage. Strikingly, the RNase-A-cleaved homopurine stretches of GRC-RNA continue to localize to the subnuclear foci (Fig. 2A). This result indicates that either the subnuclear-foci-enriched GRC-RNAs primarily consist of homopurine strands of ~1 kb or association with specific subnuclear structure(s) maintains the distribution of GRC-RNA in these subnuclear domains.
GRC-RNAs associate with GAA.TTC-repeat DNA elements in the chromatin
Our initial attempts to clone the mouse GRC-RNAs remain unsuccessful, probably owing to the large number of repeats present in the transcripts. Genome-sequencing analyses in mammals have indicated the presence of a large number of extended GAA.TTC-repeat regions (more than 20 GAA tandem repeats) in several parts of the genome (Clark et al., 2006). We were interested to examine whether long GAA-repeats show any preference in specific regions of the mouse genome. We have conducted a bioinformatics-based search in order to estimate the relative positions of GAA-repeat regions in the mouse genome (Fig. 3A). Our analysis indicated the presence of ~1142 polypurine-rich regions with GAA-repeats in the mouse genome (at least 7 continuous GAA-repeats and the whole polypurine repeat length is ~300 bp). Further, a significantly large proportion of GAA-repeat regions were preferentially located at the 5′ or 3′ end of the annotated genes, whereas genic regions seemed to contain fewer GAA-repeats (Fig. 3A). Our results imply that GAA-repeat regions are not randomly distributed in the genome but are located in close proximity to the regulatory elements in a large number of genes. Next, we examined whether the GRC-RNAs associate with any of the GAA.TTC-repeat DNA regions in the mouse genome. We performed RNA-FISH using a digoxigenin-labeled (TTC)15 probe in non-denatured NIH-3T3 cells followed by DNA-FISH using a fluorescently labeled (GAA)15 probe post-DNA denaturation (Fig. 3B; supplementary material Fig. S4). Dual RNA-DNA-FISH results revealed that a large number of the GRC-RNA foci (~80% of GRC-RNA-positive foci; n=50 nuclei) colocalized with the (GAA)15 DNA FISH signal, indicating that several of the GRC-RNA foci associate with GAA.TTC-repeat DNA elements (arrows in Fig. 3B; supplementary material Fig. S4) and could be transcribed from these regions. The remaining fraction of the GRC-RNA foci did not associate with GAA.TTC-repeat DNA (~20%; circles in Fig. 3B; supplementary material Fig. S4). Conversely, ~30% of the GAA.TTC DNA foci did not contain GRC-RNA (arrowheads in Fig. 3B; supplementary material Fig. S4), indicating that these DNA elements might represent transcriptionally inactive regions.
Fig. 3.
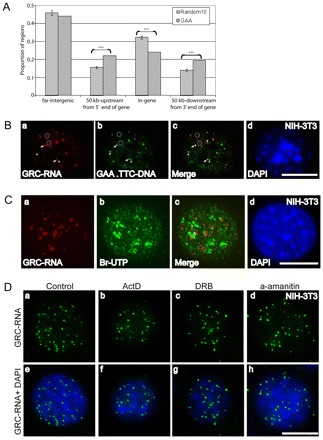
GAA-repeats are preferentially enriched in the 5′ and 3′ regulatory regions of genes. (A) GAA-repeats are preferentially enriched in the 5′ and 3′ regulatory regions of genes. Based on the Refseq gene definition from the UCSC genome browser, the mouse genome is divided into four parts: the gene region (from start to end), 50 kb upstream of the gene 5′ end, 50 kb downstream of the gene 3′ end, and others called ‘far-intergenic regions’. The distribution of 1142 GAA-enriched regions (with at least 7 continuous GAAs and at least 300 bp of A/G stretch) in each of these four parts is shown by the gray bar. The significance of enrichment of GAA-repeats in each part is estimated based on the distribution of randomly selected genomic DNA regions (10 times) with same amount in each chromosome and same length distribution as the GAA fragments. (***P<0.001). The P-values are: 50 kb up-gene=0.00007; in gene=0.00002; and 50 kb down-gene=0.0006. (B) GRC-RNA foci associate with GAA.TTC-repeat DNA elements: RNA-FISH using a (TTC)15 (red; a,c) probe followed by DNA-FISH using a (GAA)15 probe (green; b,c) revealed association of GRC-RNA with GAA.TTC-repeat DNA elements in the genome. Arrows show a few of the representative GRC-RNA foci that colocalize with GAA.TTC-repeats; open circles represent the GRC-RNA foci that do not associate with GAA.TTC-repeats; arrowheads represent a few of the GAA.TTC-repeat DNA regions that do not associate with GRC-RNA. (C) GRC-RNA foci do not represent highly active transcription sites: RNA-FISH using a (TTC)15 (red; a,c) probe coupled with in vivo Br-UTP incorporation assays (5 minutes, Br-UTP pulse; green; b,c) revealed weak to no significant overlap between GRC-RNA foci and actively transcribing sites (c). (D) GRC-RNA foci contains stable nuclear RNAs: RNA-FISH for GRC-RNA in control (a,e) and RNA polymerase II inhibitor-treated NIH-3T3 cells [(actinomycin D for 1 hour; 5 μg/ml; b,f), (DRB for 3 hours; 32 μg/ml: c,g), (α-amanitin for 6 hours; 50 μg/ml: d,h)] showed a similar number of nuclear foci. Chromatin was stained with DAPI (blue). Scale bars: 10 μm.
To determine the identity of the GRC-RNA foci that did not associate with GAA.TTC DNA elements, we conducted a series of dual-labeling fluorescence microscopic analyses utilizing GRC-RNA RNA-FISH with markers for known subnuclear domains. No association of GRC-RNA with either centromeres (supplementary material Fig. S5a–d) or telomeres (supplementary material Fig. S5e–h) was evident. Moreover, dual RNA-FISH and immunostaining studies revealed that GRC-RNA foci did not overlap with any of the known subnuclear domains including paraspeckles (supplementary material Fig. S5i–l), nuclear speckles (supplementary material Fig. S5m–p), Cajal bodies (supplementary material Fig. S5q–t) and PML bodies (supplementary material Fig. S5u–x), indicating that some of the GRC-RNA foci represent novel subnuclear domains.
To determine whether GRC-RNA foci coincide with actively transcribing regions, we performed in vivo short-pulse Br-UTP incorporation assays (5 minutes; Fig. 3C). Surprisingly, the majority of the GRC-RNA-containing nuclear foci did not colocalize with Br-UTP-labeled nascent transcripts, indicating that the GRC-RNA foci do not represent highly active transcription sites or are sites that are active only at specific stages of the cell cycle. Based on the DNA-RNA FISH results, we are tempted to speculate that the absence of Br-UTP incorporation in the GRC-RNA foci that are associated with GAA.TTC DNA elements could be because GRC-RNAs transcribed from these regions constitute a stable population of RNA that associate with transcription sites after being synthesized. In order to study the cellular turnover of GRC-RNA, RNA-FISH using a (TTC)15 probe was conducted in NIH-3T3 and wild-type mouse embryonic fibroblast (WT-MEF) cells that were incubated with various RNA polymerase II inhibitors: α-amanitin, 5,6-dichloro-1-beta-D-ribobenzimidazole (DRB) and actinomycin D. RNA-FISH revealed that the GRC-RNA foci number within the nuclei remained unaltered after RNA polymerase II transcription inhibition (Fig. 3D). However, treatment of WT-MEFs with α-amanitin for more than 14 hours resulted in the decrease of GRC-RNA foci, implying that GRC-RNAs might constitute a stable population of RNA polymerase II transcripts (supplementary material Fig. S6).
GRC-RNAs localize to the midbody in mitosis
Many of the subnuclear domains present in interphase nuclei also form analogous structures in the cytoplasm during mitosis (Chen et al., 2008; Leung et al., 2004; Prasanth et al., 2003). We examined the fate of GRC-RNA foci in mitotic NIH-3T3 cells by RNA-FISH analysis. The nuclear GRC-RNA foci were disorganized during prophase soon after the nuclear envelope breakdown (data not shown). GRC-RNA was concentrated in distinct bodies (20–40/cell) in cytoplasm of metaphase cells (Fig. 4Aa,b) and these structures were more prominent in anaphase cells (Fig. 4Ac,d). The GRC-RNA foci were mostly excluded from the chromosomes and were distributed in the cytoplasm. Interestingly, in early telophase cells, most of the GRC-RNA foci were congregated in the mid-zone area, a cytoplasmic region located between the two newly forming daughter nuclei (Fig. 4Ae,f). A similar localization of Xist nrRNA at the midzone area in telophase cells has been reported previously (Clemson et al., 1996). Finally, in late-telophase cells, a fraction of the GRC-RNA was localized in the cytokinetic furrow or midbody (arrow in Fig. 4Ag,h). GRC-RNA continued to associate with the midbody in early G1 cells (Fig. 4Ba–f). The midbody forms a cytoplasmic bridge between dividing daughter cells and is ultimately cleaved to complete cytokinesis (Eggert et al., 2006). The midbody is characterized by a specific set of proteins and comprises thick bundles of actin and tubulin (Eggert et al., 2006). It is speculated that components present at the midbody play vital roles in the successful division of daughter cells (Eggert et al., 2006). Interestingly, the midbody-localized GRC-RNA displayed similar characteristics to GRC-RNA that is localized at the subnuclear domains in interphase nuclei. For example, both were resistant to DNase I (Fig. 4Bc,d) and RNase A (Fig. 4Be,f) treatments, and sensitive to RNase I treatment (Fig. 4Bg,h). Transfection of an antisense DNA oligonucleotide that specifically targets GAA-repeat-containing RNA resulted in complete disappearance of GRC-RNA in the midbody (Fig. 4Bi,j). Other nrRNAs that are known to form cytoplasmic foci in mitotic cells [Neat1 (supplementary material Fig. S7); MALAT1 and CTN-RNA (data not shown)] did not associate with the midbody, indicating that midbody distribution is specific to GRC-RNA. A recent study, by performing RNA-FISH analyses, identified ~10 RNA species (out of ~3000 RNAs tested) to be localized in the midzone area in Drosophila embryos, further emphasizing the importance of RNA localization to the midbody (Lecuyer et al., 2007).
Fig. 4.
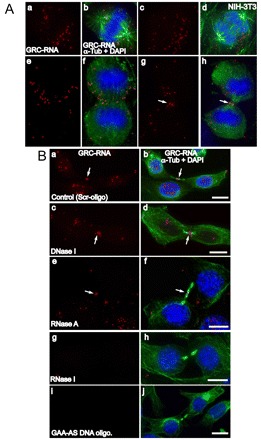
GRC-RNAs form cytoplasmic foci in mitotic cells and localize to the midbody during cell division. (A) Co-RNA-FISH for GRC-RNA (red) and immunostaining for α-tubulin (green) in mitotic NIH-3T3 cells [(metaphase; a,b), (anaphase; c,d), (early telophase; e,f), (late telophase; g,h)] revealed the presence of GRC-RNA in cytoplasmic foci. The arrows in g and h show the presence of GRC-RNA in the midbody. (B) Co-RNA FISH for GRC-RNA (red) and immunostaining for α-tubulin (green) was conducted in early G1 cells that were treated with scrambled DNA oligonucleotide (control; a,b), DNase I (c,d), RNase A (e,f), RNase I (g,h) or GAA-antisense DNA oligonucleotides (i,j). GRC-RNA showed midbody localization (arrows) in control, DNase-I- and RNase-A-treated cells. However, RNase I treatment (g,h) and GAA-antisense DNA oligonucleotide transfection (i,j) resulted in the loss of GRC-RNA signal. The chromatin is stained with DAPI (blue). Scale bars: 10 μm.
GRC-RNAs associate with the nuclear matrix and interact with nuclear matrix proteins
Earlier studies have reported the association of nrRNAs with the nuclear matrix, a biochemically defined nuclear skeletal non-chromatin structure that is suggested to be involved in crucial nuclear functions including DNA replication, transcription, pre-mRNA processing and mRNP transport (Clemson et al., 1996; Nickerson, 2001; Sone et al., 2007). GRC-RNA nuclear foci were resistant to detergent and nuclease extraction (data not shown), a characteristic feature of nuclear-matrix-associated molecules (Nickerson, 2001). RNA-FISH using a GRC-RNA probe clearly demonstrated the presence of GRC-RNA foci in high-salt-extracted, DNase-I-treated nuclear matrix preparations (Fig. 5A). We speculate that the interaction with specific nuclear matrix proteins stabilizes the GRC-RNA foci in the nucleus. Alternatively, GRC-RNA could be involved in the structural organization of the nuclear matrix. To identify the factors that interact with GAA-rich GRC-RNA, (GAA)15-, (UUC)15- and scrambled (scr)-RNA oligonucleotides were covalently coupled to agarose beads and were used in parallel for RNA affinity purification of proteins from NIH-3T3 nuclear extract. Several protein bands were consistently enriched in the (GAA)15-RNA oligo affinity-purified samples but were also present at lower concentrations in the (UUC)15-RNA oligo-purified fraction (Fig. 5B). Because the GRC-RNAs in the nuclear extract will hybridize to (UUC)15-RNA oligos, we speculated that a lower amount of the GRC-RNA-interacting proteins can also potentially be present in the (UUC)15-RNA oligo-purified fraction. Therefore, bands that showed enrichment in the (GAA)15-RNA oligo affinity-purified fraction but were also present in the (UUC)15-purified fraction at lower amounts (Fig. 5B) were excised from silver-stained gels and subjected to mass spectrometry analysis. The proteins that displayed higher affinity to GAA-repeat RNAs included hnRNP U/SAF-A (Fig. 5B, band a), Nucleolin (C23; Fig. 5B, band b), hnRNPs A1 (Fig. 5B, band d), A2/B1 (Fig. 5B, band d), C1, R and Q. The GRC-RNA also showed a strong association with the nuclear-enriched La/SSB antigen. The polypyrimidine-tract-binding protein PTB/hnRNP I (Fig. 5B, band c), which is known to specifically interact with RNA containing polypyrimidine rich regions, was enriched specifically in the (UUC)15-purified fraction, indicating the specificity of the RNA affinity-purification procedure. To validate the mass spectrometry results, we carried out immunoblot analysis of the affinity-purified samples using antibodies against the proteins that were identified by mass spectrometry. The immunoblot assays demonstrated the enrichment of hnRNP U/SAF-A, hnRNP R, Q, hnRNP A1, hnRNP C1, Nucleolin (C23) and La/SSB antigen in the (GAA)15 RNA oligo-purified fraction compared with (UUC)15 or scr-RNA oligo-purified fractions (Fig. 5C). Most of the GAA-repeat RNA-interacting proteins identified in the present study contain RNA-interacting domains and were previously shown to be integral components of the nuclear matrix (Nickerson, 2001).
Fig. 5.
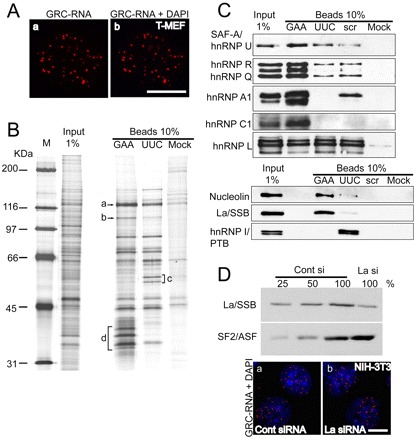
GRC-RNA is associated with the nuclear matrix. (A) GRC-RNA-FISH in nuclear matrix preparations from transformed MEFs revealed the presence of GRC-RNA nuclear foci (a,b). The chromatin was stained with DAPI (blue). The absence of DAPI staining in b is due to DNase I treatment, which degrades most of the nuclear DNA. (B) Isolation of the GRC-RNA binding protein complex. RNA affinity chromatography was performed as described in the Materials and Methods, using affinity matrices containing different RNA sequences [(GAA)15, (UUC)15 and scrambled (scr) RNA oligonucleotides]. For identification, specific bands that showed enrichment were excised, digested with trypsin and subjected to mass spectrometry. a-d represent some of the bands that were excised and analyzed by mass spectrometry (a: hnRNP U; b: nucleolin; c: hnRNP I; d: hnRNP A1, A2/B1, hnRNP C1). (C) Western blot analysis of GAA- and UUC-RNA affinity-purified samples using antibodies against proteins that were identified by mass spectrometry. (D) Depletion of La/SSB in NIH-3T3 cells does not influence the nuclear distribution of GRC-RNA. Immunoblot analysis using La-antibody in cell extracts revealed >75% depletion of La protein in La-siRNA-treated cell extracts. GRC-RNA FISH (red) in control (a) and La/SSB-siRNA-treated (b) NIH-3T3 cells showed similar distributions of GRC-RNA. The chromatin is stained with DAPI. Scale bars: 10 μm.
The interaction between GAA-repeat RNA and La/SSB is of particular interest owing to the suggested role of La in the nuclear retention of cellular and viral RNAs (Brenet et al., 2005; Fok et al., 2006; Intine et al., 2002). In order to understand the role of La-protein in the nuclear retention of GRC-RNA, we examined the distribution of GRC-RNA in La-depleted cells. RNA-FISH against GRC-RNA revealed comparable levels of nuclear GRC-RNA in the control and La-siRNA-treated cells, indicating that La/SSB is not involved in the nuclear distribution of GRC-RNA (Fig. 5D).
GRC-RNA nuclear foci number is altered during cell differentiation and proliferation
To determine whether the distribution of GRC-RNA foci are sensitive to changes in gene expression, RNA-FISH was conducted in NIH-3T3 cells that were serum-starved for 3 days. Serum deprivation of cells is known to alter the expression of several genes that are involved in cell proliferation and differentiation (Mandl et al., 2007; Muise-Helmericks et al., 1998). Serum starvation typically causes cell cycle arrest (G0 arrest or quiescence) eventually leading to senescence, apoptosis or cellular differentiation (Alexandre et al., 2000; Cooper, 2003; Simm et al., 1997). Interestingly, upon serum starvation, the number of GRC-RNA foci within the nuclei was dramatically decreased (5–8 instead of 50–60) but the existing nuclear foci became larger and more prominent (Fig. 6A). We have also observed a concomitant increase in the homogenous pool of GRC-RNA in the serum-starved cell nuclei (compare Fig. 6Ab with Aa).
Fig. 6.
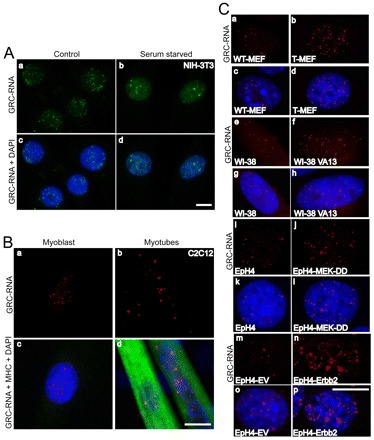
GRC-RNA nuclear foci number is altered upon serum starvation and during cellular transformation. (A) GRC-RNA-FISH (green) in untreated (a,c) and serum-starved (3 days: b,d) NIH-3T3 cells reveal a reduced number of GRC-RNA foci in serum-deprived cells. Scale bar: 10 μm. (B) GRC-RNA-FISH in myoblasts (a,c) and myotubes (b,d; differentiated by incubating the myoblasts in serum-free media) revealed a reduced number, but prominent, GRC-RNA foci in myotubes. Note that the myogenesis marker, myosin heavy chain (MHC) specifically stains differentiated myotubes (d) and not proliferating myoblasts (c). Chromatin was stained with DAPI. Scale bar: 10 μm. (C) GRC-RNA-FISH (red) in WT-MEF (a,c), transformed MEF (b,d), WI38 (e,g), WI38 VA13 (f,h), WT-EpH4 (i,k), empty vector (EV)-transfected EpH4 (m,o), transformed constitutively active MEKDD (j-l) or Erbb2 (n,p) EpH4 cell lines revealed increased numbers of GRC-RNA foci in the transformed highly proliferating cell lines (SV-40 T-antigen-transformed MEF, WI38 VA13 human fibroblast, EpH4-MEKDD and EpH4-Erbb2) compared with their wild-type counterparts (WT-MEF, WI38 primary fibroblast and EpH4 and EpH4-EV mammary cells). DNA was counterstained with DAPI. Scale bar: 10 μm.
Similarly, upon serum starvation, C2C12 murine myoblast cells exit the cell cycle; such a cell cycle arrest is essential for their differentiation into multinucleated myotubes (Yaffe and Saxel, 1977). C2C12 cells are studied extensively to understand the molecular events associated with skeletal myogenesis (Yaffe and Saxel, 1977). RNA-FISH using a (TTC)15 probe coupled with immunofluorescence staining against a myogenesis marker, myosin heavy chain (MHC; expressed specifically in myotubes and in myoblasts that are cell-cycle-arrested and are committed towards forming myotubes), revealed that the GRC-RNA formed fewer but larger and brighter foci in the MHC-expressing cell-cycle-arrested differentiating myoblasts and multinucleated myotubes compared with proliferating myoblasts (Fig. 6B). The reduced number of GRC-RNA foci in serum-starved non-dividing NIH-3T3 fibroblasts and C2C12 myotubes suggests that the specific distribution of GRC-RNA in the form of intranuclear foci might be linked to cell proliferation status. To test this hypothesis, we have compared the GRC-RNA foci distribution in various diploid and transformed cell line pairs. In WT-MEFs, GRC-RNA formed ~10 foci per nucleus (Fig. 6Ca,c). However, SV40 T-antigen-transformed MEFs contained 60–80 GRC-RNA foci (Fig. 6Cb,d). Similarly, the transformed human fibroblast WI38 VA13 cells showed an increased number of GRC-RNA foci (~60) relative to its primary wild-type counterpart (WT-WI38; 10–20 foci/nucleus; Fig. 6Ce–h). We have analyzed the GRC-RNA foci status in non-tumorigenic [WT-EpH4 and EpH4-EV (empty vector)] versus tumorigenic mouse mammary epithelial cell line pairs (due to a stable overexpression of the constitutively active form of the MEK, MEK-DD, EpH4-MekDD or Erbb2 oncogene or EpH4-Erbb2) (Pinkas and Leder, 2002). Consistent with the results in mouse and human fibroblasts, the tumorigenic MEK or Erbb2-transformed EpH4 cells displayed greater numbers of GRC-RNA foci than their wild-type counterparts (Fig. 6Ci–p).
Discussion
Recent studies have indicated that a large number of lncRNAs present in eukaryotic cells play vital roles in regulating gene expression (Mercer et al., 2009; Wilusz et al., 2009). The increasing functional diversity among lncRNAs observed in the mammalian genome suggests that they contribute to the complex networks needed to regulate cell function and could be the ultimate answer to the ‘genome paradox’ (Mercer et al., 2009; Wilusz et al., 2009).
We have identified GRC-RNA, a GAA-repeat-rich lncRNA that is localized at numerous puncate nuclear foci in both primary and transformed human and mouse cell lines. GRC-RNAs consist of a heterogeneous population of nuclear RNA. It remains to be demonstrated whether these transcripts are produced by a single gene or are encoded by multiple genes in the genome. Genomic analyses have identified several genomic regions, both in mouse and human, that contain long polypurine-rich sequence elements (including GAA-repeat regions) preferentially located at the promoter, 5′ end or 3′ end of protein-coding genes (Goni et al., 2004; Goni et al., 2006; Wu et al., 2007). Interestingly, several of these GAA-repeat regions in the mouse genome display higher histone-3 lysine-36 methylation modifications (H3K36 methylation), a histone mark that is associated with transcriptionally active regions (data not shown). The colocalization of several of the GRC-RNA foci with GAA.TTC-DNA-repeat elements supports the notion that multiple transcribing regions could be responsible for the increased number of GRC-RNA nuclear foci.
Our studies have revealed that GRC-RNA is part of the nuclear scaffold and interacts with several nuclear-matrix-associated proteins. NrRNAs are known to be an integral part of the nuclear matrix (examples include Xist RNA and Gomafu RNA) and are suggested to be involved in maintaining the higher-order chromatin architecture in cells (Clemson et al., 1996; Nickerson, 2001; Nickerson et al., 1995; Rodriguez-Campos and Azorin, 2007; Sone et al., 2007). The components that provide structural integrity to the nuclear matrix remain enigmatic. Evidence indicates that the nuclear matrix comprises a highly ordered network of ribonucleoprotein (RNP) complexes, and in vitro studies have indicated that proteins like hnRNP A2 and B1 play crucial roles in the assembly of ordered filament-like nuclear structures (Nickerson, 2001; Tan et al., 2000). Because GRC-RNA interacts with hnRNP A2 and B1 and other nuclear matrix proteins, it is tempting to speculate a possible structural role for GRC-RNA in the formation of the core filament structure underlying nuclear matrix organization. HnRNP U/SAF-A, another GRC-RNA-interacting protein, is a bona fide component of the nuclear matrix and is also the only nuclear matrix protein shown to specifically interact with scaffold-attachment-regions-DNA (SAR-DNA) (Fackelmayer et al., 1994; Mattern et al., 1996). SAF-A is a multifunctional protein that binds to both SAR-DNA and RNA and is suggested to regulate gene expression (Eggert et al., 2001; Eggert et al., 1997; Kim and Nikodem, 1999). A previous study indicated the role for SAF-A as a plausible candidate for an anchor point of the Xist nrRNA to inactive X-chromosomes (Helbig and Fackelmayer, 2003). SAF-A interaction with GRC-RNA could be crucial for the proper organization of GRC-RNA into subnuclear foci.
At present, we do not know the functional significance of GRC-RNA nuclear foci. The insensitivity of GRC-RNA nuclear foci to RNase A treatment indicates that GRC-RNAs contain long stretches of polypurine ribonucleotides (GAA-repeats). To our knowledge, our results on GRC-RNA are the first report showing the existence of long GAA-repeat-rich RNA in normal cell lines. In vitro studies have indicated that long polypurine RNAs, especially GAA-repeat-containing RNAs, can partially hybridize with their template DNA strand (dTTC) to form RNA:DNA hybrids (Grabczyk et al., 2007; Grabczyk and Usdin, 2000). Such hybrids are favored when the non-template strand (dGAA) is engaged as the third strand to form a triple-helical structure (GAA-GAA-TTC) by hybridizing with a DNA duplex via non-Watson-Crick hydrogen bonds (Hoogsteen base pairing and reverse Hoogsteen base pairing) (Grabczyk et al., 2007; Grabczyk and Usdin, 2000). Furthermore, it has been demonstrated that RNA:DNA hybrids and triple-helix DNA structure formed on such templates arrest RNA polymerses, resulting in transcription inhibition (Grabczyk et al., 2007; Grabczyk and Usdin, 2000; Ohshima et al., 1998; Wells, 2008). In situations described above, GAA-repeat RNA coexists with the triplex DNA structure. Abnormal amplification of GAA-tandem repeats in the transcribing region of the frataxin (FXN) gene resulting in its transcriptional silencing owing to the formation of an RNA:DNA hybrid and triple-helix DNA structure has been implicated in case of Friedreich's ataxia (Bidichandani et al., 1998; Grabczyk et al., 2007; Grabczyk and Usdin, 2000; Ohshima et al., 1998; Sakamoto et al., 2001). Previous studies by Ohno et al. demonstrated the association between GAA.TTC foci and triple-helix structures (Ohno et al., 2002). However, this study claimed that the GAA.TTC nuclear foci primarily consisted of GAA-GAA-TTC triple-helix DNA and the single-stranded template (TTC)n DNA. By contrast, we have provided several lines of evidence confirming the existence of polypurine-repeat-rich GRC-RNA in these novel subnuclear domains. These include: (1) sensitivity of GRC-RNA to RNase I, a single-strand-specific ribonuclease that cleaves phosphodiester bonds at the 3′ end of all the ribonucleotides, and to Riboshredder, a cocktail of multiple ribonucleases; (2) absence of GRC-RNA nuclear foci in cells that are transfected with antisense DNA oligonucleotides complementary only to GAA-repeat (GAA-antisense), but not TTC-repeat, sequences (GAA-sense); and (3) differentiation- or proliferation-specific changes in the distribution of GRC-RNA foci in cell lines. Further, insensitivity of GRC-RNA foci to DNase I treatment ruled out the role of DNA in the formation of these nuclear foci. Taken together, we demonstrate the presence of GRC-RNAs in these subnuclear foci that could coexist with triple-helix DNA structure in these domains. Based on our data, we speculate that the GRC-RNA transcribed from several of the GAA.TTC-repeat elements tend to associate with those genomic regions and facilitate the formation or stabilization of triple-helical structures, exerting a regulatory effect on transcription of those genes that are located in close proximity to the repeat elements. In such a scenario, the GRC-RNAs might behave as riboregulators and modulate the expression of several genes in a developmental stage- or cell-cycle-dependent manner. Its upregulation in cancerous cells and redistribution after serum starvation, as well as during myogenesis, suggest physiological and developmental relevance. Future studies on homopurine-rich RNAs like GRC-RNA will provide insights into the role of such unique structures in regulating gene expression.
Materials and Methods
Cell culture and treatment
NIH-3T3, WT-MEFs, U2OS, HeLa, C2C12, WT and mutant EpH4, WI38 and WI38-VA13 cells were grown in Dulbecco's Modified Eagle Medium (DMEM) containing high glucose (Invitrogen, Carlsbad, CA) supplemented with penicillin-streptomycin and 10% fetal bovine serum or calf serum (NIH-3T3; Hyclone, Logan, UT). For the serum-starvation experiment, cells were cultured in DMEM supplemented with 0.1% calf serum for 3 days. Cells were seeded onto acid-washed coverslips for immunofluorescence or RNA-FISH experiments (Prasanth et al., 2005). For transient transfection experiments, plasmid DNA (2 μg) was transfected into cells using lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
To inhibit RNA polymerase II transcription, cells were incubated with α-amanitin (50 μg/ml, Sigma-Aldrich, St Louis, MO), DRB (32 μg/ml, Sigma-Aldrich) or actinomycin D (5 μg/ml) for indicated time-points. Nascent transcription sites were detected using Br-UTP incorporation assays using a previously published protocol (Prasanth et al., 2003).
Nuclease treatment
Cells were fixed in 2–4% formaldehyde for 15 minutes at room temperature, permeabilized using 0.5% Triton X-100 for 10 minutes at 4°C and were treated with different nucleases for 1 hour in a moist chamber at 37°C: RNase A (1 mg/ml in CSK buffer, Sigma-Aldrich); RNase I (200 U/ml in 1× RNase I reaction buffer, Promega, Madison, WI); Riboshredder (50 U/ml, Epicenter Biotechnologies, Madison, WI); RNase H (100 U/ml, Epicenter Biotechnologies); RNase V1 (Epicenter Biotechnologies); RNase III (100 U/ml, Epicenter Biotechnologies); and DNase I (200 U/ml, Roche, Madison, WI). After treatment, cells were washed with PBS and processed for RNA-FISH.
Cellular fractionation and northern blot
Nuclear and cytoplasmic fractionation and RNA isolation from cell lines and northern blot hybridization using random-labeled α-32P dCTP-labeled probes were performed according to previously published procedures (Prasanth et al., 2005; Topisirovic et al., 2003). Northern hybridization using γ-32P ATP-labeled DNA oligonucleotide probes (TTC)15 was performed as per the manufacturer's instructions (Ambion, Austin, TX).
Nuclear matrix preparation
Cells were extracted according to the protocol described previously with modifications (Sone et al., 2007). Cells grown on poly-L-lysine-coated coverslips were permeabilized in cytoskeleton (CSK) buffer (100 mM NaCl, 300 mM sucrose, 10 mM PIPES, pH 6.8, 3 mM MgCl2) with 0.5% Triton X-100 on ice for 10 minutes and then extracted in extraction buffer (250 mM ammonium sulfate, 300 mM sucrose, 10 mM PIPES, pH 6.8, 3 mM MgCl2, 0.5% Triton X-100) on ice for 5 minutes. After two washes in digestion buffer (50 mM NaCl, 300 mM sucrose, 10 mM PIPES, pH 6.8, 3 mM MgCl2), cells were treated with 500 U/ml DNase I (Roche) for 1 hour at 37°C. Cells were then fixed in 4% formaldehyde at room temperature for 15 minutes and were processed for RNA-FISH.
RNA-FISH, dual RNA-DNA-FISH and immunofluorescence microscopy
For RNA-FISH, cells were either fixed in 4% formaldehyde in PBS (pH 7.4) for 15 minutes at room temperature and then permeabilized with 0.5% Triton X-100 in PBS on ice for 10 minutes, or pre-extracted in CSK buffer plus 0.5% Triton X-100 for 5 minutes on ice and then fixed using 4% formaldehyde. Cells were washed three times in PBS and rinsed once in 2× SSC prior to hybridization. Hybridization was carried out in a moist chamber at 37°C for 12–16 hours as previously described (Prasanth et al., 2005). Texas Red or FITC-labeled oligonucleotide probes [(TTC)15, (GAA)15, (CCT)15 and (CT)20] were synthesized by Sigma-Genosys, St Louis, MO and nick-translated probe was made as per the manufacturer's instructions (Abbott Laboratories, Abbott Park, IL).
Dual RNA-DNA-FISH was performed as previously described (Xing et al., 1995). In brief, RNA-FISH using digoxigenin (DIG)-labeled (TTC)15 (Sigma Genosys) probe was performed in CSK plus 0.5% Triton X-100 pre-extracted NIH-3T3 cells and the DIG label was detected using anti-DIG-rhodamine antibody (1:200; Roche). Cells were refixed in 4% formaldehyde, DNA was denatured using heat denaturation (4 minutes at 72–75°C in 70% formamide plus 2× SSC) and hybridized using FITC-labeled (GAA)15 oligonucleotide probe.
For RNA-FISH coupled with immunofluorescence staining, cells were fixed and permeabilized as described above, and then blocked in PBS containing 1% RNase-free fetal bovine serum (Invitrogen). Cells were incubated with primary antibodies for 1 hour at room temperature in a moist chamber: anti-centromere antibody (AnaC; human IgG, 1:10, Sigma-Aldrich); anti-coilin (rabbit IgG, 1:100, Santa Cruz Biotechnology, CA), anti-SF2/ASF mAb103 (mouse IgG, 1:100, a kind gift from Dr A. Krainer, CSHL, NY); and anti-BrdU [mouse IgG, 1:100, Sigma-Aldrich]. Cells were then washed in PBS containing 1% RNase-free fetal bovine serum, and secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were added for 45 minutes at room temperature: goat anti-mouse (GAM) IgG Texas Red (1:1000); GAM-FITC (1:500); donkey anti-rabbit IgG Texas Red (1:1000); DAR-FITC (1:500); and goat anti-human IgG-FITC (1:500). After secondary antibodies, cells were washed three times in PBS, fixed for 5 minutes in 4% formaldehyde and processed for RNA-FISH.
Cells were examined using an AxioImager.Z1 fluorescence microscope (Carl Zeiss Inc., Thornwood, NY) equipped with Chroma filters (Chroma Technology, Bellows Falls, VT) using a 63×/1.4 objective (PlanApochromat, Carl Zeiss, Inc.). Images were acquired by a Hamamatsu ORCA-ER camera and processed using AxioVision Rel 4.7 software (Carl Zeiss, Inc.). Alternatively, cells were imaged using a Deltavision microscope (Applied Precision, Issaquah, WA) with a 60×/1.42 objective (PlanApochromat, Olympus, Canter Valley, PA) and images were deconvolved using SoftWorx software.
Antisense oligonucleotide and siRNA treatment
Sense and antisense DNA oligonucleotides targeting GAA-repeats (GAA-antisense oligo, TTCTTCTTCTTCTTCTTCTTC; GAA-sense oligo, GAAGAAGAAGAAGAAGAAGAA) were modified with six 2′-o-methoxyethyl nucleotides on the 5′ and 3′ ends and nine consecutive oligodeoxynucleotides to support RNase H activity and were used to deplete mouse GRC-RNA (ISIS Pharmaceuticals, Carlsbad, CA). The oligonucleotides were modified with phosphorothioate internucleosidic linkages for maximum stability. The oligonucleotides (final concentration: 100 nM) were transfected to cells using Lipofectamine RNAiMAX Reagent as per the manufacturer's instructions (Invitrogen). Depletion of mouse La protein was performed by using pooled double-stranded siRNAs (sc-40916; Santa Cruz Biotechnology, CA) as previously described (Prasanth et al., 2004).
RNA affinity chromatography
RNA oligonucleotides [(GAA)15, (UUC)15 and scrambled (scr; GAUGACCGUUCAGCAACCUUCUACGCAUACGCCAUCUCGACGGGAUCCAGACGGA) Dharmacon Inc., Lafayette, CO] were covalently coupled to agarose beads (Sigma-Aldrich) using a method previously described (Caputi et al., 1999). To each aliquot of resin, 250 μl of NIH-3T3 nuclear extract was loaded in a total volume of 600 μl and was incubated at 30°C for 1 hour. Unbound proteins were removed by washing with 1 ml Dignam buffer D (20 mM HEPES, pH 7.9, 20% glycerol, 100 mM KCl, 0.2 mM EDTA, 1 mM DTT, 0.5 mM PMSF) (Dignam et al., 1983) containing 0.2 M NaCl and 0.1 mg/ml yeast tRNA at 4°C followed by two washes with buffer D containing 0.04 mg/ml yeast tRNA, and two washes with buffer D alone. Specifically-bound proteins were released by boiling in Laemmli buffer for 5 minutes and were resolved by SDS-PAGE and silver staining (SilverQuest Kit, Invitrogen). Specific protein bands were excised from the silver-stained gels and were used for mass spectrometric analysis.
Western blot analysis
Protein samples were resolved by 10% SDS-PAGE, transferred onto nitrocellulose membrane and used for western blot (WB) analysis. The primary antibodies included: anti-mouse hnRNP U/SAF-A antibody (3G6; 1:2000) (Kiledjian and Dreyfuss, 1992); anti-mouse hnRNP A1 (4B10; 1:5000; Dr G. Dreyfuss, University of Pennsylvania, PA); anti-mouse hnRNP C1/C2 (4F4; 1:1000) (Choi and Dreyfuss, 1984); anti-mouse hnRNP Q & R (18E4; 1:2000; Sigma-Aldrich); anti-mouse hnRNP L (4D11; 1:1000; Sigma-Aldrich); anti-rabbit nucleolin (C23; 1:10; Novus Biologicals, Littleton, CO); anti-rabbit La (1:1000) (Park et al., 2006) and anti-rabbit PTB (1:20; Dr S. Huang, Northwestern University, Chicago, IL). HRP-labeled secondary antibodies (Jackson ImmunoResearch) followed by ECL assays (Pierce Inc., Rockford, IL) were used for detecting the specific signal.
Supplementary Material
Acknowledgements
We thank Dr P. M. Yau for mass spectrometry analysis. We thank Drs M. Bellini, G. Carmichael, J. Chen, Z. Deng, G. Dreyfuss, M. Hastings, S. Huang, A. Krainer, P. Lieberman, R. Maraia, S. Nakagawa and Z. Zhang for sharing reagents, protocols and for helpful discussions. We thank Drs S. Ceman, J. Chen, P. Leder, C. Mizzen and Y. Ge for providing cell lines. We thank A. Zhanar for the technical help. We also thank Drs P. Bubulya, A. Lal, P. Newmark and Prasanth laboratory members for helpful discussions and for critically reading the manuscript. This work is supported by grants UIUC-ICR-MCB to K.V.P. and S.G.P. and NSF0843604 to S.G.P.
Footnotes
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.070466/-/DC1
References
- Alexandre S., Rast C., Nguyen-Ba G., Vasseur P. (2000). Detection of apoptosis induced by topoisomerase inhibitors and serum deprivation in syrian hamster embryo cells. Exp. Cell Res. 255, 30-39. [DOI] [PubMed] [Google Scholar]
- Amaral P. P., Dinger M. E., Mercer T. R., Mattick J. S. (2008). The eukaryotic genome as an RNA machine. Science 319, 1787-1789. [DOI] [PubMed] [Google Scholar]
- Bidichandani S. I., Ashizawa T., Patel P. I. (1998). The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am. J. Hum. Genet. 62, 111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braidotti G., Baubec T., Pauler F., Seidl C., Smrzka O., Stricker S., Yotova I., Barlow D. P. (2004). The Air noncoding RNA: an imprinted cis-silencing transcript. Cold Spring Harbor Symp. Quant. Biol. 69, 55-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenet F., Dussault N., Borch J., Ferracci G., Delfino C., Roepstorff P., Miquelis R., Ouafik L. (2005). Mammalian peptidylglycine alpha-amidating monooxygenase mRNA expression can be modulated by the La autoantigen. Mol. Cell. Biol. 25, 7505-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G. F., McCabe V. M., Norris D. P., Cooper P. J., Swift S., Rastan S. (1992). The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell 71, 515-526. [DOI] [PubMed] [Google Scholar]
- Caputi M., Mayeda A., Krainer A. R., Zahler A. M. (1999). hnRNP A/B proteins are required for inhibition of HIV-1 pre-mRNA splicing. EMBO J. 18, 4060-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. L., Carmichael G. G. (2009). Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: functional role of a nuclear noncoding RNA. Mol. Cell 35, 467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C., Kappel C., Beaudouin J., Eils R., Spector D. L. (2008). Live cell dynamics of promyelocytic leukemia nuclear bodies upon entry into and exit from mitosis. Mol. Biol. Cell 19, 3147-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Kapranov P., Drenkow J., Dike S., Brubaker S., Patel S., Long J., Stern D., Tammana H., Helt G., et al. (2005). Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. Science 308, 1149-1154. [DOI] [PubMed] [Google Scholar]
- Choi Y. D., Dreyfuss G. (1984). Isolation of the heterogeneous nuclear RNA-ribonucleoprotein complex (hnRNP): a unique supramolecular assembly. Proc. Natl. Acad. Sci. USA 81, 7471-7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. C., Ciaudo C., Fazzari M. J., Mise N., Servant N., Glass J. L., Attreed M., Avner P., Wutz A., Barillot E., et al. (2010). LINE-1 activity in facultative heterochromatin formation during X chromosome inactivation. Cell 141, 956-969. [DOI] [PubMed] [Google Scholar]
- Chueh A. C., Northrop E. L., Brettingham-Moore K. H., Choo K. H., Wong L. H. (2009). LINE retrotransposon RNA is an essential structural and functional epigenetic component of a core neocentromeric chromatin. PLoS Genet. 5, e1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. M., Bhaskar S. S., Miyahara M., Dalgliesh G. L., Bidichandani S. I. (2006). Expansion of GAA trinucleotide repeats in mammals. Genomics 87, 57-67. [DOI] [PubMed] [Google Scholar]
- Clemson C. M., McNeil J. A., Willard H. F., Lawrence J. B. (1996). XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J. Cell Biol. 132, 259-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemson C. M., Hutchinson J. N., Sara S. A., Ensminger A. W., Fox A. H., Chess A., Lawrence J. B. (2009). An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol. Cell 33, 717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. (2003). Reappraisal of serum starvation, the restriction point, G0, and G1 phase arrest points. FASEB J. 17, 333-340. [DOI] [PubMed] [Google Scholar]
- D'Alessio G., Riordan J. F. (1997). Ribonucleases: Structures and Functions. San Diego: Academic Press. [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. (1983). Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11, 1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinger M. E., Pang K. C., Mercer T. R., Crowe M. L., Grimmond S. M., Mattick J. S. (2009). NRED: a database of long noncoding RNA expression. Nucleic Acids Res. 37, D122-D126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert H., Schulz M., Fackelmayer F. O., Renkawitz R., Eggert M. (2001). Effects of the heterogeneous nuclear ribonucleoprotein U (hnRNP U/SAF-A) on glucocorticoid-dependent transcription in vivo. J. Steroid Biochem. Mol. Biol. 78, 59-65. [DOI] [PubMed] [Google Scholar]
- Eggert M., Michel J., Schneider S., Bornfleth H., Baniahmad A., Fackelmayer F. O., Schmidt S., Renkawitz R. (1997). The glucocorticoid receptor is associated with the RNA-binding nuclear matrix protein hnRNP U. J. Biol. Chem. 272, 28471-28478. [DOI] [PubMed] [Google Scholar]
- Eggert U. S., Mitchison T. J., Field C. M. (2006). Animal cytokinesis: from parts list to mechanisms. Annu. Rev. Biochem. 75, 543-566. [DOI] [PubMed] [Google Scholar]
- Fackelmayer F. O., Dahm K., Renz A., Ramsperger U., Richter A. (1994). Nucleic-acid-binding properties of hnRNP-U/SAF-A, a nuclear-matrix protein which binds DNA and RNA in vivo and in vitro. Eur. J. Biochem. 221, 749-757. [DOI] [PubMed] [Google Scholar]
- Fok V., Friend K., Steitz J. A. (2006). Epstein-Barr virus noncoding RNAs are confined to the nucleus, whereas their partner, the human La protein, undergoes nucleocytoplasmic shuttling. J. Cell Biol. 173, 319-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni J. R., de la Cruz X., Orozco M. (2004). Triplex-forming oligonucleotide target sequences in the human genome. Nucleic Acids Res. 32, 354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goni J. R., Vaquerizas J. M., Dopazo J., Orozco M. (2006). Exploring the reasons for the large density of triplex-forming oligonucleotide target sequences in the human regulatory regions. BMC Genomics 7, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabczyk E., Usdin K. (2000). The GAA*TTC triplet repeat expanded in Friedreich's ataxia impedes transcription elongation by T7 RNA polymerase in a length and supercoil dependent manner. Nucleic Acids Res. 28, 2815-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabczyk E., Mancuso M., Sammarco M. C. (2007). A persistent RNA.DNA hybrid formed by transcription of the Friedreich ataxia triplet repeat in live bacteria, and by T7 RNAP in vitro. Nucleic Acids Res. 35, 5351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal S. I., Elgin S. C. (2007). Transcription and RNA interference in the formation of heterochromatin. Nature 447, 399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Amit I., Garber M., French C., Lin M. F., Feldser D., Huarte M., Zuk O., Carey B. W., Cassady J. P., et al. (2009). Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458, 223-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig R., Fackelmayer F. O. (2003). Scaffold attachment factor A (SAF-A) is concentrated in inactive X chromosome territories through its RGG domain. Chromosoma 112, 173-182. [DOI] [PubMed] [Google Scholar]
- Intine R. V., Dundr M., Misteli T., Maraia R. J. (2002). Aberrant nuclear trafficking of La protein leads to disordered processing of associated precursor tRNAs. Mol. Cell 9, 1113-1123. [DOI] [PubMed] [Google Scholar]
- Khalil A. M., Guttman M., Huarte M., Garber M., Raj A., Rivea Morales D., Thomas K., Presser A., Bernstein B. E., van Oudenaarden A., et al. (2009). Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. USA 106, 11667-11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiledjian M., Dreyfuss G. (1992). Primary structure and binding activity of the hnRNP U protein: binding RNA through RGG box. EMBO J. 11, 2655-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. K., Nikodem V. M. (1999). hnRNP U inhibits carboxy-terminal domain phosphorylation by TFIIH and represses RNA polymerase II elongation. Mol. Cell. Biol. 19, 6833-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuyer E., Yoshida H., Parthasarathy N., Alm C., Babak T., Cerovina T., Hughes T. R., Tomancak P., Krause H. M. (2007). Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell 131, 174-187. [DOI] [PubMed] [Google Scholar]
- Lee J. T., Davidow L. S., Warshawsky D. (1999). Tsix, a gene antisense to Xist at the X-inactivation centre. Nat. Genet. 21, 400-404. [DOI] [PubMed] [Google Scholar]
- Lein E. S., Hawrylycz M. J., Ao N., Ayres M., Bensinger A., Bernard A., Boe A. F., Boguski M. S., Brockway K. S., Byrnes E. J., et al. (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168-176. [DOI] [PubMed] [Google Scholar]
- Leung A. K., Gerlich D., Miller G., Lyon C., Lam Y. W., Lleres D., Daigle N., Zomerdijk J., Ellenberg J., Lamond A. I. (2004). Quantitative kinetic analysis of nucleolar breakdown and reassembly during mitosis in live human cells. J. Cell Biol. 166, 787-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison C., Bailly D., Peters A. H., Quivy J. P., Roche D., Taddei A., Lachner M., Jenuwein T., Almouzni G. (2002). Higher-order structure in pericentric heterochromatin involves a distinct pattern of histone modification and an RNA component. Nat. Genet. 30, 329-334. [DOI] [PubMed] [Google Scholar]
- Mandl A., Sarkes D., Carricaburu V., Jung V., Rameh L. (2007). Serum withdrawal-induced accumulation of phosphoinositide 3-kinase lipids in differentiating 3T3-L6 myoblasts: distinct roles for Ship2 and PTEN. Mol. Cell. Biol. 27, 8098-8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern K. A., Humbel B. M., Muijsers A. O., de Jong L., van Driel R. (1996). hnRNP proteins and B23 are the major proteins of the internal nuclear matrix of HeLa S3 cells. J. Cell Biochem. 62, 275-289. [DOI] [PubMed] [Google Scholar]
- Mattick J. S. (2005). The functional genomics of noncoding RNA. Science 309, 1527-1528. [DOI] [PubMed] [Google Scholar]
- Mattick J. S. (2009). The genetic signatures of noncoding RNAs. PLoS Genet. 5, e1000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick J. S., Makunin I. V. (2006). Non-coding RNA. Hum. Mol. Genet. 15 Spec No 1, R17-R29. [DOI] [PubMed] [Google Scholar]
- Meador J., 3rd, Cannon B., Cannistraro V. J., Kennell D. (1990). Purification and characterization of Escherichia coli RNase I. Comparisons with RNase M. Eur. J. Biochem. 187, 549-553. [DOI] [PubMed] [Google Scholar]
- Mercer T. R., Dinger M. E., Sunkin S. M., Mehler M. F., Mattick J. S. (2008). Specific expression of long noncoding RNAs in the mouse brain. Proc. Natl. Acad. Sci. USA 105, 716-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer T. R., Dinger M. E., Mattick J. S. (2009). Long non-coding RNAs: insights into functions. Nat. Rev. Genet. 10, 155-159. [DOI] [PubMed] [Google Scholar]
- Muise-Helmericks R. C., Grimes H. L., Bellacosa A., Malstrom S. E., Tsichlis P. N., Rosen N. (1998). Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 273, 29864-29872. [DOI] [PubMed] [Google Scholar]
- Nickerson J. (2001). Experimental observations of a nuclear matrix. J. Cell Sci. 114, 463-474. [DOI] [PubMed] [Google Scholar]
- Nickerson J. A., Blencowe B. J., Penman S. (1995). The architectural organization of nuclear metabolism. Int. Rev. Cytol. 162A, 67-123. [DOI] [PubMed] [Google Scholar]
- Ohno M., Fukagawa T., Lee J. S., Ikemura T. (2002). Triplex-forming DNAs in the human interphase nucleus visualized in situ by polypurine/polypyrimidine DNA probes and antitriplex antibodies. Chromosoma 111, 201-213. [DOI] [PubMed] [Google Scholar]
- Ohshima K., Montermini L., Wells R. D., Pandolfo M. (1998). Inhibitory effects of expanded GAA.TTC triplet repeats from intron I of the Friedreich ataxia gene on transcription and replication in vivo. J. Biol. Chem. 273, 14588-14595. [DOI] [PubMed] [Google Scholar]
- Pandey R. R., Mondal T., Mohammad F., Enroth S., Redrup L., Komorowski J., Nagano T., Mancini-Dinardo D., Kanduri C. (2008). Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol. Cell 32, 232-246. [DOI] [PubMed] [Google Scholar]
- Park J. M., Kohn M. J., Bruinsma M. W., Vech C., Intine R. V., Fuhrmann S., Grinberg A., Mukherjee I., Love P. E., Ko M. S., et al. (2006). The multifunctional RNA-binding protein La is required for mouse development and for the establishment of embryonic stem cells. Mol. Cell. Biol. 26, 1445-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T. (2009). The discovery of eukaryotic genome design and its forgotten corollary-the postulate of gene regulation by nuclear RNA. FASEB J. 23, 2019-2021. [DOI] [PubMed] [Google Scholar]
- Pinkas J., Leder P. (2002). MEK1 signaling mediates transformation and metastasis of EpH4 mammary epithelial cells independent of an epithelial to mesenchymal transition. Cancer Res. 62, 4781-4790. [PubMed] [Google Scholar]
- Prasanth K. V., Spector D. L. (2007). Eukaryotic regulatory RNAs: an answer to the ‘genome complexity’ conundrum. Genes Dev. 21, 11-42. [DOI] [PubMed] [Google Scholar]
- Prasanth K. V., Sacco-Bubulya P. A., Prasanth S. G., Spector D. L. (2003). Sequential entry of components of the gene expression machinery into daughter nuclei. Mol. Biol. Cell 14, 1043-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanth K. V., Prasanth S. G., Xuan Z., Hearn S., Freier S. M., Bennett C. F., Zhang M. Q., Spector D. L. (2005). Regulating gene expression through RNA nuclear retention. Cell 123, 249-263. [DOI] [PubMed] [Google Scholar]
- Prasanth S. G., Prasanth K. V., Siddiqui K., Spector D. L., Stillman B. (2004). Human Orc2 localizes to centrosomes, centromeres and heterochromatin during chromosome inheritance. EMBO J. 23, 2651-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Campos A., Azorin F. (2007). RNA is an integral component of chromatin that contributes to its structural organization. PLoS ONE 2, e1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N., Ohshima K., Montermini L., Pandolfo M., Wells R. D. (2001). Sticky DNA, a self-associated complex formed at long GAA*TTC repeats in intron 1 of the frataxin gene, inhibits transcription. J. Biol. Chem. 276, 27171-27177. [DOI] [PubMed] [Google Scholar]
- Sasaki Y. T., Ideue T., Sano M., Mituyama T., Hirose T. (2009). MENepsilon/beta noncoding RNAs are essential for structural integrity of nuclear paraspeckles. Proc. Natl. Acad. Sci. USA 106, 2525-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simm A., Bertsch G., Frank H., Zimmermann U., Hoppe J. (1997). Cell death of AKR-2B fibroblasts after serum removal: a process between apoptosis and necrosis. J. Cell Sci. 110, 819-828. [DOI] [PubMed] [Google Scholar]
- Sone M., Hayashi T., Tarui H., Agata K., Takeichi M., Nakagawa S. (2007). The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. J. Cell Sci. 120, 2498-2506. [DOI] [PubMed] [Google Scholar]
- Struhl K. (2007). Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat. Struct. Mol. Biol. 14, 103-105. [DOI] [PubMed] [Google Scholar]
- Sunwoo H., Dinger M. E., Wilusz J. E., Amaral P. P., Mattick J. S., Spector D. L. (2009). MEN varepsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 19, 347-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. H., Wooley J. C., LeStourgeon W. M. (2000). Nuclear matrix-like filaments and fibrogranular complexes form through the rearrangement of specific nuclear ribonucleoproteins. Mol. Biol. Cell 11, 1547-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topisirovic I., Culjkovic B., Cohen N., Perez J. M., Skrabanek L., Borden K. L. (2003). The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. EMBO J. 22, 689-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bakel H., Nislow C., Blencowe B. J., Hughes T. R. (2010). Most “dark matter” transcripts are associated with known genes. PLoS Biol. 8, e1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D. (2008). DNA triplexes and Friedreich ataxia. FASEB J. 22, 1625-1634. [DOI] [PubMed] [Google Scholar]
- Wilusz J. E., Sunwoo H., Spector D. L. (2009). Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 23, 1494-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. H., Brettingham-Moore K. H., Chan L., Quach J. M., Anderson M. A., Northrop E. L., Hannan R., Saffery R., Shaw M. L., Williams E., et al. (2007). Centromere RNA is a key component for the assembly of nucleoproteins at the nucleolus and centromere. Genome Res. 17, 1146-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Gaddis S. S., MacLeod M. C., Walborg E. F., Thames H. D., DiGiovanni J., Vasquez K. M. (2007). High-affinity triplex-forming oligonucleotide target sequences in mammalian genomes. Mol. Carcinog. 46, 15-23. [DOI] [PubMed] [Google Scholar]
- Xing Y., Johnson C. V., Moen P. T., Jr, McNeil J. A., Lawrence J. (1995). Nonrandom gene organization: structural arrangements of specific pre-mRNA transcription and splicing with SC-35 domains. J. Cell Biol. 131, 1635-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. (1977). Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725-727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


