Abstract
This study assessed the effect of GLP-1 based therapies on atherosclerotic markers in type 2 diabetes patients. 31 studies were selected to obtain data after multiple database searches and following inclusion and exclusion criteria. Age and BMI of the participants of longitudinal studies were 59.8 ± 8.3 years and 29.2 ± 5.7 kg/m2 (Mean±SD). Average duration of GLP-1 based therapies was 20.5 weeks. Percent flow-mediated diameter (%FMD) did not change from baseline significantly but when compared to controls, %FMD increased non-significantly following GLP-1-based therapies (1.65 [−0.89, 4.18]; P = 0.2; REM) in longitudinal studies and increased significantly in cross sectional studies (2.58 [1.68, 3.53]; P < 0.00001). Intima media thickness decreased statistically non-significantly by the GLP-1 based therapies. GLP-1 based therapies led to statistically significant reductions in the serum levels of brain natriuretic peptide (−40.16 [−51.50, −28.81]; P < 0.0001; REM), high sensitivity c-reactive protein (−0.27 [−0.48, −0.07]; P = 0.009), plasminogen activator inhibitor-1 (−12.90 [−25.98, 0.18]; P=0.05), total cholesterol (−5.47 [−9.55, −1.39]; P = 0.009), LDL-cholesterol (−3.70 [−7.39, −0.00]; P = 0.05) and triglycerides (−16.44 [−25.64, −7.23]; P = 0.0005) when mean differences with 95% CI in the changes from baselines were meta-analyzed. In conclusion, GLP-1-based therapies appear to provide beneficial effects against atherosclerosis. More randomized data will be required to arrive at conclusive evidence.
Type 2 Diabetes Mellitus (T2D) is a progressive disease with gradually increasing prevalence. Currently, 340 million people are suffering globally and it is estimated that, by 2030, it would be the seventh leading cause of mortality1 Microvascular complications associated with diabetes leads to organ loss, and both stroke and heart disease related mortality is 2–4 times higher in diabetics than in normal individuals2.
Atherosclerosis is one of the major possible consequences of insulin resistance, obesity and T2D3. The vascular endothelial dysfunction is a critical step of atherogenesis which is usually characterized by endothelium-dependent vasodilatation and vasoconstriction imbalance and the disturbed balance of antithrombotic and prothrombotic factors4. At an earlier stage, apolipoprotein B starts retaining in the sub-endothelial spaces which activates endothelial cells to secrete chemokines which then recruit monocytes. Adhesion molecules of the endothelial cells make contacts with monocytes which later invade the vascular wall and differentiate into macrophages. The macrophages uptake lipids and lipoproteins and secrete cytokines and chemokines to further recruit CD4 T cells and smooth myocytes in the intima layer of vessels. This leads to an advance stage of atherogenesis in which plaques contain lipids, cholesterol, macrophages and altered myocytes5.
A category of gut peptide hormones, the incretins (glucagon-lie peptide 1; GLP-1 and glucose-dependent insulinotropic polypeptide; GIP), released during meals with increasing glucose levels in the blood are insulinotropic that are responsible for about three quarters of total insulin secretion6. In subjects with T2D, the GLP-1 secretion is impaired and its insulinotropic and glucagon suppressive actions are weakened. Therapeutic use of GLP-1 tends to normalize alpha and beta cell sensitivity to glucose leading to improved glycemic control and reduced diabetic toxicity7. Besides modulating a range of physiological effects such as blood glucose and metabolism regulation, the GLP-1 is also thought to prevent multiple steps of atherosclerosis4.
A number of studies have shown beneficial effects of GLP-1-based interventions on measures affecting cardiovascular pathologies such as blood pressure, body weight, and lipid metabolism that are thought to be exerted independent of GLP-1 analogue’s effects on glycemic control8,9 and the effects on markers of atherosclerosis are also reported10,11,12. However, data regarding the effects of GLP-1 based therapies on atherosclerosis generated in various studies is not previously reviewed systematically. Importantly, outcomes of various studies are not consistent which provides rationale for a meta-analysis of the relevant studies. The purpose of the present study is to meta-analyze data pertaining to regarding the effects of GLP-1 based therapies on markers of atherosclerosis observed in related clinical trials.
Method
Literature Search
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed in carrying out this study. The literature search was made in EBSCO, Embase, Medline/PubMed, Ovid, and Web of Science electronic databases. Important MeSH terms and keywords used were: glucagon-like peptide 1 (GLP-1), GLP-1 analogue, incretin, dipeptidyl peptidase 4 (DPP-4) inhibitor, atherosclerosis, arteriosclerosis, atherogenesis, plaque, intima media thickness (IMT), flow-mediated dilation (FMD), adiponectin, leptin, endothelin, E-selectin, resistin, pentraxin, brain natriuretic peptide (BNP), monocyte chemotactic protein (MCP), vascular cell adhesion molecule-1 (VCAM-1), plasminogen activator inhibitor-1 (PAI-1), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), high sensitivity C-reactive protein (hs-CRP), triglyceride and cholesterol. Relevant research articles published during 1990 and September 2014 were retrieved, and corroborations and cross references of important research papers were also searched.
Inclusion and exclusion criteria
Rationale for the inclusion of studies in the present meta-analytical review was to assess the effects of increased GLP-1 levels in blood on atheroscelortic markers in T2D patients. The inclusion criteria were: a) study investigated the effects of GLP-1-based therapies on atherosclerosis by examining the effects of either exogenous GLP-1, or GLP-1 analogue, or any other serum GLP-1 elevating agent; b) study utilized a longitudinal design and reported at least one marker of atherosclerosis with numerical values of baseline and endpoint samples or mentioned measured changes from baseline; c) study utilized a cross sectional design to study changes in % FMD following a GLP-1-based therapy. The exclusion criteria were: a) studies involving cell culture experiments to examine the effects of GLP-1-based therapies on atherosclerosis markers; and b) cross sectional studies examining the effects of GLP-1-based therapies on atherosclerosis markers other than % FMD; and c) studies with longitudinal designs providing either baseline or endpoint measures only or providing information inadequate for meta-analysis.
Primary and secondary endpoints
Primary endpoints of the present study were changes in % FMD, IMT, PAI-1, VCAM-1, MCP-1, BNP, hs-CRP, IL-6, TNF-α, endothelin, E-selectin, and resistin while the secondary endpoints were the changes in serum levels of adiponectin, leptin, total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglyceride.
Data extraction, synthesis and statistical analysis
Study screening and selection was carried out by two authors independently with high inter-rater reliability (kappa = 0.93). The data regarding the participants’ demographic, clinical and therapeutic characteristics, outcomes and other important parameters were extracted from tabular, textual and graphic sources of the published research papers independently by two researchers and were cross-checked later (kappa = 0.95). Dis-agreements were mutually discussed and resolved but when agreement could not be reached, a third researcher was involved.
For the assessment of the changes from baseline following GLP-1-based therapies in % FMD and IMT, mean differences with 95% confidence intervals (CI) were calculated between baseline samples and end-of-treatment samples for each study and then an overall effect size was generated. For controlled trials’ data, changes from the baseline were first measured and then mean differences [95% CI] between GLP-1-based therapy patients and controls were calculated for each study and then overall effect size of the meta-analysis was generated.
Meta-analyses were performed with RevMan (Version 5.2; Cochrane Collaboration) software by using means and standard deviation values of the selected markers of atherosclerosis of GLP-1 based therapy patients / controls. The overall effect of treatment was calculated as weighted average of the inverse variance adjusted individual effects. Significance of difference between GLP-1-based therapies and controls was tested with z test. Both fixed- (FEM) and random-effect (REM) models were utilized in the meta-analyses for outcome interpretation depending on between-study heterogeneity which was tested by I2 index. Sensitivity analyses were performed to explore the sources of higher statistical heterogeneity and funnel plots were examined for the assessment of publication bias.
Results
Thirty one studies13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43 were selected after following inclusion and exclusion criteria. Study screening and selection procedure is summarized in Fig. S1. Among the included studies, 24 utilized longitudinal designs (10 with placebo- and 10 comparator-controlled13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 and 4 were single armed)33,34,35,36. Seven controlled cross sectional studies examining the acute effects of GLP-1-based therapies on FMD were also included37,38,39,40,41,42,43. Average age and BMI of the participants of the longitudinal studies were 59.8 ± 8.3 years and 29.2 ± 5.7 kg/m2. The average duration of GLP-1 based therapies was 20.5 weeks (range 4–52).
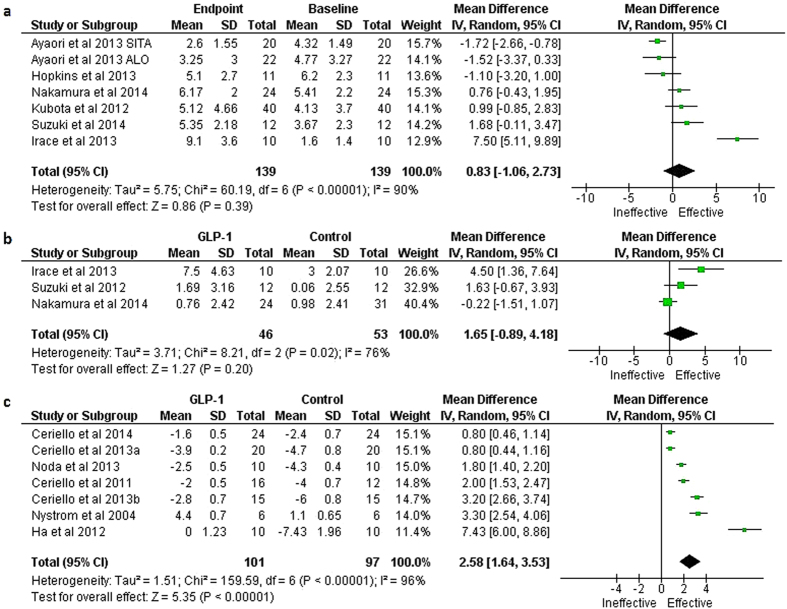
Major findings of the meta-analyses are presented in Table 1. A meta-analysis of 6 studies13,20,30,32,33,35 which attempted to estimate overall effect size of the changes from baseline in % FMD following GLP-1-based therapies could not find any significant difference between baseline and end-of-treatment samples (mean change from baseline was 0.83 [−1.06, 2.73] %; P = 0.39; REM; Fig. 1a). In the meta-analysis of 3 studies20,28,32 which compared a GLP-1-based therapy against a comparator, GLP-1-based therapies were found to increase % FMD non-significantly (mean difference [95% CI] in the changes from baseline: 1.65 [−0.89, 4.18] %; P = 0.2; REM; Fig. 1b). However, 7 cross sectional studies examining acute effects of the GLP-1-based therapies37,38,39,40,41,42,43 found a significant increase in % FMD following GLP-1-based interventions (mean difference in the changes from baseline: 2.58 [1.68, 3.53] %; P < 0.00001; REM; Fig. 1c).
Table 1. Summary of the met-analyses assessing the effects of GLP-1-based therapies on various markers of atherosclerosis.
| Parameter | No. of studies | No. of subjects | Mean difference [95% CI]; Significance level; Model | I2 | Remarks |
|---|---|---|---|---|---|
| FMD (%) | |||||
| Change from baseline | 6 | 278 | 0.83 [−1.06, 2.73]; P = 0.39; REM | 90% | GBT non-significantly increased % FMD |
| GBT vs control | 3 | 99 | 1.65 [−0.89, 4.18]; P = 0.2; REM | 76% | GBT non-significantly increased % FMD |
| Cross sectional studies | 7 | 198 | 2.58 [1.68, 3.53]; P < 0.00001; REM | 96% | GBT significantly increased % FMD |
| cIMT (mm) | |||||
| Change from baseline | 6 | 424 | −0.06 [−0.15, 0.03]; P = 0.18; REM | 61% | GBT non-significantly decreased cIMT |
| GBT vs control | 2 | 166 | −0.04 [−0.09, 0.01]; P = 0.1; FEM/REM | 0% | GBT non-significantly decreased cIMT |
| Adiponectin (% change) | 6 | 957 | −53.63 [−133.86, 26.61]; P = 0.19; REM | 99% | Indifferent |
| TNF-α (% change) | 2 | 236 | −5.21 [−17.68, 7.27]; P = 0.41; FEM/REM | 0% | Indifferent |
| IL-6 (% change) | 4 | 317 | −10.05 [−26.72, 6.63]; P = 0.24; REM | 41% | Indifferent |
| hs-CRP (mg/l) | 8 | 1066 | −0.27 [−0.48, −0.07]; P = 0.009; REM | 48% | GBT non-significantly decreased hs-CRP |
| PAI-1 (% change) | 7 | 1507 | −12.90 [−25.98, 0.18]; P = 0.05; REM | 86% | GBT significantly decreased PAI-1 |
| BNP (% change) | 6 | 1520 | −40.16 [−51.50, −28.81]; P<0.0001; REM | 54% | GBT significantly decreased BNP |
| MCP-1 (% change) | 2 | 99 | 0.01 [−0.33, 0.35]; P = 0.95; FEM | 0% | Indifferent |
| VCAM-1 (ng/ml) | 2 | 90 | 22.92 [−27.89, 73.74]; P = 0.38; FEM | 0% | Indifferent |
| Total cholesterol (mg/dl) | 7 | 1048 | −5.47 [−9.55, −1.39]; P = 0.009; FEM | 24% | GBT significantly decreased cholesterol |
| HDL-cholesterol (mg/dl) | 9 | 1092 | 0.18 [−1.53, 1.90]; P = 0.84; REM | 60% | Indifferent |
| LDL-cholesterol (mg/dl) | 7 | 1007 | −3.70 [−7.39, −0.00]; P = 0.05; REM | 65% | GBT significantly decreased LDL-chol. |
| Triglyceride (mg/dl) | 10 | 1122 | −16.44 [−25.64, −7.23]; P = 0.0005; REM | 46% | GBT significantly decreased LDL-chol. |
Abbreviations: cIMT, carotid intima media thickness; FEM, fixed effects model; FMD, flow-mediated diameter; GBT, GLP-1-based therapies; HDL, high-density lipoprotein; hs-CRP, high sensitivity C-reactive protein; IL-6, interleukin-6; LDL, low-density lipoprotein; MCP-1, monocyte chemotactic protein-1; PAI-1, plasminogen activator inhibitor-1; REM, random effects model ; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule-1.
Figure 1.
Forest plots showing the effects of GLP-1-based therapies on % FMD: a) meta-analysis of the changes from baseline following GLP-1 based therapies in longitudinal studies, b Mean difference between GLP-1 based therapies and controls in longitudinal studies, and c) mean difference between GLP-1 based therapy patients and controls in cross sectional studies examining acute effects.
Five studies14,21,33,35,36, were identified which evaluated the effect of GLP-1-based therapies on IMT using longitudinal designs. A meta-analysis of these studies revealed a statistically non-significant decrease in IMT with a mean change from baseline of −0.06 [−0.15, 0.03] mm; P = 0.18; REM (Fig. 2). Two studies14,21 were identified that evaluated the effect of GLP-1-based therapies in a controlled longitudinal design. A meta-analysis of these revealed a statistically non-significant reduction in IMT (Mean difference between GLP-1-based therapies and controls −0.04 [−0.09, 0.01]; P = 0.1; FEM/REM).
Figure 2.
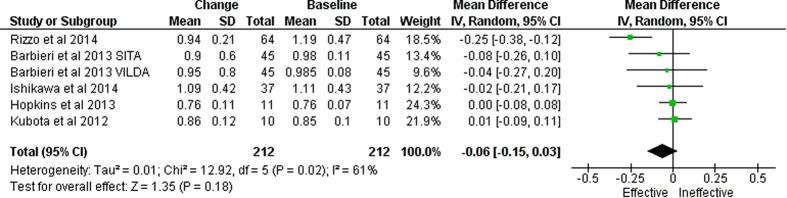
Forest plot showing meta-analysis of the changes in intima media from baseline in six studies which examined the effect of GLP-1 based therapies in longitudinal designs.
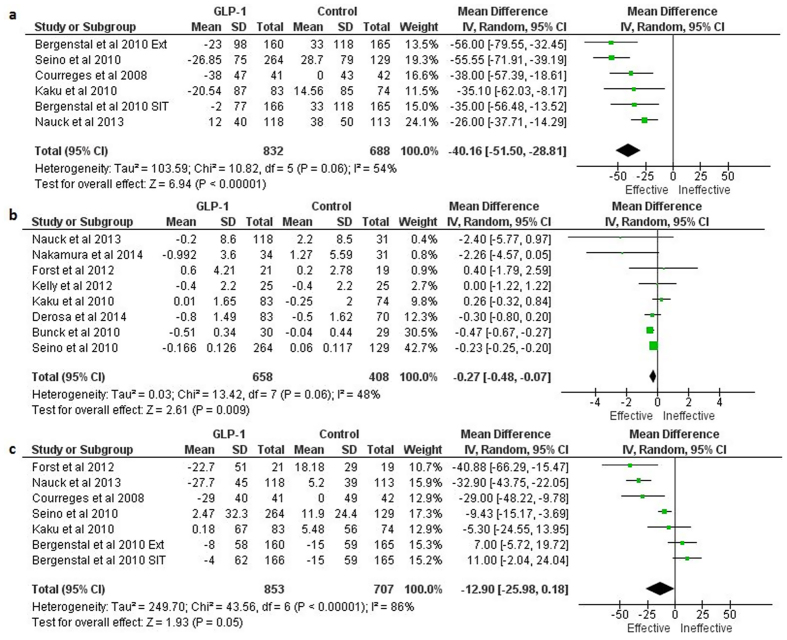
The GLP-1 based therapies led to statistically significant reductions in the serum levels of some atherosclerosis markers. Of these, the mean difference between GLP-1 based therapies and controls in the percent changes from baseline were: −40.16 [−51.50, −28.81] %; P < 0.0001; REM (for BNP), −0.27 [−0.48, −0.07] %; P = 0.009 (for hsCRP), and −12.90 [−25.98, 0.18] %; P = 0.05; REM (for PAI-1) (Figs. 3a–c).
Figure 3.
Forest plots showing the effects of GLP-1 based therapies on the percent changes from baseline of: a) Brain natriuretic peptide, b) high sensitivity c-reactive protein, and c) plasminogen activator inhibitor-1 levels. (In Bergenstal et al., 2010: Ext, exenatide; SIT, sitagliptin).
Decline in several atherosclerosis markers was not statistically significant after the GLP-1 based therapies. Mean differences between GLP-1-based therapy patients and controls in the changes from baseline were: IL-6 (−10.05 [−26.72, 6.63] %; P = 0.24; REM), TNF-α (−5.21 [−17.68, 7.27] %; P = 0.41; FEM), VCAM-1 (22.92 [−27.89, 73.74] ng/ml; P = 0.38; FEM) and MCP-1 (0.01 [−0.33, 0.35] μg/ml; P = 0.95; FEM). The GLP-1 based therapies were also not associated with a significant change in adiponectin levels in the blood with a mean difference in percent change from baseline between GLP-1 based therapies and controls of −53.63 [−133.86, 26.61] % (P = 0.19; REM).
The GLP-1 based therapies were also found to be associated with significant reductions in the serum levels of total cholesterol, LDL-cholesterol and triglycerides. Mean differences between GLP-1 based therapy and control patients in the changes from baseline were −5.47 [−9.55, −1.39] mg/dl (P = 0.009; REM) for total cholesterol, −3.70 [−7.39, −0.00] mg/dl (P = 0.05; REM) for LDL-cholesterol and −16.44 [−25.64, −7.23] mg/dl (P=0.0005; REM) for triglycerides (Figs S3a–c). No significant effect of GLP-1 based therapies could be noted on HDL-cholesterol (0.18 [−1.53, 1.90] mg/dl; P = 0.84; REM).
Atherosclerosis markers that were examined only in one study and therefore could not be meta-analyzed included resistin (−4.00 [−15.84, 7.84]; P = 0.51) ng/ml16, endothelin (0.11 [−0.03, 0.25]; P = 0.12) ng/ml16, pentraxin-3 (0.50 [−0.42, 1.41]; P = 0.29)28, c-peptide (−0.54 [−1.59, 0.52]; P = 0.32)28, and leptin (−1.67 [−2.64, −0.70]; P = 0.0007) μg/L16 (Mean differences [95% CI] in the changes from baseline between GLP-1-based therapy and control patients).
Discussion
The GLP-1-based therapies usually utilize two strategies: 1) inhibition of dipeptidyl peptidase 4 (DPP-4) enzyme with specific inhibitors, such as sitagliptin / vildgliptin, in order to raises plasma levels of endogenous GLP-1 and GIP, and 2) utilizing DPP-4 resistant GLP-1 receptor agonists, such as liraglutide / exenatide44. Both these strategies are found to be successful in overcoming deficiencies of endogenous GLP-1 or providing supra-physiological levels of GLP-1.
The present study provides preliminary evidence in favor of the GLP-1 based therapies in providing beneficial effects against atherosclerosis as indicated by the significant reductions in atherosclerosis markers including BNP, PAI-1, and hsCRP, besides statistically non-significant reductions in several other markers including IMT, FMD, IL-6, and TNF-α were also noted. Moreover, GLP-1-based therapies were also associated with significant reductions in total cholesterol, LDL-cholesterol and triglycerides.
The present study finds a significant acute effect of GLP-1-based therapies in increasing % FMD but in the long term this increase was not statistically significant. Previously, it has been found that the GLP-1 infusion in healthy subjects increases baseline and acetylcholine-induced vasodilatation45. Upon infusion, GLP-1 exerts potent vasodilatory effects on conduit artery and arterioles in healthy humans leading to increased blood flow for enhanced microvascular recruitment and muscular perfusion46. This shows that GLP-1-based therapies, besides providing glycemic control, also plays a significant role in the maintenance of endothelial function and vascular health. However, for long-term effects of the GLP-1-based therapies, more trials’ data will be required.
Intima media thickness is a well-recognized marker of sub-clinical atherosclerosis36. Increment in carotid IMT of more than 0.34 mm/year can lead to a cardiovascular event with first myocardial infarction and stroke can happen with a carotid IMT above 0.882 mm and 0.75 mm, respectively47. Whereas, some longitudinal studies have noticed a significant decrease in the IMT following a GLP-1-based therapy14,36, the present meta-analysis reveals a non-significant decrease in IMT, but this may be because of the less availability of data and paradigmatic and treatment spell deviations, thus necessitates the conduct of more trials with better designs.
Blood levels of CRP above 3 mg/L are suggested to be predictive of adverse cardiovascular outcome one year later48. It is thought that CRP has a causative role in endothelial cell activation and dysfunction, neo-intimal formation, monocyte and macrophage activity, and matrix metalloproteinase function49. The present study finds that GLP-1-based therapies significantly reduce CRP while statistically non-significantly reducing TNF-α and IL-6 levels. This is suggestive of the effects of GLP-1-based therapies on attenuating inflammatory processes that may cause atherosclerosis development.
The present study finds a significant reduction in PAI-1 with GLP-1 based therapies in diabetic patients. A critical feature of atherosclerosis is the increased production of adhesion molecules from the endothelium. Deposition of cholesterol in the intima of arteries induces endothelium to produce VCAM-150 which appears to be cytokine dependent as the presence of TNF-α in the smooth muscle cells is associated with an increase expression of VCAM-151. In human cell culture studies, increased expressions of PAI-1 and VCAM-1 have been reported to be associated with hyperglycemia-related endothelial cell dysfunction, a process that predisposes vasculature to accelerated atherogenesis52.
Among the other markers of atherosclerosis, BNP, which is secreted mainly by the ventricular myocardium53,54,55, has been found to decrease significantly by the GLP-1-based therapies but MCP-1, which is secreted by the peripheral blood mononuclear cells56, has been found un-affected by the GLP-1-based therapies, in the present study. However, the present study remains inconclusive with regards to the effects on TNF-α, VCAM-1, and MCP-1, because of the unavailability of adequate data.
Taken together, results of this meta-analysis are able to provide preliminary evidence in favor of GLP-1-based therapies in overcoming atherosclerosis development/progression which is also substantiated by the significant decreases in the total cholesterol, LDL-cholesterol and triglycerides observed in the studies which fulfilled inclusion criteria of this study.
Of the limitations of the present study, owing to the availability of fewer studies in some comparisons of individual parameters, it remains under-powered to generate conclusive evidence. Methodologically, among the included longitudinal studies, only 9 were double blind RCTs, 11 were open-label RCTs and 4 were non-randomized observational studies. This may also affects the level of evidence and thus the outcomes of this analytical review should be assigned as preliminary evidence only. Funnel plots also reflected a considerable publication bias (Fig. S2). In some comparisons, statistical heterogeneity was high; although, sensitivity analyses revealed only one comparison with truly higher I2. For this reason, random effects model has been used to interpret the results of the comparisons with high heterogeneity, yet, future randomized studies with better designs will be required to further assess these outcomes.
Conclusion
This study provides preliminary evidence in support of GLP-1 based therapies in providing beneficial effects against atherosclerosis as this therapeutic regimen was found to be associated with statistically significant reductions in the plasma levels of atherosclerosis markers including plasminogen activator inhibitor-1, high sensitivity c-reactive protein, and brain natriuretic peptide. Those makers which are found to be statistically non-significantly reduced by the GLP-1-based therapies include tumor necrosis factor-α and interlekin-6. This study further strengthens the existing evidence in favor of GLP-1 based therapies in decreasing total cholesterol, LDL-cholesterol and triglycerides. More randomized controlled and blinded trials will be required to arrive at conclusive evidence.
Additional Information
How to cite this article: Song, X. et al. Anti-atherosclerotic effects of the glucagon-like peptide-1 (GLP-1) based therapies in patients with type 2 Diabetes Mellitus: A meta-analysis. Sci. Rep. 5, 10202; doi: 10.1038/srep10202 (2015).
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China to YL (No. 30801201,81270424), from Beijing Nova Program of China to YL (No 2011116), and from China International Medical Fund Exchange- Diabetes Research Fund of Excellence in China to YL (No. 2012-3).
Footnotes
Author Contributions X.Y.S. participated in research design, data analysis and interpretation. Y.M.M. and Y.L. participated in the design of the study, study screening / selection, data extraction and data analysis. L.W., Y.Z., Y.B.J. and H.T.J carried out the write-up of the manuscript and participated in data analysis. All authors read and approved the final manuscript.
References
- Danaei G, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 378, 31–40 (2011). [DOI] [PubMed] [Google Scholar]
- Green J. & Feinglos M. New combination treatments in the management of diabetes: focus on sitagliptin-metformin. Vasc. Health. Risk. Manag. 4, 743–751 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger J. & Daniels S. R. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation. 107, 1448–53 (2003). [DOI] [PubMed] [Google Scholar]
- Forst T., Weber M. M. & Pfutzner A. Cardiovascular benefits of GLP-1-based therapies in patients with diabetes mellitus type 2: Effects on endothelial and vascular dysfunction beyond glycemic control. Exp. Diabetes. Res. 2012, 635472 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita T. & Watada H. Glucagon like peptide-1 and atherosclerosis. Cardiovas. Hematol. Agents. Medicinal. Chem. 10, 309–318 (2012). [DOI] [PubMed] [Google Scholar]
- Petrie J. R. The cardiovascular safety of incretin-based therapies: a review of the evidence. Cardiovasc. Diabetol. 12, 130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J. J., Vilsboll T. & Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol. Cell. Endocrinol. 297, 127–136 (2009). [DOI] [PubMed] [Google Scholar]
- Hattori S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr. J. 58, 69–73 (2011). [DOI] [PubMed] [Google Scholar]
- Satoh-Asahara N., et al. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism 62, 347–351 (2013). [DOI] [PubMed] [Google Scholar]
- Liu L., et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension 60, 833–841 (2012). [DOI] [PubMed] [Google Scholar]
- Fadini G.P., et al. Stem cell compartmentalization in diabetes and high cardiovascular risk reveals the role of DPP-4 in diabetic stem cell mobilopathy. Basic. Res. Cardiol. 108, 313 (2013). [DOI] [PubMed] [Google Scholar]
- Rieg T., et al. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am. J. Physiol. Renal. Physiol. 303, F963–F971 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaori M., et al. Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. J. Am. Heart. Assoc 2, e003277. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri M., et al. Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis. 227, 349–354 (2013). [DOI] [PubMed] [Google Scholar]
- Bergenstal R.M., et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 376, 431–9 (2010). [DOI] [PubMed] [Google Scholar]
- Bunck M.C., et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes. Care. 33, 1734–1737 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courreges J.P., et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with Type 2 diabetes. Diabet. Med. 25, 1129–1131 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derosa G., et al. Comparison of vildagliptin and glimepiride: effects on glycaemic control, fat tolerance and inflammatory markers in people with Type 2 diabetes. Diabet. Med. 31, 1515–23 (2014). [DOI] [PubMed] [Google Scholar]
- Forst T., et al. Addition of liraglutide in patients with Type 2 diabetes well controlled on metformin monotherapy improves several markers of vascular function. Diabet. Med. 29, 1115–1118 (2012). [DOI] [PubMed] [Google Scholar]
- Irace C., et al. Exenatide improves endothelial function assessed by flow mediated dilation technique in subjects with type 2 diabetes: results from an observational research. Diab. Vasc. Dis. Res. 10, 72–77 (2013). [DOI] [PubMed] [Google Scholar]
- Ishikawa S., et al. Impact of sitagliptin on carotid intima-media thickness in patients with coronary artery disease and impaired glucose tolerance or mild diabetes mellitus. Am. J. Cardiol. 114, 384–388 (2014). [DOI] [PubMed] [Google Scholar]
- Juntti-berggren L., et al. The Antidiabetogenic Effect of GLP-1 Is Maintained During a 7-Day Treatment Period and Improves Diabetic Dyslipoproteinemia in NIDDM Patients. Diadetes. Care. 19, 1200–1206 (1996). [DOI] [PubMed] [Google Scholar]
- Kaku K., Itayasu T., Hiroi S., Hirayama M. & Seino Y. Efficacy and safety of alogliptin added to pioglitazone in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label long-term extension study. Diabetes. Obes. Metab. 13, 1028–1035 (2011). [DOI] [PubMed] [Google Scholar]
- Kaku K., Rasmussen M. F., Clauson P. & Seino Y. Improved glycaemic control with minimal hypoglycaemia and no weight change with the once-daily human glucagon-like peptide-1 analogue liraglutide as add-on to sulphonylurea in Japanese patients with type 2 diabetes. Diabetes. Obes. Metab. 12, 341–347 (2010). [DOI] [PubMed] [Google Scholar]
- Kelly A. S., Bergenstal R. M., Gonzalez-Campoy J. M., Katz H. & Bank, A.J. Effects of exenatide vs. metformin on endothelial function in obese patients with pre-diabetes: a randomized trial. Cardiovasc. Diabetol. 11, 64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makdissi A., et al. Sitagliptin exerts an antinflammatory action. J. Clin. Endocrinol. Metab. 97, 3333–3341 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen N., et al. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia. 49, 2049–2057 (2006). [DOI] [PubMed] [Google Scholar]
- Nakamura K., et al. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc. Diabetol. 13, 110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandy D., et al. The effect of liraglutide on endothelial function in patients with type 2 diabetes. Diab. Vasc. Dis. Res. 11, 419–30 (2014). [DOI] [PubMed] [Google Scholar]
- Nauck M., et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes. Metab. 15, 204–212 (2013). [DOI] [PubMed] [Google Scholar]
- Seino Y., Rasmussen M.F., Nishida T. & Kaku K. Efficacy and safety of the once-daily human GLP-1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr. Med. Res. Opin. 26, 1013–1022 (2010). [DOI] [PubMed] [Google Scholar]
- Suzuki K., et al. Sitagliptin improves vascular endothelial function in Japanese type 2 diabetes patients without cardiovascular disease. J. Diabetes. Mellitus 2, 338–345 (2012). [Google Scholar]
- Hopkins N.D., et al. Effects of 6 months glucagon-like peptide-1 receptor agonist treatment on endothelial function in type 2 diabetes mellitus patients. Diabetes Obes. Metab. 15, 770–773 (2013). [DOI] [PubMed] [Google Scholar]
- Inoue K., et al. Long-term impact of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight and glycemic control in Japanese type 2 diabetes: an observational study. Diabetol. Metab. Syndr. 6, 95 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., et al. The Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Improves Vascular Endothelial Function in Type 2 Diabetes. J. Korean. Med. Sci. 27, 1364–1370 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo M., et al. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-month prospective pilot study. Cardiovasc. Diabetol. 13, 49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A., Esposito K., Testa R., Bonfigli A.R., Marra M., et al. The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an "endothelial resistance" to glucagon-like peptide 1 in diabetes. Diabetes Care 34, 697–702 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A., et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, antiinflammatory, and antioxidant action in type 2 diabetes. Diabetes Care 37, 1938–1943 (2014). [DOI] [PubMed] [Google Scholar]
- Ceriello A., et al. Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes. Care. 36, 4104–4108 (2013a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A., et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes. Care. 36, 2346–2350 (2013b). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S.J., et al. Preventive effects of exenatide on endothelial dysfunction induced by ischemia-reperfusion injury via KATP channels. Arterioscler. Thromb. Vasc. Biol. 32, 474–480 (2012). [DOI] [PubMed] [Google Scholar]
- Noda Y., et al. Alogliptin ameliorates postprandial lipemia and postprandial endothelial dysfunction in non-diabetic subjects: a preliminary report. Cardiovasc. Diabetol. 12, 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom T., et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Lancet. Diabetes. Endocrinol. 2, 691–700 (2014). [DOI] [PubMed] [Google Scholar]
- Sivertsen J., Rosenmeier J., Holst J. J. & Vilsboll T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat. Rev. Cardiol. 9, 209–222 (2012). [DOI] [PubMed] [Google Scholar]
- Basu A., Charkoudian N., Schrage W., Rizza R. A., Basu R.,& Joyner M. J. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am. J. Physiol. Endocrinol. Metab. 293, E1289–95 (2007). [DOI] [PubMed] [Google Scholar]
- Subaran S. C., et al. GLP-1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin. Sci. (Lond). 127, 163–70 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminbakhsh A.,& Mancini G. B. Carotid intima media thickness measurements: What defines an abnormality? A systematic review. Clin. Invest. Med. 22, 149–157 (1999). [PubMed] [Google Scholar]
- Cheng J. M., et al. Relation of c-reactive protein to coronary plaque characteristics on grayscale, radiofrequency intravascular ultrasound, and cardiovascular outcome in patients with acute coronary syndrome or stable angina pectoris (from the ATHEROREMO-IVUS Study). Am. J. Cardiol. 114, 1497–503 (2014). [DOI] [PubMed] [Google Scholar]
- Ridker P. M. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J. Am. Coll. Cardiol. 49, 2129–38 (2007). [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A. Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 251, 788–791(1991). [DOI] [PubMed] [Google Scholar]
- Couffinhal T., Duplaa C., Moreau C., Lamaziere J. M. & Bonnet J. Regulation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in human vascular smooth muscle cells. Circ. Res. 74, 225–234 (1994). [DOI] [PubMed] [Google Scholar]
- Liu H., Dear A. E., Knudsen L. B. & Simpson R. W. A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J. Endocrinol. 201, 59–66 (2009). [DOI] [PubMed] [Google Scholar]
- Ashley K.E., Galla J.M. & Nicholls S.J. Brain natriuretic peptides as biomarkers for atherosclerosis. Prev. Cardiol. 11, 172–176 (2008). [DOI] [PubMed] [Google Scholar]
- Svensson P., de Faire U., Niklasson U., Hansson L.O. & Ostergren J. Plasma NT-pro-BNP concentration is related to ambulatory pulse pressure in peripheral arterial disease. Blood. Press. 14, 99–106 (2005). [DOI] [PubMed] [Google Scholar]
- Jin Q., et al. Levels of brain natriuretic peptide are associated with peripheral arterial disease in subjects with type-2 diabetes mellitus. BMC Endocrine Disorders 14, 27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha M., et al. Selectins and monocyte chemotactic peptide as the markers of atherosclerosis activity. Physiol. Res. 53, 273–278 (2004). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.