Abstract
Background and Aims:
Patients with diabetes mellitus frequently experience erectile dysfunction. This systematic review and meta-analysis were conducted to find efficacy and tolerability of phosphodiesterase 5 (PDE5) inhibitors in patients with diabetes mellitus experiencing erectile dysfunction.
Methodology:
Electronic searches were carried out to identify English language peer-reviewed randomized controlled trials (RCTs), which reported clinical efficacy of any PDE5 inhibitor in patients with diabetes mellitus having erectile dysfunction. Effect sizes were computed using Cohen's d, and I2-test was used to assess heterogeneity. Pooled mean effect sizes were computed using random-effects model. Number needed to treat (NNT), and the adverse event rates were computed.
Results:
The systematic review included a total of 17 studies yielding 25 comparisons. Three studies were open RCTs while others were double-blind RCTs. The pooled mean effect size of any PDE5 inhibitor over placebo was 0.926 (95% confidence intervals [CI]: 0.864-0.987; I2 =26.3). The pooled mean effect size for sildenafil was 1.198 (CI: 1.039-1.357; I2 =0), for tadalafil was 0.910 (CI: 0.838-0.981; I2 =33.6), and for vardenafil was 0.678 (CI: 0.627-0.729; I2 =0). In pooled analysis, the NNT for sildenafil, tadalafil, vardenafil and any PDE5 inhibitor was 2.4, 2.6, 4.1 and 3.0 respectively. The most common side effects were headache, flushing, and nasal congestion.
Conclusions:
PDE5 inhibitors are effective and safe medications for the treatment of sexual dysfunction in patients with diabetes mellitus experiencing erectile dysfunction.
Keywords: Diabetes mellitus, erectile dysfunction, phosphodiesterase 5 inhibitor, sildenafil, tadalafil, vardenafil
INTRODUCTION
Diabetes mellitus is a common medical disorder characterized by impaired glucose metabolism and is associated with multiple physical complications and poor quality of life.[1,2,3] Both type 1 and type 2 diabetes mellitus have been associated with erectile dysfunction. Prevalence of erectile dysfunction in patients with diabetes mellitus has been reported in range from 35% to 90%.[4,5,6,7] The severity of erectile dysfunction has been noted to be variable in this population.[5] Presence of sexual dysfunction has been associated with factors like greater age, longer duration of diabetes, poor glycemic control, presence of hypertension, hyperlipidemia, smoking and sedentary lifestyles.[8] Erectile dysfunction in patients with diabetes mellitus is associated with poor quality of life and can lead to marital dissatisfaction.[5,9]
Various treatment modalities have been utilized for the treatment of sexual dysfunction in patients with diabetes mellitus including phosphodiesterase 5 (PDE5) inhibitors, prostaglandins, testosterone, pentoxifylline, and others.[10,11] Among the different medications, PDE5 inhibitors have been the mainstay of treatment due to the simplicity of dosing and wider patient acceptability.[12,13,14] The various PDE5 inhibitors that have been evaluated in clinical trials in this population have included sildenafil, tadalafil, vardenafil, udenafil, mirodenafil and avanafil.[14]
With the evolving evidence about interventions on erectile dysfunction and newer drugs coming into the market, it is useful to conduct quantitative comparative assessment of efficacy across studies. As of now, there is one published meta-analysis that has assessed the efficacy of PDE5 inhibitors in patients with diabetes mellitus.[15] However that meta-analysis had been conducted about 8 years ago, and many newer PDE5 inhibitors have been evaluated in systematic trials ever since, requiring inculcating of these studies into a meta-analytic review. Moreover, the previous study was not able to comment on publication bias due to the lower number of studies. Hence, this meta-analysis was conducted to assess the efficacy and safety of PDE5 inhibitors in patients with diabetes mellitus experiencing erectile dysfunction.
METHODOLOGY
Search strategy
Electronic searches were carried out using Pubmed and PsycInfo databases, supplemented by Google Scholar search. The search was carried out in January 2015. The search process was carried out by combining term “diabetes mellitus” with names of specific PDE5 inhibitors (avanafil, lodenafil, mirodenafil, sildenafil, tadalafil, udenafil or vardenafil). Additional published material was identified from the bibliography of the studies screened and evaluated. Library-based hand searches were not carried out as a part of this review process, and unpublished dissertations were not included. The study relied on material published in peer-reviewed journals and authors were not contacted for raw data or other unpublished material.
Study selection
For the purposes of the present systematic review, English language peer-reviewed studies were included which had explicitly compared the efficacy of at least one PDE5 inhibitor in a randomized controlled trial (RCT) for treatment of erectile dysfunction in patients with diabetes mellitus. Studies were included only if the study had reported the efficacy measure using a standardized quantitative measure. Nonrandomized studies were not included in this review. Pharmacological or nonpharmacological treatment options apart from PDE5 inhibitors for the treatment of erectile dysfunction (like prostaglandins, losartan, vacuum-assisted devices, and others) were not included in this review.
Data extraction
Information was extracted using a structured proforma from the studies that met the above-mentioned inclusion and exclusion criteria. Data were extracted pertaining to the region where the study was conducted, methodological characteristics, treatment arms, duration of the study, sample size in each group, method of assessment of outcome, and the outcome in each of the group. Adverse event rate in each of the study arms was also recorded. The information was extracted by two of the investigators using predefined criteria (SS and RG).
Risk of bias
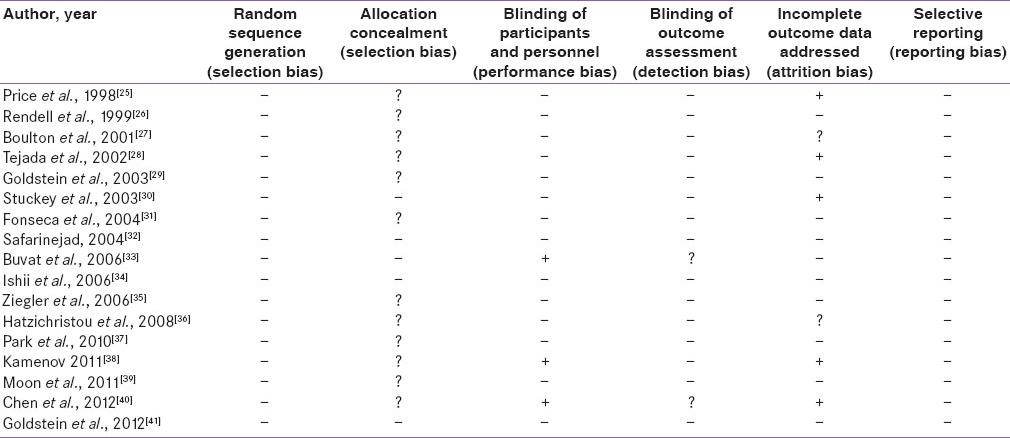
Risk of bias was evaluated as per the suggestions of Higgins and Green.[16] The quality of study was determined using included information about random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), addressing incomplete outcome data (attrition bias) and selective reporting (reporting bias). The risk of bias was categorized into present, unclear and absent.
Unit of assessment issues
Randomized controlled trials evaluating the efficacy of medications for the treatment of erectile dysfunction in patients with diabetes mellitus were expected to use several outcome measures. The commonly used structured instruments that are typically used in such studies include International Index of Erectile Function (IIEF)[17] and sexual encounter profile (SEP). Additionally, many of the studies report an overall assessment in terms of improvement perceived by the patient, in the form of global assessment questionnaire (GAQ). In this meta-analysis, wherever possible global assessment based dichotomous outcomes were utilized for computation of effect sizes as they reflected patient-centered inclusive evaluation of efficacy of the treatment agent. When global assessment based outcomes were not available, SEP 2 (percentage of sexual attempts in which the men were able to insert the penis into the partner's vagina) was utilized for computation of effect sizes.
Adverse events among the patients receiving the active drug and placebo were extracted from the included studies. The rate of adverse events experienced in the active drug was compared to that of the placebo, and an adverse event ratio was computed. Individual types of adverse events were not computed separately due to heterogeneity in assessment and reporting, especially as some studies mentioned only those adverse events that affected more than a certain proportion of the study population.
Statistical analysis
For each of the comparisons of PDE5 inhibitor with placebo or another PDE5 inhibitor, effect sizes were calculated as standardized mean differences (d).[18] This measure is useful for computation of effect sizes for continuous as well as dichotomous data. The logit method was used for computation of effect size and confidence intervals (CIs) for dichotomous data. Using the standardized mean difference, the effect size value of zero corresponds to no effect of treatment, and CIs spanning zero suggest possibility of the treatment arm having no effect.
Pooled mean effect sizes were computed for PDE5 inhibitors that were evaluated in more than one study, and then for PDE5 inhibitor as a group. Random effects model was utilized for computation of the pooled mean effect sizes. The advantage of random effects model over fixed effects model includes greater precision of the results but at the cost of wider CIs of the results.[19,20] With expected heterogeneity of the studies and pooling of results from different types of PDE5 inhibitors, random effects model was deemed to be more appropriate. I2-test was used to assess for heterogeneity between the studies. The I2 values range between 0 and 100 with greater values suggesting higher degree of heterogeneity.[21,22]
For placebo controlled studies, number needed to treat (NNT) was computed to estimate the number of patients needed to be treated for one to be benefitted. NNT was calculated for PDE5 inhibitors as a group and for specific PDE5 inhibitors that were evaluated in more than one study. NNT was computed as the inverse of absolute risk reduction, using the formula: ![]() Where PA represents proportion of patients on active drug showing improvement, and PB represents proportion of patients on placebo showing improvement.
Where PA represents proportion of patients on active drug showing improvement, and PB represents proportion of patients on placebo showing improvement.
Assessment of publication bias was conducted using Egger's test.[23,24] The Egger's test is mathematically represented as a regression equation of: Standard normal deviate (SND) = a + b × precision. Precision was computed as 1/(standard error), and SND was computed as effect size/standard error.
If smaller studies show effects that differ systematically from larger studies, the regression line will not run through the origin (a = 0), suggesting a possible publication bias.
RESULTS
Study selection and characteristics
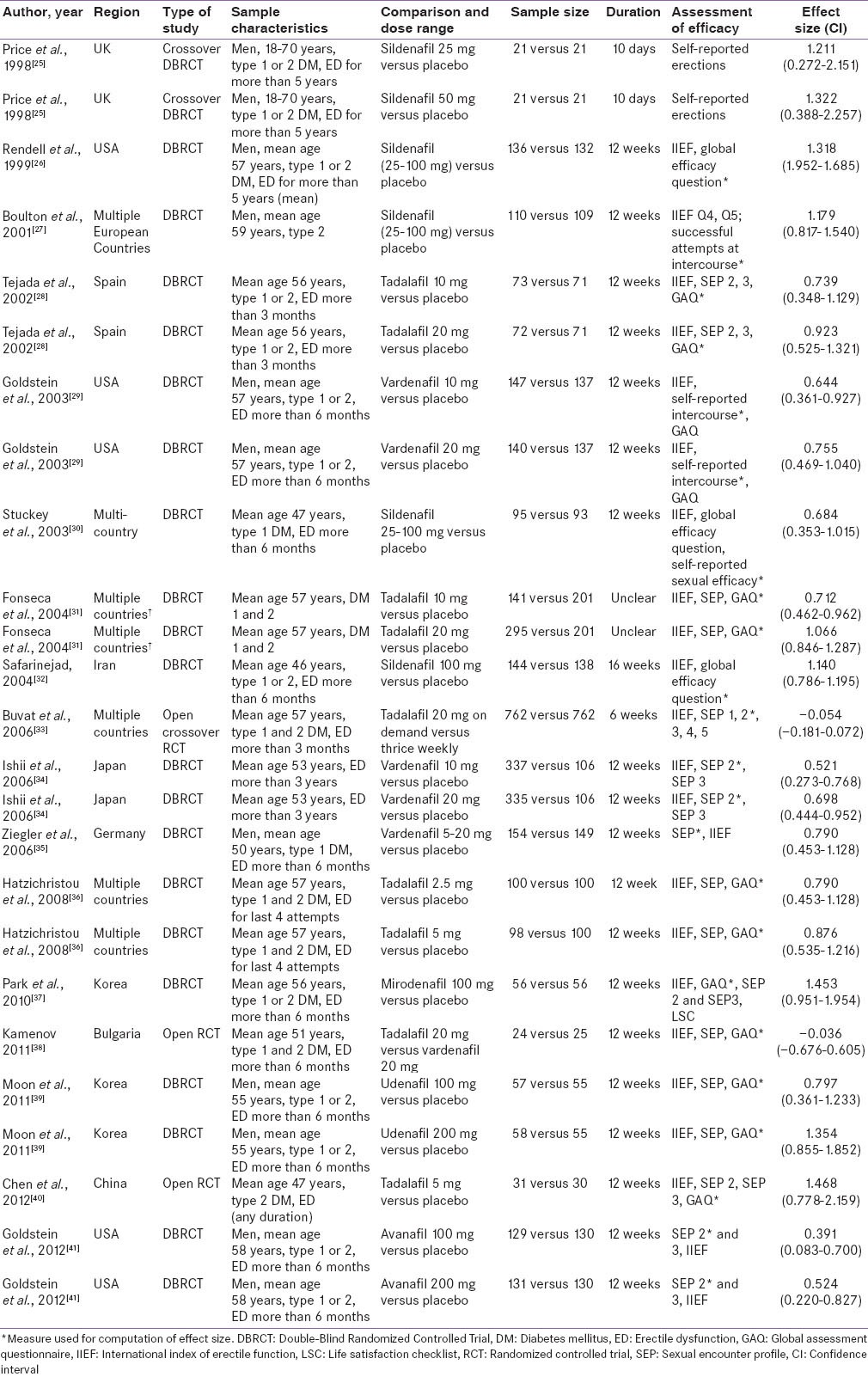
The selection of studies is depicted in Figure 1. Out of the 329 studies screened, 17 met the inclusion criteria and were included in the present systematic review and meta-analysis.[25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] There are enumerated in Table 1. The sample size in individual studies varied from as 21[25] to 762.[33] Three studies were open randomized trials[33,38,40] while the others had double blind randomized controlled design. Two of the studies had cross-over design.[25,33] The most common PDE5 inhibitors in descending order of frequency were tadalafil (6 studies),[28,31,33,36,38,40] sildenafil (4 studies)[25,27,30,32] and vardenafil (4 studies).[29,34,35,38] Avanafil, mirodenafil, and udenafil were evaluated in one study each. All the studies were placebo controlled except two: One compared on demand dosing of tadalafil to thrice weekly dosing,[9] while the other compared tadalafil to vardenafil.[38] The duration of study period ranged from 10 days[25] to 16 weeks,[32] though most of the studies were of 12 week duration. The common instruments used for assessment of sexual functioning were IIEF, SEP and GAQ.
Figure 1.
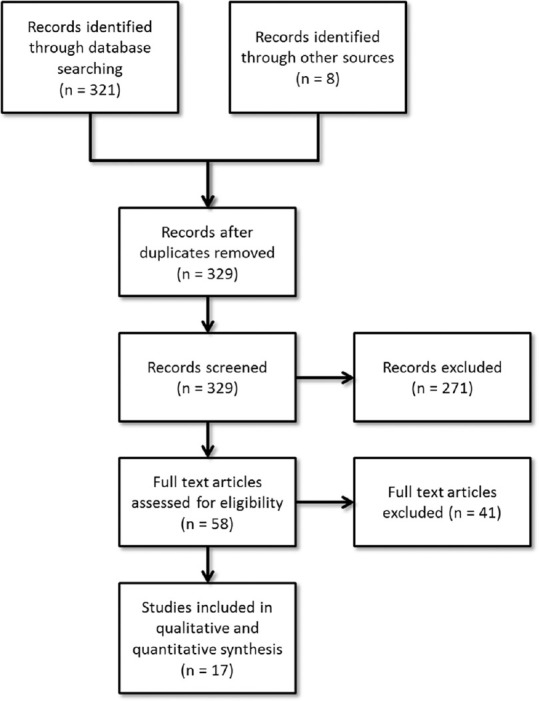
Selection of studies
Table 1.
Characteristics of included studies (n=17)
Efficacy measures
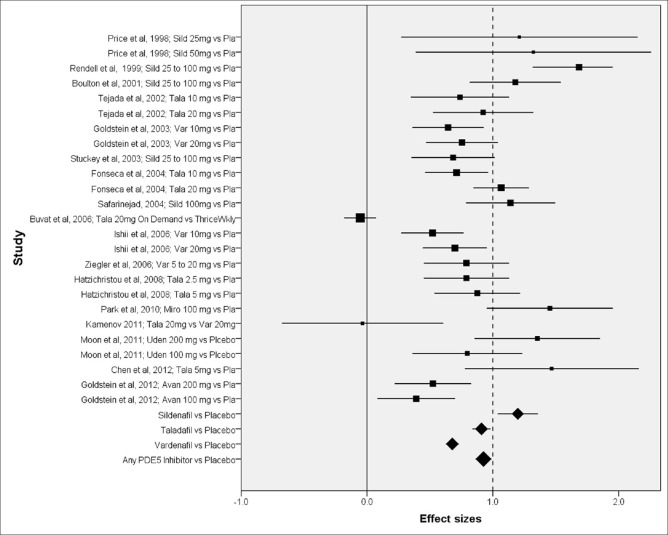
The effect sizes and CIs of the individual comparisons of PDE5 inhibitors are highlighted in Table 1 and graphically shown in Figure 2 (Forest plot). Among the placebo controlled studies, the effect sizes varied from 1.468 for tadalafil[40] to 0.391 for avanafil.[41] As is evident from the Forest plot, comparisons with lower sample size had higher CIs. For the same study utilizing two doses of the same drug, the higher dose medication seemed to have greater efficacy but not significantly so as indicated by overlapping CIs.
Figure 2.
Forest plot of included studies. Shown as effect size and confidence intervals; studies identified as author; year and comparison; box size in proportion to the study sample size; avan avanafil, miro mirodenafil, pla placebo, sild sildenafil, tala tadalafil, uden udenafil, var vardenafil
Meta-analyses were conducted to find the efficacy of different PDE5 inhibitors and PDE5 inhibitors as a group. The pooled mean effect size for sildenafil against placebo was 1.198 (n = 1041; 95% CI 1.039–1.357). Similarly, pooled mean effect size for tadalafil was 0.910 (n = 1584; CI 0.838–0.981), and for vardenafil was 0.678 (n = 1748; CI 0.627–0.729). The pooled mean effect size for PDE5 inhibitors as a group was 0.926 (n = 5230; CI 0.864–0.987). The I2 statistic for comparison of sildenafil, tadalafil, valrdenafil and PDE5 inhibitors as a group using random effects model was 0, 33.6, 0 and 26.3 respectively. The corresponding values using fixed effects model were in the range of 85-95 suggesting extremely high heterogeneity, again justifying random effects model.
Quality of the studies
The risk of bias assessment of the studies is depicted in Table 2. All the studies were RCTs though allocation concealment was not mentioned in many of the published papers. Three of the studies were not blinded while others were blinded. Blinding of the outcome assessment was assumed to be present in double-blind RCTs when not specified. Several of the studies had conducted intention-to-treat analysis while others had not. Since the present meta-analysis included published articles, selective reporting was largely absent as those outcome measures were mentioned in the methodology that was further elaborated in results.
Table 2.
Risk of bias in included studies (n=17)
Number needed to treat
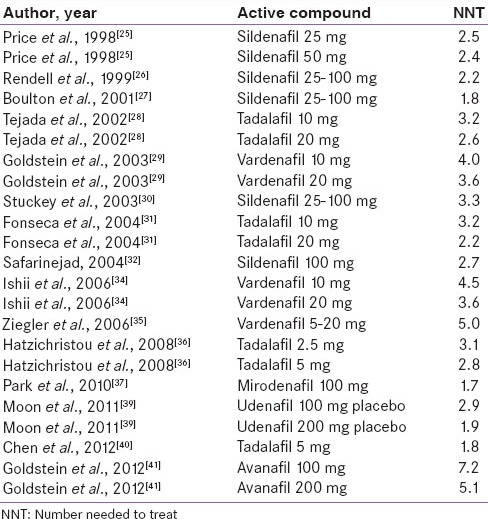
The NNTs from the different comparisons of PDE5 inhibitors are shown in Table 3. Lower NNTs represent a greater degree of efficacy. Lowest NNT was observed for mirodenafil (NNT of 1.7), and the highest for Avanafil 100 mg (NNT of 7.2). In pooled analysis, the NNT for sildenafil was 2.4, for tadalafil was 2.6, for vardenafil was 4.1, and for all PDE5 inhibitors combined was 3.0. To put it simply, when compared to placebo, it would require three individuals with diabetes mellitus and sexual dysfunction to be treated with a PDE5 inhibitor, for one to show the effect ascribed to the medication.
Table 3.
NNT for placebo controlled studies
Adverse events
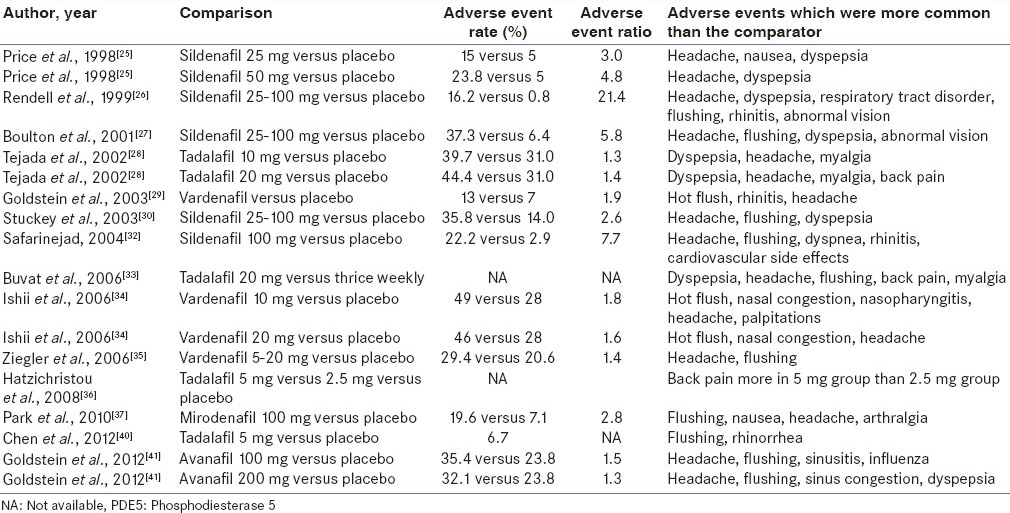
The adverse events reported in the included studies are enumerated in Table 4. The most common adverse events included headache, dyspepsia, hot flushes, rhinitis and nasal congestion. The rates of adverse events among the active medication group ranged from 13% of the sample to 44% of the sample; while, in the placebo group, the rate varied from 0.8% to 31.0%. For all the studies, the adverse event ratio for active PDE5 inhibitor to placebo was 1.3-21.4. The median comparative adverse event ratio was 1.9 (inter-quartile 1.4-4.8), suggesting that adverse events were roughly twice more common among those receiving the active drug than placebo. The adverse event rate did not show statistically significant correlation with the sample size of the study (Spearman r = −0.147, P = 0.607).
Table 4.
Adverse events with PDE5 inhibitors
Evidence for publication bias
Egger's test was conducted to assess for the presence of possible publication bias. The Egger's plot is shown in [Figure 3]. The regression equation for the present sample of studies as per Egger's test was:
Figure 3.
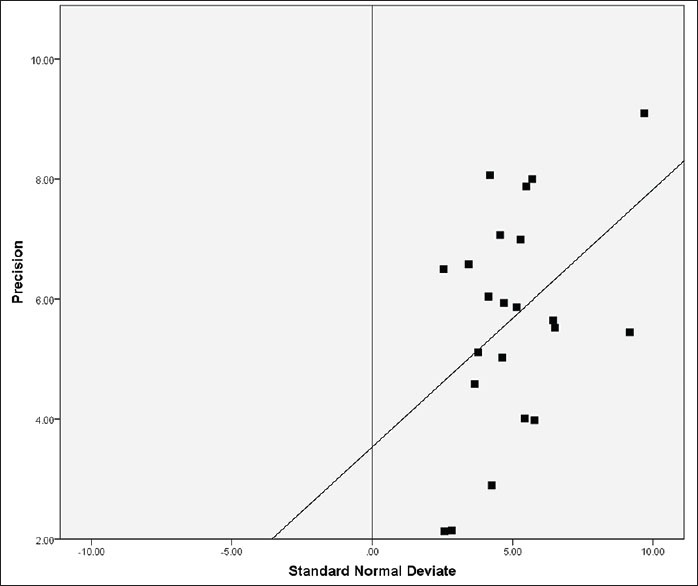
Egger's plot for assessment of publication bias. Precision was 1/(standard error of effect size). Regression line computed as: Standard normal deviate = 2.658 + 0.410 × (Precision)
SND = 2.658 + 0.410 × (precision)
The CIs of B ranged from 0.007 to 0.813 (P value of 0.046, R = 0.419) while the intercept was 2.658, which suggested that there was some evidence of publication bias or selection bias in this group of studies.
DISCUSSION
The present meta-analysis suggests that PDE5 inhibitors are effective in the treatment of erectile dysfunction in patients with diabetes mellitus. CIs of none of the placebo-controlled studies spanned zero, suggesting that all the PDE5 inhibitors were demonstrated to be clearly effective than placebo. On summary analysis, the pooled mean effect size of any PDE5 inhibitor was 0.926, which indicates a large effect size. Though there can be differences in interpretations of effect sizes,[42] the present meta-analysis suggests that PDE5 inhibitors did afford substantial improvement in this patient population. This finding is in line with other systematic reviews evaluating the efficacy of PDE5 inhibitors in general and other selected populations.[43,44,45,46]
Individual medication wise, sildenafil seemed to be superior to tadalafil, which in turn seemed to be superior to vardenafil. However, there was considerable variation in the efficacy reported for each of the medication across individual studies. Hence, superiority of one medication over the other cannot be conclusively determined. The two nonplacebo controlled studies (one comparing the dosing regimen of tadalafil, and the other comparing tadalafil with vardenafil) did not show clear advantage of one treatment arm over the other. This suggests that on the head to head comparisons, there may not be significant differences between PDE5 inhibitors, though studies to that effect are very sparse.
The NNT of PDE5 inhibitors as a group was found to be 3.0, with the lowest NNT for sildenafil (2.4). This metric can be helpful for clinicians, insurance payers and policy makers for gauging the impact of a treatment. However, one needs to be aware of the possible constraints of this NNT while making health-care decisions.[47,48] The NNT of a particular intervention can vary markedly based upon the characteristics of the patients, the outcome considered, the clinical and geographical setting. Also, NNTs are derived from well-conducted clinical trials with selective inclusion criteria and may not reflect the real world scenario.[49] Despite their limitations, NNTs do serve as a robust measure of estimating the anticipated health-care benefits of a treatment modality from a public health perspective.
The most common instruments utilized in the included studies were IIEF, SEP and GAQ. Fortunately, in the field of urology, these instruments have become standards of practice for objectively estimating treatment efficacy in RCTs. Sexual encounters vary in frequency from couple to couple and may fluctuate normally over period of time-based upon extraneous factors.[50,51] The above-mentioned instruments, however, provide adequate information about sexual functioning while minimizing the effect of frequency of actual intercourse. We tended to rely more on GAQ as it reflected overall personal satisfaction of sexual experience, and sexual satisfaction can be conceptualized more than just achieving adequate penile tumescence.[52]
Risk of bias analysis of the studies suggested that most of the studies were of fair quality. Only three of the studies were open label while the rest others were double-blinded. The randomization process and allocation concealment were not explicit in many of the studies though it could be assumed that appropriate randomization procedures were followed. Many of the studies had reported intent-to-treat analysis though a few had failed to do so. In the future, researchers need to be aware to provide intent-to-treat data as they have become standards of practice.
The adverse event rate had been quite variable across the studies included in this systematic review. The adverse event rate for active drug ranged from 49% to 15%, implying that one-sixth to one-half of the PA drug may experience adverse events. Adverse events for the active drug were twice as common as placebo. The rates of adverse events reported could vary across randomized trials based upon many factors.[53] It has been seen that clinician assessment of adverse events may not concur with patient perceptions.[54] Hence, clinicians need to regularly assess for adverse events as they arise and take management decisions accordingly. The common side effects across the group of PDE5 inhibitors were headache, dyspepsia and flushing. Musculoskeletal pain was common in the group receiving tadalafil and mirodenafil. Sinus/nasal congestion was more common in groups receiving sildenafil, vardenafil and avanafil. These adverse events were similar to those reported in previous meta-analyses.[44,55]
Evaluation of the present studies suggests that publication bias may exist in the literature. This systematic review specifically focused on published literature and the quantitative Egger's test suggests that smaller comparisons had higher effect sizes than larger comparisons. Alternate explanations include the possibility of English language bias in publication and citation bias.[23] Despite the possibility of publication bias, a chance does remain of true heterogeneity among the studies due to differences in underlying risk among studied population, and the intensity of the intervention across the different studies.
Etiology of erectile dysfunction in patients with diabetes mellitus can be multi-factorial.[8] Diabetes may be associated with depression that may lead in turn to decreased libido and reduced ability to have intercourse.[56] Diabetes may also cause vasculopathy that may reduce the blood flow to the penis. Endothelial dysfunction can result in reduced synthesis of nitric oxide that is required in the cascade for generating an erection. Sensory neuropathy ascribed to diabetes may result in reduced sexual stimulation that starts the cognitive process of initiating an erection. Lastly, hypogonadism may occur due to diabetes that may reduce the sexual drive. In an individual patient, multiple factors may play variable role to produce the erectile dysfunction. The PDE5 inhibitors act by inhibiting the metabolism of Cyclic guanosine monophosphate (cGMP) in the corpus cavernosum.[57] After sexual stimulation, nitric oxide in the corpus cavernosum of the penis binds to guanylate cyclase receptors, which causes increased levels of cGMP. Accumulation of cGMP leads to vasodilation and increased flow of blood into the spongy tissue of the penis, resulting in an erection. By inhibiting the metabolism of cGMP, PDE5 inhibitors results in generation and sustenance of the erection.
Attempts have been made to combine other treatment modalities with PDE5 inhibitors in patients with diabetes mellitus experiencing erectile dysfunction. These have included propionyl-L-carnitine, L-arginine and nicotinic acid,[58] losartan,[40] and vacuum pumps[59] which have been tested in randomized controlled design as an add-on to PDE5 inhibitors with varying success. PDE5 inhibitors have also been shown to be effective for not only men, but also women with diabetes mellitus who suffer from sexual arousal disorder.[60] The present meta-analysis however focused on PDE5 inhibitors only to reduce the heterogeneity of interventions, and make comparisons possible.
Improvement in quality of life as well as patient satisfaction may be afforded by prompt treatment of erectile dysfunction in patients with diabetes mellitus. However, often the problem of erectile dysfunction is not recognized in the clinical setting. One of the factors may be the under-recognition of the problem itself by the patient population.[61] Secondly, the problem of erectile dysfunction may be missed in a busy outpatient clinic because of the patient's reluctance to disclose, physician's reluctance to explore on sexual matters or the pressing demands of other complications of diabetes and dose adjustments required. Nonetheless, diabetes being a chronic disorder requiring a close liaison with physician or endocrinologist provides a good opportunity to explore into erectile function and treat appropriately when dysfunction is encountered.
The present systematic review should be contextualized in the presence of some limitations. Only peer-reviewed published material was included in this review, and unpublished material was not sought. This could have resulted in omission of some of the studies, especially which did not favor a PDE5 inhibitor over a placebo. Authors were not contacted for raw data that could have potentially allowed a more in-depth analysis. Moreover, sub-group analysis was not conducted as a part of this systematic review. This was because many studies did not report sub-group efficacy details and focused on overall outcome data. The meta-analysis also did not attempt to dissect out the causes of erectile dysfunction in the patient population, and hence might have clubbed together a heterogeneous population with different causes of erectile dysfunction. As alluded to above, this was not possible as erectile dysfunction in patients with diabetes mellitus can be multi-factorial.
CONCLUSION
The study suggests that PDE inhibitors are effective in the treatment of patients with diabetes mellitus and erectile dysfunction. It must be acknowledged that sexual efficacy of PDE5 inhibitors occurs in the context of various other aspects of the relationship between the partners. A comprehensive assessment of the dyadic relationship issues on efficacy of PDE5 inhibitors may be addressed in future research. Moreover, the influence of depressive symptoms on the efficacy of sexual functioning may be evaluated in further systematic evaluation. Other newer PDE5 inhibitors with favorable side effect profile and more convenient dosing regimen may be tested in this population. Patients may be benefitted with careful assessment of erectile dysfunction in patients with diabetes mellitus, accompanied judicious use of PDE5 inhibitors and review of potential adverse events.
Mental and behavioral disorders constitute an important co-morbidity among patients with diabetes mellitus.[62,63] It is important to identify and address these in order to improve outcome and quality of life of those living with diabetes.[64,65]
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
REFERENCES
- 1.Choi YJ, Lee MS, An SY, Kim TH, Han SJ, Kim HJ, et al. The relationship between diabetes mellitus and health-related quality of life in Korean adults: The Fourth Korea National Health and Nutrition Examination Survey (2007-2009) Diabetes Metab J. 2011;35:587–94. doi: 10.4093/dmj.2011.35.6.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porojan M, Poanta L, Dumitrascu DL. Assessing health related quality of life in diabetic patients. Rom J Intern Med. 2012;50:27–31. [PubMed] [Google Scholar]
- 3.Väätäinen S, Keinänen-Kiukaanniemi S, Saramies J, Uusitalo H, Tuomilehto J, Martikainen J. Quality of life along the diabetes continuum: A cross-sectional view of health-related quality of life and general health status in middle-aged and older Finns. Qual Life Res. 2014;23:1935–44. doi: 10.1007/s11136-014-0638-3. [DOI] [PubMed] [Google Scholar]
- 4.McCulloch DK, Campbell IW, Wu FC, Prescott RJ, Clarke BF. The prevalence of diabetic impotence. Diabetologia. 1980;18:279–83. doi: 10.1007/BF00251005. [DOI] [PubMed] [Google Scholar]
- 5.De Berardis G, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, Kaplan SH, et al. Erectile dysfunction and quality of life in type 2 diabetic patients: A serious problem too often overlooked. Diabetes Care. 2002;25:284–91. doi: 10.2337/diacare.25.2.284. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki H, Yamasaki H, Ogawa K, Nanjo K, Kawamori R, Iwamoto Y, et al. Prevalence and risk factors for erectile dysfunction in Japanese diabetics. Diabetes Res Clin Pract. 2005;70:81–9. doi: 10.1016/j.diabres.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Malavige LS, Jayaratne SD, Kathriarachchi ST, Sivayogan S, Fernando DJ, Levy JC. Erectile dysfunction among men with diabetes is strongly associated with premature ejaculation and reduced libido. J Sex Med. 2008;5:2125–34. doi: 10.1111/j.1743-6109.2008.00907.x. [DOI] [PubMed] [Google Scholar]
- 8.Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6:1232–47. doi: 10.1111/j.1743-6109.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 9.Malavige LS, Jayaratne SD, Kathriarachchi ST, Sivayogan S, Ranasinghe P, Levy JC. Erectile dysfunction is a strong predictor of poor quality of life in men with type 2 diabetes mellitus. Diabet Med. 2014;31:699–706. doi: 10.1111/dme.12412. [DOI] [PubMed] [Google Scholar]
- 10.Basu A, Ryder RE. New treatment options for erectile dysfunction in patients with diabetes mellitus. Drugs. 2004;64:2667–88. doi: 10.2165/00003495-200464230-00004. [DOI] [PubMed] [Google Scholar]
- 11.Nikolaidou B, Nouris C, Lazaridis A, Sampanis C, Doumas M. Diabetes mellitus and erectile dysfunction. In: Viigimaa M, Vlachopoulos C, Doumas M, editors. Erectile Dysfunction in Hypertension and Cardiovascular Disease. Cham, Heidelberg, New York, Dordrecht, London: Springer International Publishing; 2015. pp. 119–28. [Google Scholar]
- 12.Carson CC, Lue TF. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005;96:257–80. doi: 10.1111/j.1464-410X.2005.05614.x. [DOI] [PubMed] [Google Scholar]
- 13.Setter SM, Iltz JL, Fincham JE, Campbell RK, Baker DE. Phosphodiesterase 5 inhibitors for erectile dysfunction. Ann Pharmacother. 2005;39:1286–95. doi: 10.1345/aph.1E487. [DOI] [PubMed] [Google Scholar]
- 14.Smith WB, 2nd, McCaslin IR, Gokce A, Mandava SH, Trost L, Hellstrom WJ. PDE5 inhibitors: Considerations for preference and long-term adherence. Int J Clin Pract. 2013;67:768–80. doi: 10.1111/ijcp.12074. [DOI] [PubMed] [Google Scholar]
- 15.Vardi M, Nini A. Phosphodiesterase inhibitors for erectile dysfunction in patients with diabetes mellitus. Cochrane Database Syst Rev. 2007:CD002187. doi: 10.1002/14651858.CD002187.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochrane Collaboration. Chichester, England, Hoboken, NJ: Wiley-Blackwell; 2008. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 17.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 18.Sutton AJ, Abrams KR, Jones DR, Jones DR, Sheldon TA, Song F. Chichester, UK: John Wiley and Sons; 2000. Methods for Meta-Analysis in Medical Research. [Google Scholar]
- 19.Borenstein M, Hedges LV, Higgins J, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: John Wiley and Sons; 2007. Fixed-effect versus random-effects model; pp. 61–103. [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–29. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 25.Price DE, Gingell JC, Gepi-Attee S, Wareham K, Yates P, Boolell M. Sildenafil: Study of a novel oral treatment for erectile dysfunction in diabetic men. Diabet Med. 1998;15:821–5. doi: 10.1002/(SICI)1096-9136(199810)15:10<821::AID-DIA697>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 26.Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil for treatment of erectile dysfunction in men with diabetes: A randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999;281:421–6. doi: 10.1001/jama.281.5.421. [DOI] [PubMed] [Google Scholar]
- 27.Boulton AJ, Selam JL, Sweeney M, Ziegler D. Sildenafil citrate for the treatment of erectile dysfunction in men with type II diabetes mellitus. Diabetologia. 2001;44:1296–301. doi: 10.1007/s001250100656. [DOI] [PubMed] [Google Scholar]
- 28.Sáenz de Tejada I, Anglin G, Knight JR, Emmick JT. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care. 2002;25:2159–64. doi: 10.2337/diacare.25.12.2159. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein I, Young JM, Fischer J, Bangerter K, Segerson T, Taylor T, et al. Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: A multicenter double-blind placebo-controlled fixed-dose study. Diabetes Care. 2003;26:777–83. doi: 10.2337/diacare.26.3.777. [DOI] [PubMed] [Google Scholar]
- 30.Stuckey BG, Jadzinsky MN, Murphy LJ, Montorsi F, Kadioglu A, Fraige F, et al. Sildenafil citrate for treatment of erectile dysfunction in men with type 1 diabetes: Results of a randomized controlled trial. Diabetes Care. 2003;26:279–84. doi: 10.2337/diacare.26.2.279. [DOI] [PubMed] [Google Scholar]
- 31.Fonseca V, Seftel A, Denne J, Fredlund P. Impact of diabetes mellitus on the severity of erectile dysfunction and response to treatment: Analysis of data from tadalafil clinical trials. Diabetologia. 2004;47:1914–23. doi: 10.1007/s00125-004-1549-6. [DOI] [PubMed] [Google Scholar]
- 32.Safarinejad MR. Oral sildenafil in the treatment of erectile dysfunction in diabetic men: A randomized double-blind and placebo-controlled study. J Diabetes Complications. 2004;18:205–10. doi: 10.1016/S1056-8727(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 33.Buvat J, van Ahlen H, Schmitt H, Chan M, Kuepfer C, Varanese L. Efficacy and safety of two dosing regimens of tadalafil and patterns of sexual activity in men with diabetes mellitus and erectile dysfunction: Scheduled use vs. on-demand regimen evaluation (SURE) study in 14 European countries. J Sex Med. 2006;3:512–20. doi: 10.1111/j.1743-6109.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 34.Ishii N, Nagao K, Fujikawa K, Tachibana T, Iwamoto Y, Kamidono S. Vardenafil 20-mg demonstrated superior efficacy to 10-mg in Japanese men with diabetes mellitus suffering from erectile dysfunction. Int J Urol. 2006;13:1066–72. doi: 10.1111/j.1442-2042.2006.01480.x. [DOI] [PubMed] [Google Scholar]
- 35.Ziegler D, Merfort F, van Ahlen H, Yassin A, Reblin T, Neureither M. Efficacy and safety of flexible-dose vardenafil in men with type 1 diabetes and erectile dysfunction. J Sex Med. 2006;3:883–91. doi: 10.1111/j.1743-6109.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 36.Hatzichristou D, Gambla M, Rubio-Aurioles E, Buvat J, Brock GB, Spera G, et al. Efficacy of tadalafil once daily in men with diabetes mellitus and erectile dysfunction. Diabet Med. 2008;25:138–46. doi: 10.1111/j.1464-5491.2007.02338.x. [DOI] [PubMed] [Google Scholar]
- 37.Park HJ, Choi HK, Ahn TY, Park JK, Chung WS, Lee SW, et al. Efficacy and safety of oral mirodenafil in the treatment of erectile dysfunction in diabetic men in Korea: A multicenter, randomized, double-blind, placebo-controlled clinical trial. J Sex Med. 2010;7:2842–50. doi: 10.1111/j.1743-6109.2010.01888.x. [DOI] [PubMed] [Google Scholar]
- 38.Kamenov ZA. Comparison of the first intake of vardenafil and tadalafil in patients with diabetic neuropathy and diabetic erectile dysfunction. J Sex Med. 2011;8:851–64. doi: 10.1111/j.1743-6109.2010.02148.x. [DOI] [PubMed] [Google Scholar]
- 39.Moon du G, Yang DY, Lee CH, Ahn TY, Min KS, Park K, et al. A therapeutic confirmatory study to assess the safety and efficacy of Zydena (udenafil) for the treatment of erectile dysfunction in male patients with diabetes mellitus. J Sex Med. 2011;8:2048–61. doi: 10.1111/j.1743-6109.2011.02268.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Cui S, Lin H, Xu Z, Zhu W, Shi L, et al. Losartan improves erectile dysfunction in diabetic patients: A clinical trial. Int J Impot Res. 2012;24:217–20. doi: 10.1038/ijir.2012.4. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein I, Jones LA, Belkoff LH, Karlin GS, Bowden CH, Peterson CA, et al. Avanafil for the treatment of erectile dysfunction: A multicenter, randomized, double-blind study in men with diabetes mellitus. Mayo Clin Proc. 2012;87:843–52. doi: 10.1016/j.mayocp.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olejnik S, Algina J. Measures of effect size for comparative studies: Applications, interpretations, and limitations. Contemp Educ Psychol. 2000;25:241–86. doi: 10.1006/ceps.2000.1040. [DOI] [PubMed] [Google Scholar]
- 43.Fink HA, Mac Donald R, Rutks IR, Nelson DB, Wilt TJ. Sildenafil for male erectile dysfunction: A systematic review and meta-analysis. Arch Intern Med. 2002;162:1349–60. doi: 10.1001/archinte.162.12.1349. [DOI] [PubMed] [Google Scholar]
- 44.Markou S, Perimenis P, Gyftopoulos K, Athanasopoulos A, Barbalias G. Vardenafil (Levitra) for erectile dysfunction: A systematic review and meta-analysis of clinical trial reports. Int J Impot Res. 2004;16:470–8. doi: 10.1038/sj.ijir.3901258. [DOI] [PubMed] [Google Scholar]
- 45.Cui YS, Li N, Zong HT, Yan HL, Zhang Y. Avanafil for male erectile dysfunction: A systematic review and meta-analysis. Asian J Androl. 2014;16:472–7. doi: 10.4103/1008-682X.123670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fanbin L, Mei Y, Yan Z, Yirong Y, Shaoling Z, Yong C, et al. Efficacy and safety of phosphodiesterase-5 inhibitors for treating erectile dysfunction in kidney transplant recipients: A meta-analysis. Exp Clin Transplant. 2014;12:184–9. [PubMed] [Google Scholar]
- 47.Ebrahim S, Smith GD. The ‘number need to treat’: Does it help clinical decision making? J Hum Hypertens. 1999;13:721–4. doi: 10.1038/sj.jhh.1000919. [DOI] [PubMed] [Google Scholar]
- 48.Black HR, Crocitto MT. Number needed to treat: Solid science or a path to pernicious rationing? Am J Hypertens. 1998;11:128S–134S. doi: 10.1016/s0895-7061(98)00107-1. [DOI] [PubMed] [Google Scholar]
- 49.Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117:412–9. doi: 10.1111/j.1600-0447.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- 50.Cheung MW, Wong PW, Liu KY, Yip PS, Fan SY, Lam TH. A study of sexual satisfaction and frequency of sex among Hong Kong Chinese couples. J Sex Res. 2008;45:129–39. doi: 10.1080/00224490801987416. [DOI] [PubMed] [Google Scholar]
- 51.Schneidewind-Skibbe A, Hayes RD, Koochaki PE, Meyer J, Dennerstein L. The frequency of sexual intercourse reported by women: A review of community-based studies and factors limiting their conclusions. J Sex Med. 2008;5:301–35. doi: 10.1111/j.1743-6109.2007.00685.x. [DOI] [PubMed] [Google Scholar]
- 52.Pascoal PM, Narciso Ide S, Pereira NM. What is sexual satisfaction? Thematic analysis of lay people's definitions. J Sex Res. 2014;51:22–30. doi: 10.1080/00224499.2013.815149. [DOI] [PubMed] [Google Scholar]
- 53.Smith SM, Wang AT, Katz NP, McDermott MP, Burke LB, Coplan P, et al. Adverse event assessment, analysis, and reporting in recent published analgesic clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154:997–1008. doi: 10.1016/j.pain.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Di Maio M, Gallo C, Leighl NB, Piccirillo MC, Daniele G, Nuzzo F, et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J Clin Oncol. 2015;33:910–915. doi: 10.1200/JCO.2014.57.9334. [DOI] [PubMed] [Google Scholar]
- 55.Tsertsvadze A, Yazdi F, Fink HA, MacDonald R, Wilt TJ, Bella AJ, et al. Oral sildenafil citrate (viagra) for erectile dysfunction: A systematic review and meta-analysis of harms. Urology. 2009;74:831–6.e8. doi: 10.1016/j.urology.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 56.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 57.Corbin JD. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res. 2004;16(Suppl 1):S4–7. doi: 10.1038/sj.ijir.3901205. [DOI] [PubMed] [Google Scholar]
- 58.Gentile V, Antonini G, Antonella Bertozzi M, Dinelli N, Rizzo C, Ashraf Virmani M, et al. Effect of propionyl-L-carnitine, L-arginine and nicotinic acid on the efficacy of vardenafil in the treatment of erectile dysfunction in diabetes. Curr Med Res Opin. 2009;25:2223–8. doi: 10.1185/03007990903138416. [DOI] [PubMed] [Google Scholar]
- 59.Sun L, Peng FL, Yu ZL, Liu CL, Chen J. Combined sildenafil with vacuum erection device therapy in the management of diabetic men with erectile dysfunction after failure of first-line sildenafil monotherapy. Int J Urol. 2014;21:1263–7. doi: 10.1111/iju.12564. [DOI] [PubMed] [Google Scholar]
- 60.Caruso S, Rugolo S, Agnello C, Intelisano G, Di Mari L, Cianci A. Sildenafil improves sexual functioning in premenopausal women with type 1 diabetes who are affected by sexual arousal disorder: A double-blind, crossover, placebo-controlled pilot study. Fertil Steril. 2006;85:1496–501. doi: 10.1016/j.fertnstert.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 61.Shabsigh R, Kaufman J, Magee M, Creanga D, Russell D, Budhwani M. Lack of awareness of erectile dysfunction in many men with risk factors for erectile dysfunction. BMC Urol. 2010;10:18. doi: 10.1186/1471-2490-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balhara YS. Diabetes and psychiatric disorders. Indian J Endocr Metab. 2011;15:274–83. doi: 10.4103/2230-8210.85579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balhara YP, Sagar R. Correlates of anxiety and depression among patients with type 2 diabetes mellitus. Indian J Endocrinol Metab. 2011;15:S50–4. doi: 10.4103/2230-8210.83057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balhara YS, Verma R, Kalra B. Pharmacological management of psychiatric disorders in pregnancy complicated by diabetics. J Soc Health Diabetes. 2014;2:70–6. [Google Scholar]
- 65.Kalra S, Sridhar GR, Balhara YP, Sahay RK, Bantwal G, Baruah MP, et al. National recommendations: Psychosocial management of diabetes in India. Indian J Endocrinol Metab. 2013;17:376–95. doi: 10.4103/2230-8210.111608. [DOI] [PMC free article] [PubMed] [Google Scholar]