Abstract
Introduction:
Dietary and life style practices differ in postpartum (PP) and nonpregnant Indian women. Effect of these practices on postpartum weight retention (PPWR) and development of cardio-metabolic risk (CMR) has been scarcely studied in urban women. Aims of this study were to (i) compare anthropometry, biochemical parameters and body composition up to 3 years PP (ii) effect of PPWR, dietary fat intake and physical activity on CMR factors.
Methods:
Design: Cross-sectional, 300-fullterm, apparently healthy primi-parous women (28.6 ± 3.4 years) randomly selected. 128 women within 7-day of delivery (Group-A), 88 with 1–2 years (Group-B) and 84 with 3–4-year-old-children (Group-C) were studied. Anthropometry, sociodemographic status, physical activity, diet, clinical examination, biochemical tests, body composition, at total body (TB), by dual energy X-ray absorptiometry (GE-Lunar DPX) were collected.
Results:
Women at 3-year PP showed higher weight retention (6.5[10] kg) than at 1-year (3.0[7] kg) (median [IQR]). Android fat % (central obesity) increased (P < 0.05) at 1-year PP (47 ± 10.0%) when compared to 1-week PP (44.3 ± 6.7%) and remained elevated at 3-year PP (45.6 ± 10.2%). Regression analysis revealed that at 1-year PP, increase in PPWR (Odd Ratio [OR] 1.8, 95% confidence interval [CI] = [1.2, 2.5], P < 0.001) and inactivity (OR 1.4, 95% CI= (0.97, 2.0), P < 0.1) were predictors for CMR. At 3-year PP, only PPWR was responsible for increase in CMR parameters (OR 1.6, 95% CI = (1.3, 2.3), P < 0.001) and not inactivity (P > 0.1).
Conclusion:
Postdelivery, low physical activity and higher PPWR may increase CMR in Indian women.
Keywords: Cardio-metabolic risk, lactation, physical activity, postpartum weight retention, traditional food supplements
INTRODUCTION
Weight gain during pregnancy and postdelivery is likely to be a major contributor to the future development of obesity in women.[1] Reports suggest that pregnancy and lactation may also be associated with increased deposition of visceral fat in women.[2] Obesity developed during the postpartum (PP) period is also linked to the development of cardio metabolic risk.[3] Further compared with other ethnic groups, Asian Indians are more prone to develop abdominal obesity and are also at higher at cardio metabolic risk.[4,5] However, studies assessing changes in body composition in urban Indian women PP are scarce.
A study on Danish women suggests that exclusive breast feeding till recommended period reduces postpartum weight retention (PPWR) in all body mass index (BMI) categories.[6] However, in genetically predisposed women, low levels of physical activity and an energy dense, high fat diet are known to be independent risk factors for weight gain, obesity.[7] and PPWR.[8] As a result of various beliefs and customs, low physical activity PP has been documented.[9] To meet increased caloric demands, traditional food practices are followed during PP period in different populations.[10] In India, intake of traditional food supplements (TFS) PP is also a common practice.[11] These TFS are high calorie, high fat foods which are rich sources of saturated fats consumed by lactating mothers from immediately after delivery up to 3–6 months PP. However, the effect of feeding practices, physical activity and high fat foods on PPWR and development of cardio-metabolic risk (CMR) factors have been scarcely studied in Indian urban lactating women.
Therefore, aims of our cross-sectional study were (i) to assess changes in anthropometric, biochemical and cardio-metabolic parameters and body composition as measured by dual X-ray absorptiometry up to 3 years PP, and (ii) to explore the linkages of PPWR with risk of developing cardio metabolic risk.
METHODS
Selection of participants
This cross-sectional study was conducted on 300 urban PP women. Based on standard deviation of PPWR from previous studies[12] sample size was estimated to be 82 per group to detect the difference in groups at 5% level of significance and 10% margin of error so as to achieve a power of 80%. Considering the nonavailability of women for all measurements due to their immediate PP status, a total of 332 women were approached and all those who satisfied the inclusion/exclusion criteria were enrolled. Hence, out of a of total 300 selected, 128 primi-parous mothers within week of delivery (Group A) in a tertiary level health care hospital in Pune city, India, were enrolled. Further, mothers of singletons, coming to different pediatric clinics or school nurseries were approached and those satisfying inclusion/exclusion criteria voluntarily participated in this study. Eighty-eight mothers had children who were 1–2 years old (Group B) and 84 mothers had 3–4 year olds (Group C). Inclusion criteria was primi gravid women and exclusion criteria were: Multi-parous mothers, mothers with twins, mothers with any preexisting conditions such as gestational diabetes, preeclumpsia and mothers of infants diagnosed with intra uterine growth retardation or small for gestational age after examination and confirmation from pediatrician/gynecologist.[13] When the subjects were enrolled in the study, detailed history and review of relevant medical records was performed. None of the participants had any episodes of PP depression. The research protocol was approved by the institutional ethics committee and all participants gave written informed consent.
Anthropometry
Mothers’ weight was measured on the date of enrolment (DOE) in light clothes, without shoes using an electronic digital scale (Salter, India) to the nearest 0.1 kg. Standing height was measured using a stadiometer (Leicester Height Meter, Child Growth Foundation, UK, range 60–207 cm) to the accuracy of 1 mm. Measurements were standardized by running daily calibration for both the instruments. BMI was calculated as weight in kg/height in meter square. Waist circumference (between the lowest rib and the iliac crest at the end of gentle expiration) was measured using a nonstretchable tape to the nearest 1 mm.[14] Prepregnancy weight measured in the hospital was recorded from the mothers’ case records as noted by the gynecologist around 1-month before pregnancy. Mother's PPWR till the DOE over prepregnancy weight was computed.
Lactation history
Details of delivery, information on history of lactation regarding whether baby was breast fed exclusively or partially,[15] were recorded. Exclusive breastfeeding was defined when the infant received breast milk only and no other solids or liquids with the exception of vitamins, minerals, medicines or oral rehydration solution;[15] partial breastfeeding was defined when the infant received breast milk in addition to complementary foods; complementary foods included milk, infant formula, gruel or semi-solid foods given in addition to breast milk.[15] Mothers giving just breast milk till 6 months were considered as exclusively breast fed (EBFed) mothers and mothers giving complimentary foods before 6 months considered Partially breast fed mothers.
Clinical examination
Clinical health assessment was performed and blood pressure (BP) was measured by a clinician using sphygmomanometer on the day of enrolment. BP was measured in the left arm after 10 min of rest with the participant lying down quietly. Measurements were made by auscultation with a mercury-column sphygmomanometer and a cuff appropriately sized for the arm size of the subject. The onset of the first Korotkoff phase was used to determine systolic BP, and the onset of the fifth Korotkoff phase was used to determine diastolic BP. Mean of two measurements was recorded. Systolic BP above 130 mm/Hg and or diastolic BP above 85 mm/Hg was considered hypertension.[16]
Assessment of physical activity
Data on habitual physical activity were collected using a validated structured questionnaire.[17] Information about duration in minutes of major daily activities such as sleep, sitting, standing, walking, exercise, recreation and occupational activity were used to classify an individual into levels of following physical activity groups: Inactivity, light and moderate activity.[18] Activities such as office work, commuting, cooking and household cleaning were considered as light activity. Time spent in exercise (e.g. yoga, walking and gym) was considered as moderate activity.[18] Watching television, afternoon nap and other leisurely activities were categorized as inactivity.[18]
Blood collection
A venous blood sample (total 8 ml) was collected after an overnight fast (not <10 h and not >12 h) from each participant using plain mineral free vacutainers (BD Franklin Lakes, NJ USA) for serum estimations. Samples in plain vacutainers were immediately centrifuged at 2500 rpm for 15 min and the serum separated and frozen at −70°C until analysis. Lipid profile was estimated on a Siemens analyzer (Dade Dimension RXL Max.) with enzymatic procedures for measurement of total cholesterol, triglycerides (TG) and high density-lipoproteins (HDL). The low density-lipoproteins cholesterol levels were calculated (Friedewald equation).[19] Blood sugar was estimated immediately from plasma using Siemens analyzer (Dade Dimension RXL Max).
Dual-energy X-ray absorptiometry measurement for body composition analysis
Body composition for TB was measured using Lunar DPX-PRO TB pencil beam Densitometer (GE Healthcare, WI, USA) using a medium mode scan (software Encore 2005 version 9.30.044 Wisconsin, USA). The precision of the Lunar DPX for repeat measurements in adults is 1.1% for TB.[20] Measurements were standardized by running daily quality assurance scans. All scans and scan analyses were performed by the same operator.
Cardio-metabolic risk factors
All the women were assessed for CMR factors. They were considered to have CMR if they had any three of the following abnormalities.[21]
Abdominal obesity (waist circumference >80 cm in women)
Hyper tri-glyceridemia(>150 mg/dl)
Low level of HDL cholesterol (<40 mg/dl)
High BP (>130/85 mmHg)
Elevated fasting blood glucose (>110 mg/dl)
Since our aim was to study apparently healthy women, there were no women with gestational diabetes mellitus and preeclumpsia, thus, we considered increased waist circumference, hyper tri-glyceridemia and lower levels of HDL cholesterol for assessing CMR.
Dietary assessment
Dietary intakes were assessed by 24 h recall on three nonconsecutive days, including a Sunday. Mothers were asked about the intake of food items using standard cups and spoons by a trained investigator through face-to-face interview. Mothers were also asked about their consumption of TFS. Recipes of the TFS were recorded through a short questionnaire consisting of name and proportion of the food ingredients, water content, and cooking methods. Nutrient contents of the TFS supplements were estimated by the nutrient analysis software, (Xenios technology Pvt. Limited, India, 2012) C-Diet, version 2.0[22] using the observed moisture content and the proportion of ingredients of each recipe. Daily dietary intakes of nutrients were calculated using C-Diet version 2.0, which uses the Indian cooked food database[23] and the nutritive value tables of raw foods.[24,25]
Statistical analysis
Data were analyzed using SPSS software for Windows (version 16.0, SPSS Inc, Chicago, IL, USA). Mothers were categorized into three groups. Group A - within a week PP (3 ± 2 days), Group B 1–2 years PP (18 ± 5 months), Group C was 3–4 years PP (40 ± 6 months). Differences in means of all the groups for parameters such as anthropometric, biochemical and body composition parameters and nutrient intakes were analyzed with ANOVA. Generalized linear model was used to test the effect of pre pregnancy BMI, PPWR, inactivity level and traditional food intakes for development of cardio metabolic risk after adjusting for breast feeding practices. Level of significance was set at P < 0.05.
RESULTS
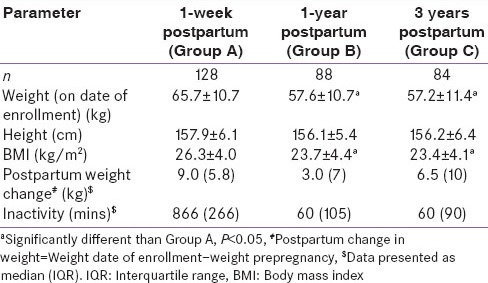
All the three groups were similar in age (28.7 ± 3.4 years) and height (156.9 ± 6.0 cm) (P > 0.1) [Table 1]. Parameters such as weight, BMI, PPWR and inactivity were highest immediately after delivery (P < 0.05) indicating pregnancy related changes. Majority of women EBFed (above 58%) for complete 6 months in Groups B and C. Average duration of total breast feeding in Group B was 15.5 ± 5.7 months and Group C was 15.0 ± 7.3 months. 49% women from 1-year PP group and 44% women in 3 years PP group had higher BMI (>23.5 kg/m2) indicating PPWR in spite of significant decrease in absolute BMI and inactivity (P < 0.05). When compared to group B, higher PPWR was observed in Group C (P < 0.05) only 5% women reported moderate activity in groups A and B; barely 13% women from Group C reported engagement in moderate activity such as walk or yoga above 30 min/day.
Table 1.
General characteristics of study population
Most of the CMR factors and dietary nutrient intakes were significantly lower in group C than A or B [Table 2], while changes in body composition were small and not significant (P > 0.1). Sixty-one percent women had raised cholesterol (total cholesterol > 200 mg/dl), 75% of women had raised triglyceride levels (TG > 150 mg/dl) in the immediate PP period. However, among women post 1-year and 3 years of delivery most of the parameters were lower than immediate PP status. Total fat % and gynoid fat % were lower at 1-year and 3 years PP (P < 0.05) when compared to immediate PP values. Percent lean body mass and total percent fat showed similar values from Group A to C (P > 0.1), whereas android fat % (another measure of central obesity) was higher (P < 0.05) in Group B women as compared to Group A and remained high in Group C. Android fat% was positively associated with TG levels (r = 0.29, P < 0.05) both in Group B and C indicating increasing risk of hyper triglycerdemia with increase in central obesity. Furthermore, waist circumference above 80 cm was found in 87% women from the entire study cohort. Highest central obesity (that is waist circumference > 80 cm) was found immediately after delivery (Group A) among 41% women, followed by 25% among Group B and 21% in Group C.
Table 2.
Cardio metabolic risk factors, biochemical parameters, body composition and nutrient intake of study population
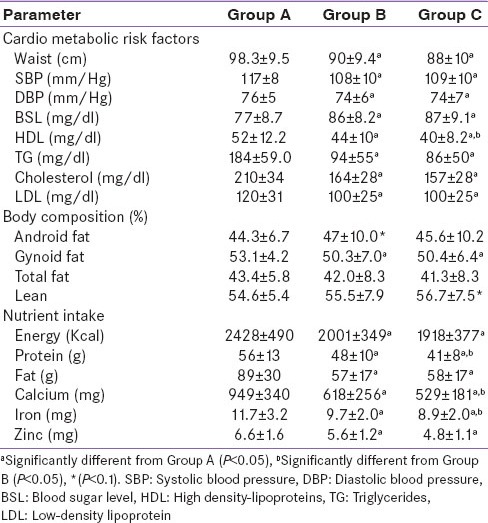
Consumption of traditional foods supplements in addition to their normal diet was reported by majority of mothers from all Groups (A - 70%, B - 80% and C - 85%) for > 1-month PP (average intake for 47 ± 45 days). Macronutrient (calories, protein, fat) and micronutrient (calcium, iron and zinc) intake was highest immediately PP (Group A) than in Group B or C. Visible dietary fat intake was 2–3 times higher than Indian recommended dietary allowances among all three groups with major contribution of TFS and extra ghee consumption during meals, dietary inadequacy of other macro nutrients and micronutrients was also observed. More than 95% women in all groups consumed diet inadequate in protein, iron and zinc and 75% consumed less dietary calcium. However, we could not find any association between dietary fat intake and PPWR or CMR in our study cohort.
As illustrated in Figure 1, at 3 years PP when breast feeding stopped completely, we observed increased prevalence of women with CMRs as PPWR increased. Prevalence of selected cardio metabolic risk factors (Waist, TG and HDL levels) was higher when PPWR was above 5 kg.
Figure 1.
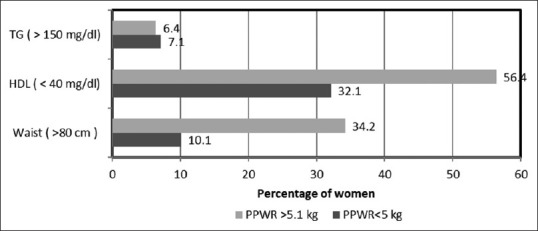
Prevalence of women with cardio-metabolic risks factors as postpartum (PP) weight retention increases at 3 years PP
Generalized linear model revealed that after 1-year of delivery, increase in PPWR (odd ratio [OR] 1.8, 95% confidence interval [CI] =1.2–2.5, P < 0.001) and inactivity (OR 1.4, 95%CI = 0.97–2.0, P < 0.1) were predictors for CMR parameters after adjusting for BF practices. Whereas, 3 years postdelivery, only PPWR was responsible for increase in CMR parameters (OR 1.6, (95% CI = 1.3–2.3, P < 0.001).
DISCUSSION
Our study reveals that although there was reduction in absolute weight and BMI at 1-year PP, possibly as a result of breast feeding, there was significant rise in PPWR in women at 3 years after delivery. About 49% women at 1-year and 44% women at 3 years were overweight/obese. Most women were very inactive and consumed a high fat diet which included TFS but were still deficient in many macro and micronutrients. Twenty-five percent of Group B and 21% from Group C women had increased waist circumference, 10% had hypercholestaremia, 7% hyper-triglycerdemia among both Groups (B and C). Body composition assessments showed that android fat increased at 1-year PP and remained elevated. Increased PPWR was associated with increased risk of CMR at 3 years PP.
In a recent longitudinal study from Sweden, authors report that weight retention between 3 and 12 months PP`adversely impacts the cardio metabolic profile in women.[26] Authors also report higher cholesterol levels in women at 1-year PP. Our findings are in line with these results where risk of developing CMR was associated with PPWR.
Pregnancy-induced body fat deposition is usually believed to occur at central sites (trunk and thighs) and this fat is normally mobilized to support lactation.[27] In a retrospective study from Finland, increased CMR was found in later life, that is 15–20 years after their last pregnancy among those with higher android fat percentage.[28] Further, even in nonoverweight women, visceral fat has been found to increase the CMR.[29] Therefore, in spite of losing excess body weight PP, mobilization of fat to central area may increase CMR in later life.[29] Our study also demonstrates that women in Group C had a high android fat percentage which may contribute to increased CMR in later years. Earlier studies indicate that high parity is directly related to cardio metabolic risks[30] whereas a longer duration of breastfeeding has a favorable effect on cardiovascular outcomes.[31] However, our mothers were primi parous and were breastfeeding for a longer duration (>15 months); still inactivity and high PPWR were predictors of cardio metabolic risk.
Motil et al. in their longitudinal study reported that adequate consumption of protein during lactation preserves lean mass in exclusively breast feeding mothers.[32] However, in spite of higher inactivity and low protein intakes lean body mass of our study cohort was similar among three groups. Low physical activity PP has also been also reported by others,[9,33] similar to that seen in our study. Further, high intake of dietary fat along with fat rich TFS has also been reported by other Indian studies.[11,34] Imbalances in nutrient intakes have also been reported by others, where high dietary fat consumption was reported similar to that seen in our study.[35]
One of the limitations of our study design is that it is cross sectional, however, most of our results are in line with other longitudinal studies. However, longitudinal studies are required to confirm the association of dietary fat intake and PPWR and CMR in Indian women.
CONCLUSION
Our results provide an important message that postdelivery, low physical activity and higher PPWR may result in central fat mass accumulation and increased CMR. It is imperative to devise strategies to correct nutritional inadequacies and to minimize PPWR for reducing obesity and CMR in Indian women.
Financial support and sponsorship
Corporate Social grant: Mr. Pancharatnam, Panchasheel Filters, Pune, Maharashtra, India.
Conflict of interest
There are no conflicts of interest.
REFERENCES
- 1.Gunderson EP. Childbearing and obesity in women: Weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36:317–32. doi: 10.1016/j.ogc.2009.04.001. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunderson EP, Sternfeld B, Wellons MF, Whitmer RA, Chiang V, Quesenberry CP, Jr, et al. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring) 2008;16:1078–84. doi: 10.1038/oby.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chopra SM, Misra A, Gulati S, Gupta R. Overweight, obesity and related non-communicable diseases in Asian Indian girls and women. Eur J Clin Nutr. 2013;67:688–96. doi: 10.1038/ejcn.2013.70. [DOI] [PubMed] [Google Scholar]
- 4.Cheng HR, Walker LO, Tseng YF, Lin PC. Post-partum weight retention in women in Asia: A systematic review. Obes Rev. 2011;12:770–80. doi: 10.1111/j.1467-789X.2011.00886.x. [DOI] [PubMed] [Google Scholar]
- 5.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: Results of the Multicultural Community Health Assessment Trial (M-CHAT) Am J Clin Nutr. 2007;86:353–9. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 6.Baker JL, Gamborg M, Heitmann BL, Lissner L, Sørensen TI, Rasmussen KM. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr. 2008;88:1543–51. doi: 10.3945/ajcn.2008.26379. [DOI] [PubMed] [Google Scholar]
- 7.Astrup A. Macronutrient balances and obesity: The role of diet and physical activity. Public Health Nutr. 1999;2:341–7. doi: 10.1017/s1368980099000464. [DOI] [PubMed] [Google Scholar]
- 8.Østbye T, Peterson BL, Krause KM, Swamy GK, Lovelady CA. Predictors of postpartum weight change among overweight and obese women: Results from the Active Mothers Postpartum study. J Womens Health (Larchmt) 2012;21:215–22. doi: 10.1089/jwh.2011.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evenson KR, Aytur SA, Borodulin K. Physical activity beliefs, barriers, and enablers among postpartum women. J Womens Health (Larchmt) 2009;18:1925–34. doi: 10.1089/jwh.2008.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen LW, Low YL, Fok D, Han WM, Chong YS, Gluckman P, et al. Dietary changes during pregnancy and the postpartum period in Singaporean Chinese, Malay and Indian women: The GUSTO birth cohort study. Public Health Nutr. 2014;17:1930–8. doi: 10.1017/S1368980013001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kajale N, Khadilkar A, Chiplonkar S, Unni J, Mansukhani N. Effect of traditional food supplements on nutritional status of lactating mothers and growth of their infants. Nutrition. 2014;30:1360–5. doi: 10.1016/j.nut.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Maddah M, Nikooyeh B. Weight retention from early pregnancy to three years postpartum: A study in Iranian women. Midwifery. 2009;25:731–7. doi: 10.1016/j.midw.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Mandruzzato G. Intrauterine growth restriction (IUGR): Guidelines for definition, recognition and management. Arch perinat Med. 2008;14:7–8. [Google Scholar]
- 14.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and cardiometabolic risk: A consensus statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr. 2007;85:1197–202. doi: 10.1093/ajcn/85.5.1197. [DOI] [PubMed] [Google Scholar]
- 15.Mihrshahi S, Oddy WH, Peat JK, Kabir I. Association between infant feeding patterns and diarrhoeal and respiratory illness: A cohort study in Chittagong, Bangladesh. Int Breastfeed J. 2008;3:28. doi: 10.1186/1746-4358-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 17.Chiplonkar SA, Agte VV, Tarwadi KV, Paknikar KM, Diwate UP. Micronutrient deficiencies as predisposing factors for hypertension in lacto-vegetarian Indian adults. J Am Coll Nutr. 2004;23:239–47. doi: 10.1080/07315724.2004.10719367. [DOI] [PubMed] [Google Scholar]
- 18.Physical Activity for Everyone, CDC. 1999. [Last accessed on 2015 Jan 10]. Available from: http://www.cdc.gov/physicalactivity/everyone/guidelines/adults.html .
- 19.Bethesda, MD: 1974. Manual of Laboratory Operations, Lipid Research Clinics Program. Lipid and Lipoprotein Analysis, DHEW Publication No. (NIH) [Google Scholar]
- 20.Khadilkar AV, Chiplonkar SA, Pandit DS, Kinare AS, Khadilkar VV. Metabolic risk factors and arterial stiffness in Indian children of parents with metabolic syndrome. J Am Coll Nutr. 2012;31:54–62. doi: 10.1080/07315724.2012.10720009. [DOI] [PubMed] [Google Scholar]
- 21.Misra A, Wasir JS, Pandey RM. An evaluation of candidate definitions of the metabolic syndrome in adult Asian Indians. Diabetes Care. 2005;28:398–403. doi: 10.2337/diacare.28.2.398. [DOI] [PubMed] [Google Scholar]
- 22.Pune , India: Xenios technology Pvt. Limited; 2012. C-Diet. Version 2. [Google Scholar]
- 23.Chiplonkar SA, Agte VV. Extent of error in estimating nutrient intakes from food tables versus laboratory estimates of cooked foods. Asia Pac J Clin Nutr. 2007;16:227–39. [PubMed] [Google Scholar]
- 24.Gopalan C, Ramasastri BV, Balasubramanian SG. Hyderabad, India: Published by National Institute of Nutrition; 1993. Revised and Updated by Rao BS, Deosthale YB, Pant KC, Nutritive Value of Indian Foods. Reprinted: 2000. [Google Scholar]
- 25.Gebhardt SE, Howe JC, Lemar LE, Haytowitz DB, Pehrsson PR, Cutrufelli RL, et al. USDA nutrient database for standard reference, release No. 23, 2009 [Google Scholar]
- 26.Kew S, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care. 2014;37:1998–2006. doi: 10.2337/dc14-0087. [DOI] [PubMed] [Google Scholar]
- 27.Butte NF, Hopkinson JM. Body composition changes during lactation are highly variable among women. J Nutr. 1998;128:381S–85S. doi: 10.1093/jn/128.2.381S. [DOI] [PubMed] [Google Scholar]
- 28.Wiklund PK, Xu L, Wang Q, Mikkola T, Lyytikäinen A, Völgyi E, et al. Lactation is associated with greater maternal bone size and bone strength later in life. Osteoporos Int. 2012;23:1939–45. doi: 10.1007/s00198-011-1790-z. [DOI] [PubMed] [Google Scholar]
- 29.Cho GJ, Yoon HJ, Kim EJ, Oh MJ, Seo HS, Kim HJ. Postpartum changes in body composition. Obesity (Silver Spring) 2011;19:2425–8. doi: 10.1038/oby.2011.163. [DOI] [PubMed] [Google Scholar]
- 30.Lao XQ, Thomas GN, Jiang CQ, Zhang WS, Yin P, Schooling M, et al. Parity and the metabolic syndrome in older Chinese women: The Guangzhou Biobank Cohort Study. Clin Endocrinol (Oxf) 2006;65:460–9. doi: 10.1111/j.1365-2265.2006.02615.x. [DOI] [PubMed] [Google Scholar]
- 31.Gunderson EP, Jacobs DR, Jr, Chiang V, Lewis CE, Feng J, Quesenberry CP, Jr, et al. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: A 20-Year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults) Diabetes. 2010;59:495–504. doi: 10.2337/db09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motil KJ, Sheng HP, Kertz BL, Montandon CM, Ellis KJ. Lean body mass of well-nourished women is preserved during lactation. Am J Clin Nutr. 1998;67:292–300. doi: 10.1093/ajcn/67.2.292. [DOI] [PubMed] [Google Scholar]
- 33.Durham HA, Morey MC, Lovelady CA, Namenek Brouwer RJ, Krause KM, Østbye T. Postpartum physical activity in overweight and obese women. J Phys Act Health. 2011;8:988–93. doi: 10.1123/jpah.8.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaushik D, Mathew S. Nutritional composition of traditional supplementary foods consumed by lactating women. Indian J Nutr Diet. 1988;25:320–4. [PubMed] [Google Scholar]
- 35.Hatsu IE, McDougald DM, Anderson AK. Effect of infant feeding on maternal body composition. Int Breastfeed J. 2008;3:18. doi: 10.1186/1746-4358-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


