Abstract
Background:
One year, prospective, observational study in an Indian subpopulation to assess back pain in patients with severe osteoporosis treated with teriparatide or antiresorptives in a clinical setting.
Materials and Methods:
One hundred and nineteen teriparatide-naοve Indian men and postmenopausal women (mean age 68.0 years) with previous osteoporotic vertebral fracture participated. Patients were assessed at baseline, 6-and 12-months to evaluate relative risk (RR) of new/worsening back pain using the Back Pain Questionnaire. The incidence of back pain and changes in back pain severity were assessed using the visual analog scale (VAS); Health outcomes were assessed using the euroquol-5 dimensions (EQ-5D) questionnaire. All tests were conducted with a two-sided alpha of 0.05.
Results:
Of 562 overall patients, 57, 60, and 2 Indian patients received teriparatide, antiresorptive, or teriparatide and antiresorptive, respectively. Baseline disease characteristics were slightly worse for antiresorptive-treated patients, whereas teriparatide-treated patients were older with more comorbidities. At 6-months, the incidence of new/worsening back pain was 5.3% for teriparatide-treated patients versus 4.4% for antiresorptive-treated patients (RR: 1.00, 95% confidence interval: 0.68, 1.48); the incidence of severe back pain was 0% versus 12.5% (P = 0.017); in these treatment groups, respectively. Mean VAS change scores (mean ± standard deviation [SD]) were − 1.9 ± 1.73 versus − 1.4 ± 1.77, and mean EQ-5D change scores were 4.2 ± 27.20 versus 9.9 ± 26.23 at 6-months. At 6 months, more teriparatide-treated patients felt better (89% vs. 61%; P = 0.001) and were at least very satisfied with their treatment (30% vs. 9%; P = 0.011).
Conclusion:
Teriparatide-treated Indian patients had similar new/worsening back pain risk and minimal risk of severe back pain compared with antiresorptive-treated patients at 6-months.
Keywords: Antiresorptives, back pain, fractures, osteoporosis, teriparatide
INTRODUCTION
Osteoporosis is a chronic skeletal disorder characterized by decreased bone density and changes in bone microarchitecture leading to increased skeletal fragility and risk of low-trauma fractures with associated complications eventually leading to reduced health-related quality of life.[1,2,3] Vertebral fracture is the most common fracture and prevalence increases with age. In addition, vertebral fractures have significant acute complications, such as back pain and functional limitations, and chronic complications, including severe back pain, kyphosis, height loss, and physical and psychological impairment.[4,5]
Indirect estimates in 2003 suggested that 26 million Indians suffer from osteoporosis, making India one of the largest affected countries in the world, with the numbers projected to increase to 36 million by 2013.[6] The incidence of osteoporosis is noticed at an average age of 50–60 years in the Indian population, and osteoporotic hip fractures occur even at an earlier age in the lower socioeconomic groups. These high numbers of osteoporosis and age at occurrence may be due to the widespread prevalence (ranging from 53% to 85%) of Vitamin D deficiency with low dietary calcium intake in the Indian population,[7,8] which contributes to poor bone health and osteoporosis.
Most importantly, even after the first fracture has occurred, there are effective osteoporotic treatments to decrease the risk of further fractures, as well as the risk of developing acute or chronic back pain or its intensity.[9] Antiresorptive agents increase bone mineral density (BMD) by suppressing bone resorption, in turn improving bone strength and preventing fractures.[10]
Teriparatide (human parathyroid hormone 1–34, of recombinant DNA origin), a bone anabolic agent, is approved for the treatment of osteoporosis. Teriparatide stimulates bone formation by increasing osteoblast number and inhibiting osteoblast apoptosis, leading to increased BMD, improved bone quality (i.e. restoring bone microarchitecture), and reduced risk of both vertebral and nonvertebral fractures.[9,11] In addition, teriparatide demonstrated a significantly lower incidence of new/worsened back pain when compared with alendronate.[9]
This article reports the subanalysis of the Indian population in an observational study conducted in a multi-ethnic population in patients with severe osteoporosis with back pain who were treated with teriparatide or antiresorptives.[12]
MATERIALS AND METHODS
Study patients
This article describes the subanalysis results of the Indian subpopulation from a 1-year, prospective, observational study that compared changes in back pain among teriparatide-treated versus. antiresorptive-treated patients with severe osteoporosis in a routine clinical setting. Since this was a nonrandomized study, treatment pattern and treatment initiation or changes were solely at the discretion of the physician and the patient. Patients were enrolled at 58 centers in nine countries/provinces: Hong Kong, India, Korea, Malaysia, Mexico, Pakistan, Singapore, Taiwan, and Thailand. In India, the study was conducted in eight centers. In this article, East Asia includes China, Hong Kong, Taiwan, and Korea; other includes Malaysia, Singapore, Pakistan, and Thailand.
Patients were teriparatide-naïve men and postmenopausal women who had a previous vertebral osteoporotic fracture sustained at least 6 weeks before joining the study. Patients consented to participate in the study and release information regarding treatment. They also agreed to return for follow-up visits; in the opinion of the prescribing physician, they were eligible to receive the intended treatment and comply with all the recommendations stated in the relevant product information.[12] Patients were excluded if they were contraindicated according to the relevant product information in the country/province in which they were being treated or if they were simultaneously participating in a different study that included a treatment intervention and/or an investigational drug.[12]
The study was reviewed and approved by ethical review boards. All the study methods and procedures were conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with good clinical practices and applicable local laws and regulations.
Outcome measures
Assessments were performed at baseline, 6 months, and 12 months. Medical evaluations for patients with severe osteoporosis were planned at approximately every 6 months due to the high risk of fractures. The primary study endpoint included evaluation of relative risk (RR) of new/worsening back pain for patients treated with teriparatide versus those treated with antiresorptives at 6 months as assessed by the Back Pain Questionnaire.[12] Patients were considered to have new/worsening back pain if their score on question 2 of the Back Pain Questionnaire was worse than their score at baseline. Patients with severe back pain at baseline were not included in this analysis since their pain was already of maximum severity.
Secondary effectiveness measures were the incidence of back pain and changes in back pain severity using a 10-point visual analog scale (VAS), where zero is “no pain” and 10 is “worst possible pain.” Safety variables included the occurrence of serious and nonserious adverse events (AEs) and events considered possibly related to therapy by the investigator. One or more incidence of treatment-emergent nontraumatic osteoporotic fractures any time during the study was counted as an event (new fractures).[12]
Health-outcome evaluations included treatment adherence, discontinuation of treatment at 1-year, reasons for treatment discontinuation, treatment satisfaction, and changes in health-related quality of life using the 100-point scale EuroQol-5 Dimensions (EQ-5D) questionnaire instrument to measure self-reported health-related outcomes, where zero is “the worst health you can imagine” and 100 is “the best health you can imagine.”[12]
Treatment persistence was determined by asking patients to report the date of their first dose and the approximate date of their last dose. Patients were also asked at each visit to estimate the number of days that they missed taking their prescribed treatment. Investigators evaluated both aspects of treatment compliance at 3 months and 9 months.[12]
Statistical analysis
Of 562 overall patients among nine countries, this report analyzed Indian patients who comprised the second largest population (n = 119) in the study, after Mexico (n = 185).
All tests were conducted with a two-sided alpha of 0.05. No adjustments were made for multiple comparisons. Patients who received teriparatide or antiresorptive treatment or both at any time during the study were included in the safety cohort. Patients who received teriparatide or antiresorptive treatment, but not both, at baseline were included in the monotherapy cohort and classified according to the treatment received at baseline.[12] For all statistical comparisons, only monotherapy groups were compared.
The covariates used in the comparisons and models were baseline value and propensity score. The propensity score method was used to adjust for baseline differences and selection bias. The propensity score for each patient was derived using logistic regression with selected baseline characteristics as independent variables (i.e. demographics, vitals, alcohol and tobacco use, exercise, type of insurance, education, and medical history, including fracture and baseline assessments of disease and back pain). Two patients whose propensity scores were in non-overlapping regions of the propensity score distributions were removed from any comparative analyses.
Relative risk of new/worsening back pain at 6 months was estimated using the modified Poisson regression model adjusted for baseline back pain severity and propensity scores. The point estimate and 95% confidence interval (CI) for the RR were presented.
In addition, because of the small subgroup size of Indian patients, the primary analyses of new/worsening back pain were performed for the overall study group and by geographic regions for comparisons. RRs (95% CI) were presented for the overall group and by geographic regions adjusted for covariates (country, baseline severity of back pain, and propensity score) using a Poisson regression model and unadjusted for covariates using 2 × 2 table; results were displayed in the Forest graphs [Supplementary Figures 1 and 2].
Supplementary Figure 1.
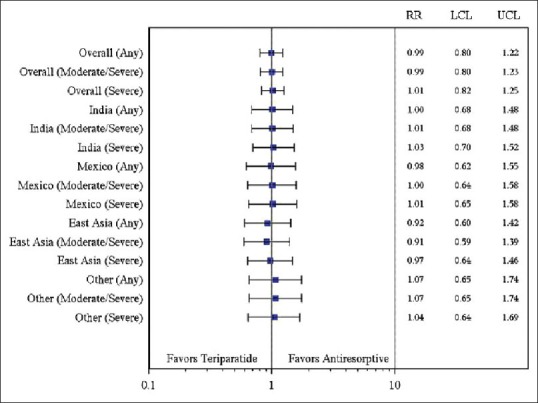
Relative risk (95% confidence interval) of new/worsening back pain at 6 months by region (Poisson regression model with covariates). Relative risk was calculated by adjusting country, baseline severity of back pain, and propensity score. East Asia includes China, Hong Kong, Taiwan, and Korea; other includes Malaysia, Singapore, Pakistan, and Thailand
Supplementary Figure 2.
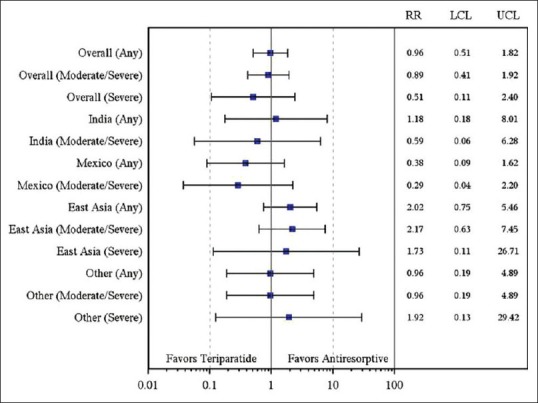
Relative risk (95% confidence interval) of new/worsening back pain at 6 months by region (2 × 2 table). Relative risk was not adjusted for any covariate. East Asia included China, Hong Kong, Taiwan, and Korea; others included Malaysia, Singapore, Pakistan, and Thailand
The severity of back pain at 6 months was compared using a generalized logit model adjusted for baseline back pain severity. Odds ratios (95% CI) were calculated using “none” as the reference category.
The incidence of any back pain during the first 6 months was defined as at least one report of back pain (minor, moderate, or severe) from baseline to 6 months. Similarly, the incidence of any back pain was defined as at least one report of back pain (minor, moderate, or severe) at any time during the study. The odds of experiencing any back pain postbaseline during the first 6 months and any time during the study were calculated using the generalized logit model adjusted for propensity score and baseline back pain severity. Odds ratios (95% CI) were calculated using “none” as the reference category.
Descriptive analyses of actual values and change from baseline of VAS back pain severity scores were provided. Change from baseline in back pain VAS score was analyzed using a mixed-model repeated measure with baseline value and propensity score as covariates and treatment cohort, time point, and treatment cohort-by-time point interaction as fixed effects and patient and error as random effects. Unstructured covariance matrix was used to model the within-patient errors.
Descriptive analyses of actual values and change from baseline of EQ-5D health state scores of quality of life were provided. Cohorts were compared with respect to change from baseline in EQ-5D health state scores using an analysis of covariance model with baseline value and propensity score as covariates and treatment cohort as fixed effects.
Treatment adherence and satisfaction, discontinuation rate, and reasons for discontinuation were also summarized using descriptive statistics. Treatment discontinuation by 1-year was analyzed using logistic regression with treatment and propensity score in the model. For safety, summaries were provided for common, serious, and nonserious treatment-emergent AEs, treatment discontinuation due to AEs, and nontraumatic osteoporotic fractures.
RESULTS
Of the 562 patients who received treatment, 119 patients were of Indian descent (teriparatide: N =57, antiresorptive: N =60, teriparatide and antiresorptive: N =2; [Figure 1]).[12] Among these Indian patients, 42 patients in the teriparatide group and 41 patients in the antiresorptive group completed the study. Fewer teriparatide-treated patients discontinued treatment than antiresorptive-treated patients (15 [26.3%] vs. 19 [31.7%] patients). The most common reason for discontinuation was “subject decision” (11 [19.3%] patients) in the teriparatide group and “lost to follow-up” (9 [15%] patients) in the antiresorptive group [Figure 1]. No statistically significant difference in treatment discontinuation by 1-year (adjusted for propensity score) was reported between the two groups (odds ratio: 1.12 [0.42, 3.01]; P = 0.815).
Figure 1.
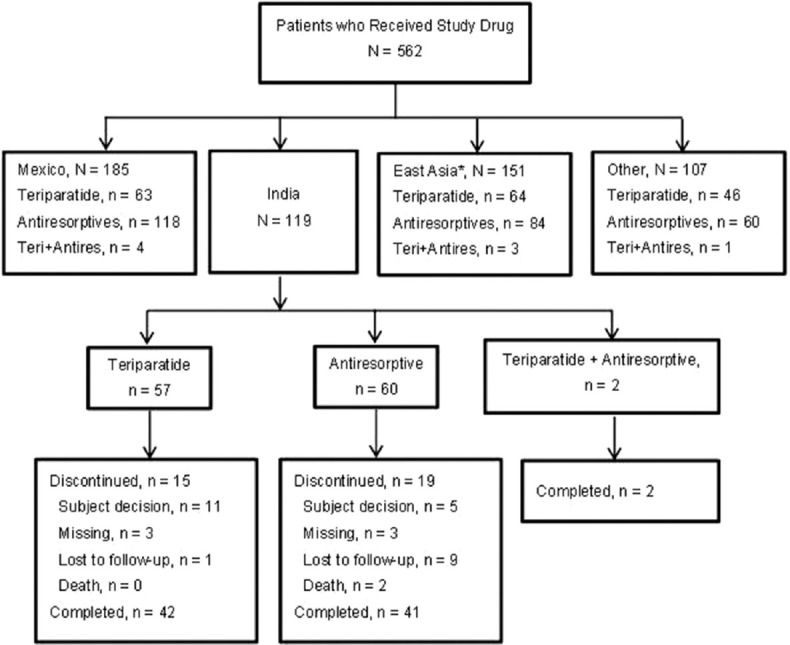
Patient disposition. N: Total number of patients, n: Number of patients in a specified category, Teri + Antires: Teriparatide and antiresorptive (*East Asia includes China, Taiwan, Hong Kong, and Korea; Other includes Malaysia, Singapore, Pakistan, and Thailand)
Patient demographics
Baseline demographics and disease characteristics were similar between the two groups with the exception of disease severity, which appeared to be worse for patients in the antiresorptive cohort compared to the teriparatide cohort [Tables 1a and 1b]. Furthermore, at baseline, the mean scores for VAS back pain severity and EQ-5D appeared to be worse in the antiresorptive group (5.8 and 48.0) compared with the teriparatide group (5.0 and 53.7) [Table 1c]. The mean age of patients was 66.1 years in the teriparatide group versus 62.5 years in the antiresorptive group. More patients in the teriparatide group had preexisting conditions compared with the antiresorptive group (hypertension: 36.8% vs. 16.7%, diabetes mellitus: 10.5% vs. 6.7%, hypothyroidism: 5.3% vs. 5.0%) [Table 1a].
Table 1 (a).
Patient demographics and baseline characteristics-Indian population
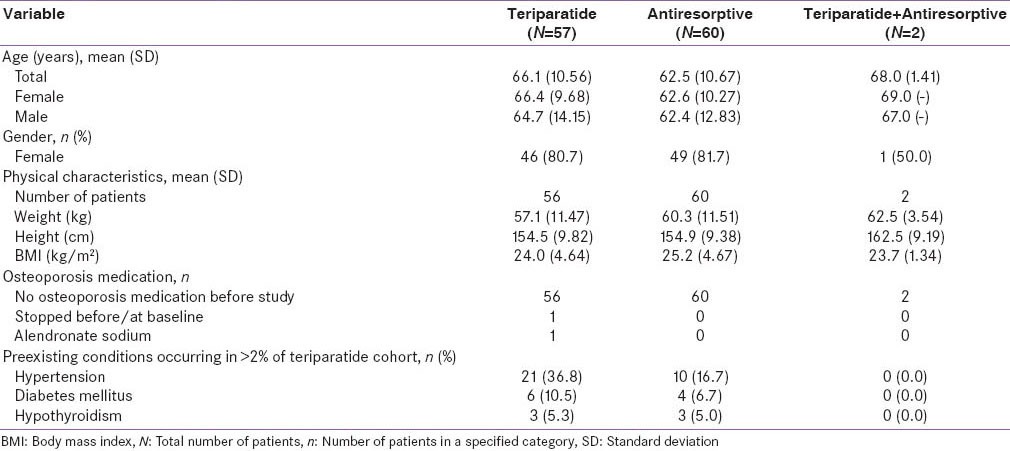
Table 1 (b).
Baseline Back Pain Questionnaire score- Indian population
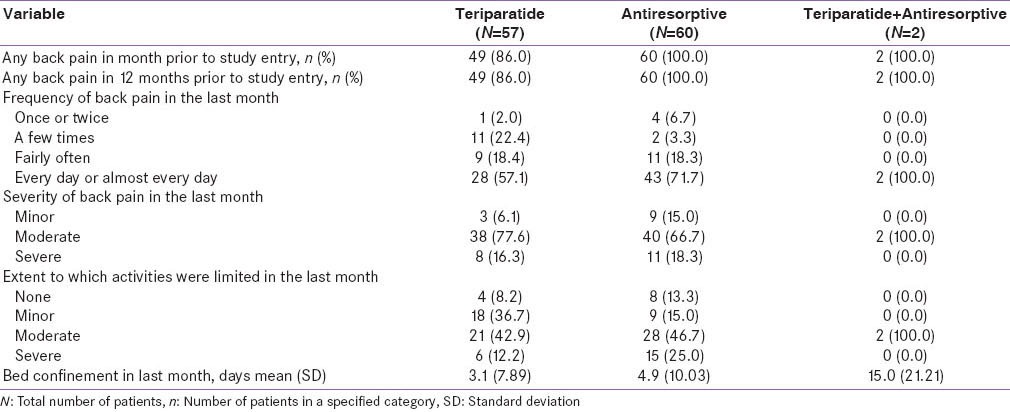
Table 1 (c).
Baseline VAS and EQ-5D health state scores-monotherapy cohort–Indian population
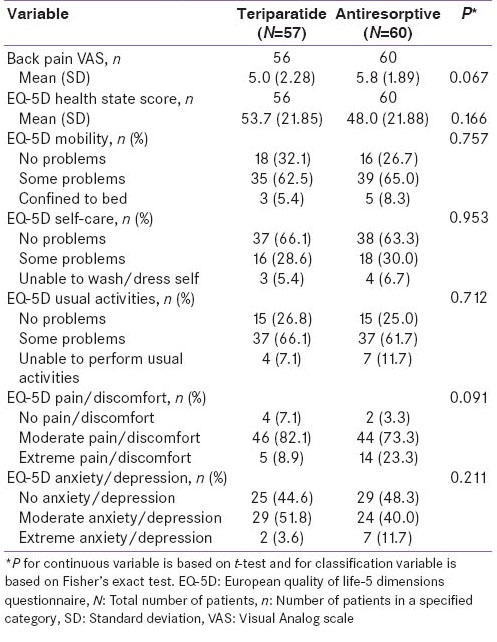
Efficacy
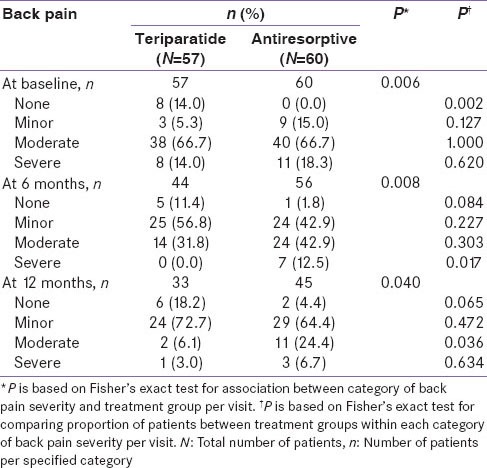
For the primary endpoint, teriparatide-treated patients had a similar risk of new/worsening back pain as antiresorptive-treated patients at 6 months [Table 2]. The incidence of new/worsening back pain at 6 months was 5.3% for teriparatide-treated patients versus 4.4% for antiresorptive-treated patients (RR: 1.00; 95% CI: 0.68, 1.48). At 12 months, the incidence of new/worsening back pain was 0% versus 2.9% in the teriparatide and antiresorptive groups, respectively (RR: 0.99; 95% CI: 0.64, 1.55). Fewer teriparatide-treated patients had moderate back pain at 6 months compared to antiresorptive-treated patients; however, there was no significant difference between the two groups (31.8% vs. 42.9%; P = 0.303). At 12 months, significantly fewer teriparatide-treated patients had moderate back pain (6.1% vs. 24.4%; P = 0.036). At 6 months, no teriparatide-treated patients had severe back pain compared to seven patients on antiresorptive treatment (0% vs. 12.5%; P = 0.017). At 12 months, there was no significant difference between the two groups in terms of the occurrence of severe back pain (3.0% vs. 6.7%; P = 0.634) [Table 3]. The odds ratios (95% CI) of back pain severity at 6 months, using no back pain as a reference and adjusted for baseline back pain severity, were as follows: 0.35 (0.03, 4.02), minor; 0.19 (0.02, 2.35), moderate. Severe back pain was not estimated due to zero incidence in the teriparatide group. The odds ratios (95% CI) of any back pain during the first 6 months and anytime during study, no back pain as reference and adjusted for baseline back pain severity and propensity score, were as follows: 0.38 (0.08, 1.74) for any back pain during first 6 months and 0.38 (0.08, 1.75) for any back pain during the study.
Table 2.
Incidence of new/worsening back pain of patients- Indian population
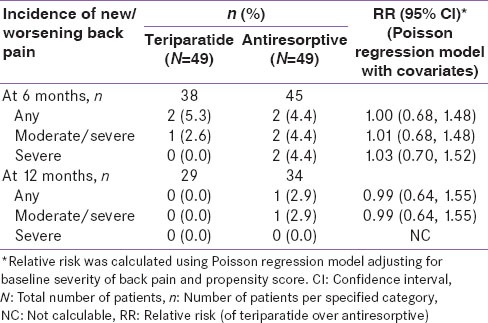
Table 3.
Severity of back pain at baseline, month 6 and month 12-monotherapy cohort- Indian population
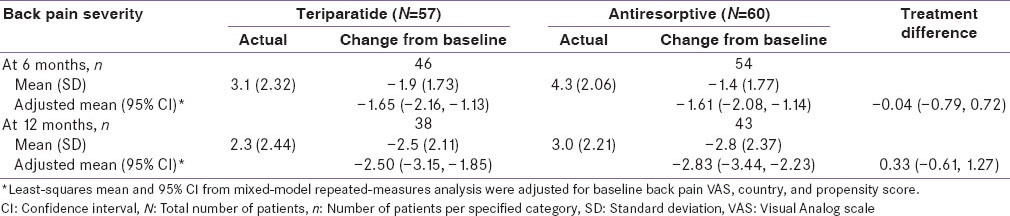
Back pain severity measured using VAS improved significantly for both cohorts at 6 and 12 months [Table 4]; however, no significant treatment differences were noted at 6 and 12 months (95% CI at 6 months: -0.79, 0.72 and at 12 months: −0.61, 1.27).
Table 4.
Change in back pain severity (VAS)-monotherapy cohort-Indian population
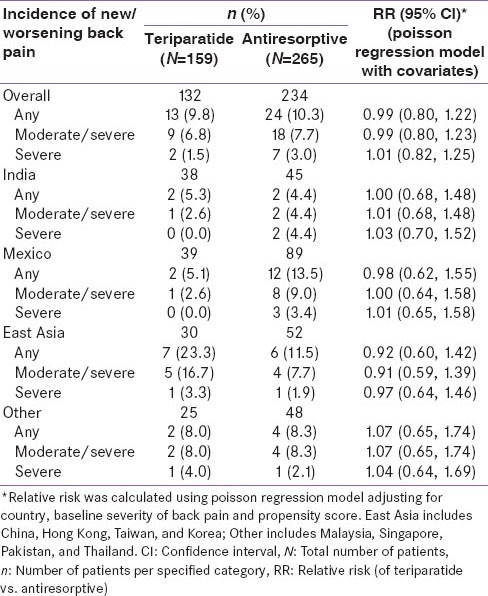
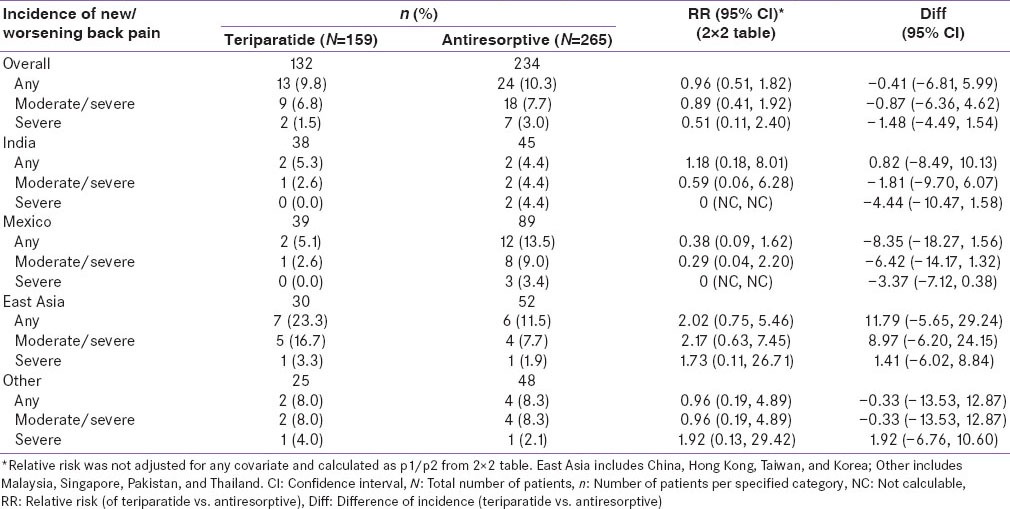
The RR in the incidence of new/worsening back pain for teriparatide versus antiresorptive was similar in Indian patients when compared with patients across the geographic regions [Supplementary Table 1 and Supplementary Figure 1, covariates adjusted RR of new/worsening back pain (any, moderate/severe, severe) by geographic regions at 6 months]. The unadjusted RR in incidence of new/worsening back pain was numerically in favor of teriparatide in the Indian and Mexican populations and numerically in favor of antiresorptive's in the East Asian population [Supplementary Table 2 and Supplementary Figure 2, unadjusted RR of new/worsening back pain (any, moderate/severe, severe) by geographic regions at 6 months]. The difference in the incidence of new/worsening back pain numerically favored teriparatide group in the Mexican population and favored antiresorptive group in the East Asian population [Supplementary Figure 3]. However, the differences between treatment groups were not significant.
Supplementary Table 1.
Incidence of new/worsening back pain of patients at 6 months by region (poisson regression model with covariates)
Supplementary Table 2.
Incidence of new/worsening back pain of patients at 6 months by region (2×2 table)
Supplementary Figure 3.
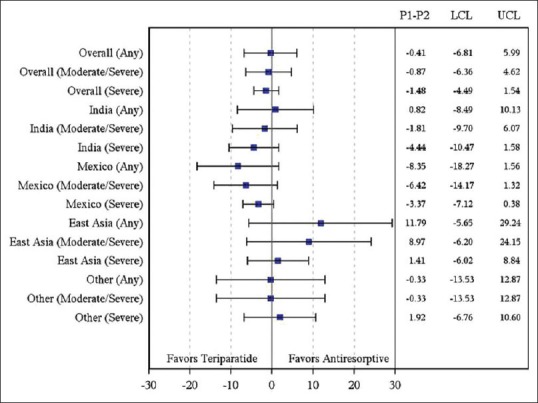
Difference in incidence rate (95% Confidence interval) of new/worsening back pain at 6 months by region (2 × 2 table). Patients with severe back pain at baseline were excluded. East Asia included China, Hong Kong, Taiwan, and Korea; Other included Malaysia, Singapore, Pakistan, and Thailand
The mean (standard deviation [SD]) number of days on therapy for the Indian subpopulation was 314.9 days (103.51) in the teriparatide group, 323.2 days (78.61) in the antiresorptive group, and 377.5 days (3.54) in the two patients who received both types of treatment.
Safety
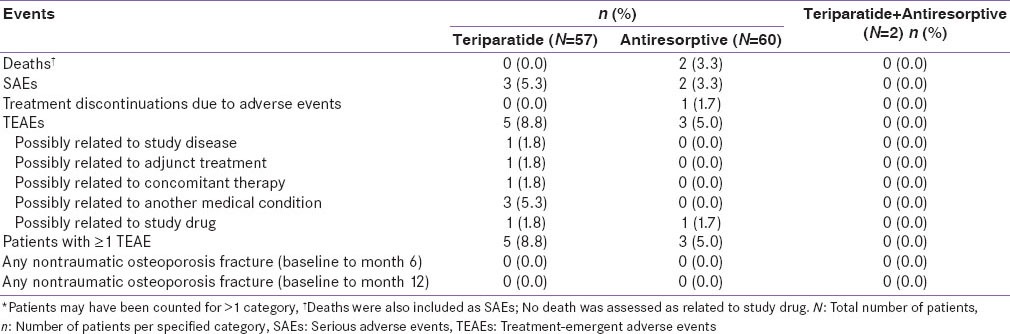
Overall, the number of serious adverse events (SAEs) was low in both treatment groups. The most common SAEs (≥2 events) were as follows: Antiresorptive group, death (n = 2). There were no reports of nontraumatic osteoporotic fractures or osteosarcoma in either treatment group. The incidence of treatment discontinuation due to AEs was 0% in the teriparatide group and 1.7% in the antiresorptive group [Table 5].
Table 5.
Adverse event overview*-Indian population
Quality of life
Health outcomes assessments were based on the EQ-5D [Table 6]. The mean ± SD EQ-5D health-state score at baseline was 53.7 ± 21.85 for the teriparatide group compared with 48.0 ± 21.88 for the antiresorptive group [Table 1c]. These scores improved significantly for both groups at 6 and 12 months [Table 6]. However, no significant differences between treatments were noted at 6 and 12 months (95% CI at 6 months: −10.59, 10.32 and at 12 months: −14.94, 5.82). A greater percentage of teriparatide-treated patients felt better compared to antiresorptive-treated patients in response to question 1 on the treatment satisfaction questionnaire (“How has treatment affected you?) at month 6 (89% vs. 61%; P = 0.001) and at month 12 (95% vs. 79%; P = 0.051) [Figure 2]. Similarly, for question 2 (“Please indicate how satisfied you are with medication you took during your participation in this study”), more teriparatide-treated patients were very satisfied with their treatment versus antiresorptive-treated patients at 6 months (30% vs. 9%) and at 12 months (49% vs. 30%) [Figure 2]. This difference was statistically significant at 6 months (P = 0.011) but not statistically significant at 12 months (P = 0.114). For patients who had not discontinued study, treatment compliance in the teriparatide group was 95.9% at 6 months and 97.4% at 12 months and treatment compliance in the antiresorptive group was 100% at 6 and 12 months.
Table 6.
EQ-5D questionnaire score-monotherapy cohort-Indian population
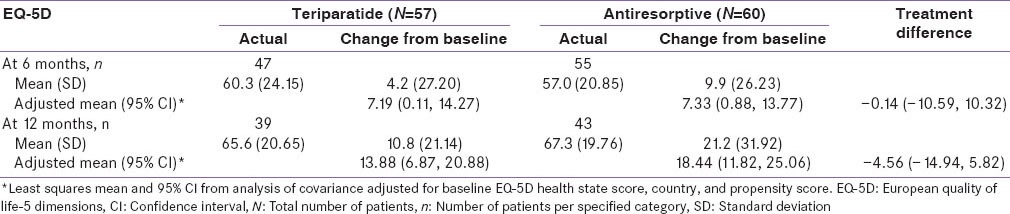
Figure 2.
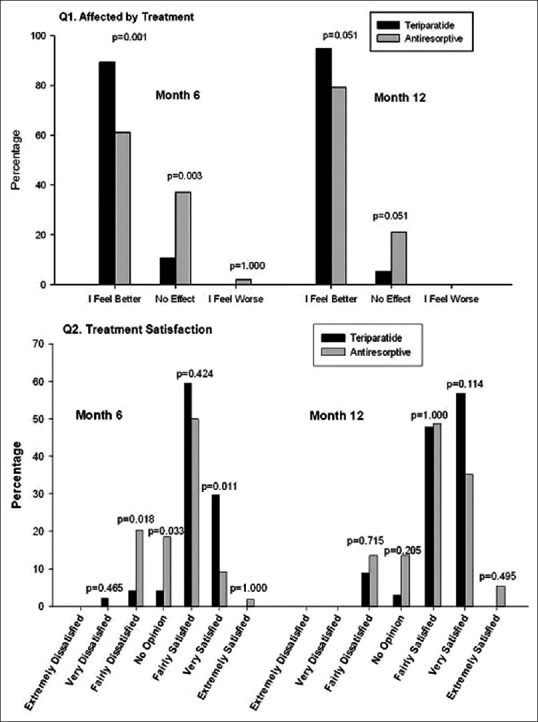
Health outcomes responses on treatment satisfaction questionnaire. Q1: Affected by treatment, Q2: Treatment satisfaction
DISCUSSION
The data reported here are an Indian subgroup analysis from a recently reported observational study conducted in multi-ethnic patients.[12]
In general, baseline disease characteristics in the Indian population were worse for the antiresorptive group compared with the teriparatide group. However, patients in the teriparatide group were older and had more associated comorbidities.[12]
Similar to the results for the Asian patients[12], teriparatide-treated Indian patients had a similar risk of new/worsening back pain as antiresorptive-treated patients at 6 months, and there was no significant difference observed for the VAS back pain severity and EQ-5D health state score at 6 and 12 months.
Back pain severity VAS in Indian patients improved significantly for both cohorts at 6 and at 12 months with no significant differences between treatment groups. However, in the overall population, at 12 months, there was a statistically significant improvement in back pain severity in teriparatide-treated patients compared to antiresorptive-treated patients. The incidence of severe back pain at 6 months and moderate back pain at 12 months was significantly lower in the teriparatide group compared to the antiresorptive group. Significantly more teriparatide-treated patients felt better and were very satisfied with their treatment at 6 months. The results from the Indian subpopulation are consistent with the results reported in Caucasian patients with respect to health-related quality of life.[2]
The main strengths of this study are that the results apply generally since the sample represents the whole population, normal clinical practice settings of the study, and the longer duration of patient monitoring. The main limitation is the lack of randomization, which leads to selection biases like more associated comorbidities in the teriparatide group and the lack of assessment of back pain origin and its relatedness to osteoporosis.
Vertebral fractures are the most common type of fracture, accounting for 45% of osteoporotic fractures that lead to acute or chronic back pain.[5] Although these observational trial results are promising, more studies are needed on patients with back pain due to at least one moderate to severe vertebral fracture.
The results of this observational study in Indian ethnicity support previous findings with regards to effect on back pain during teriparatide treatment.[2,9,10]
Financial support and sponsorship
This research was funded by Eli Lilly and Company.
Conflict of interest
Sirel Gurbuz, Simrat Sohal, and Kyoungah See are employees of Eli Lilly and Company. The authors have no other direct or indirect commercial financial incentive associated with publishing the manuscript.
REFERENCES
- 1.Lewiecki EM. Current and emerging pharmacologic therapies for the management of postmenopausal osteoporosis. J Womens Health (Larchmt) 2009;18:1615–26. doi: 10.1089/jwh.2008.1086. [DOI] [PubMed] [Google Scholar]
- 2.Jakob F, Oertel H, Langdahl B, Ljunggren O, Barrett A, Karras D, et al. Effects of teriparatide in postmenopausal women with osteoporosis pre-treated with bisphosphonates: 36-month results from the European Forsteo Observational Study. Eur J Endocrinol. 2012;166:87–97. doi: 10.1530/EJE-11-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genant HK, Halse J, Briney WG, Xie L, Glass EV, Krege JH. The effects of teriparatide on the incidence of back pain in postmenopausal women with osteoporosis. Curr Med Res Opin. 2005;21:1027–34. doi: 10.1185/030079905X49671. [DOI] [PubMed] [Google Scholar]
- 4.Francis RM, Aspray TJ, Hide G, Sutcliffe AM, Wilkinson P. Back pain in osteoporotic vertebral fractures. Osteoporos Int. 2008;19:895–903. doi: 10.1007/s00198-007-0530-x. [DOI] [PubMed] [Google Scholar]
- 5.Bodenner D, Redman C, Riggs A. Teriparatide in the management of osteoporosis. Clin Interv Aging. 2007;2:499–507. doi: 10.2147/cia.s241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mithal A, Dhingra V, Lau E. Nyon; Switzerland: Int Osteoporos Foundation; 2009. The Asian audit: Epidemiology, costs and burden of osteoporosis in Asia; pp. 24–7. http://www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Asia/Asian_regional_audit_2009.pdf . [Google Scholar]
- 7.Harinarayan CV, Joshi SR. Vitamin D status in India – its implications and remedial measures. J Assoc Physicians India. 2009;57:40–8. [PubMed] [Google Scholar]
- 8.Aggarwal N, Raveendran A, Khandelwal N, Sen RK, Thakur JS, Dhaliwal LK, et al. Prevalence and related risk factors of osteoporosis in peri- and postmenopausal Indian women. J Midlife Health. 2011;2:81–5. doi: 10.4103/0976-7800.92537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyritis G, Marin F, Barker C, Pfeifer M, Farrerons J, Brixen K, et al. Back pain during different sequential treatment regimens of teriparatide: Results from EUROFORS. Curr Med Res Opin. 2010;26:1799–807. doi: 10.1185/03007995.2010.488516. [DOI] [PubMed] [Google Scholar]
- 10.Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87:4528–35. doi: 10.1210/jc.2002-020334. [DOI] [PubMed] [Google Scholar]
- 11.McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165:1762–8. doi: 10.1001/archinte.165.15.1762. [DOI] [PubMed] [Google Scholar]
- 12.Songpatanasilp T, Mumtaz M, Chhabra H, Yu M, Sorsaburu S. Back pain in patients with severe osteoporosis on teriparatide or antiresorptives: A prospective observational study in a multiethnic population. Singapore Med J. 2014;55:493–501. doi: 10.11622/smedj.2014120. [DOI] [PMC free article] [PubMed] [Google Scholar]


