Abstract
Background:
Postmenopausal osteoporosis affects large fraction of elderly women. Oxidative stress (OS) appears to be involved in its pathogenesis. The scarcity of human studies focusing on the correlation between bone mineral density (BMD) and OS in postmenopausal women has prompted us to study on this issue.
Materials and Methods:
We conducted a cross sectional study in 95 subjects, between 21–65 years of age, including postmenopausal osteoporotic females (n = 35), healthy postmenopausal females (n = 30) and healthy females in reproductive age group (n = 30). We measured serum antioxidant activity of superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and total antioxidant power (TAP). BMD was obtained at lumbar spine and femur neck by dual-energy X-ray absorptiometry scan. Osteoporosis was considered when subjects had a BMD of 2.5 standard deviations or more below the mean value for young adults.
Results:
Serum GPx, SOD, catalase and TAP level were found significantly lower in osteoporotic postmenopausal group as compared to healthy postmenopausal women and women in healthy reproductive age group healthy reproductive women (P < 0.005). but correlation between BMD and serum antioxidants were not found to be statistically significant (P > 0.005).
Conclusion:
These findings support that oxidative stress plays an important role in pathogenesis of postmenopausal osteoporosis. We did not find any significant association between BMD and serum level of antioxidants (P > 0.05). The failure to detect this association does not preclude the role of OS in osteoporosis because OS is complex and dynamic process.
Keywords: BMD, correlation, oxidative stress, postmenopausal women
INTRODUCTION
Bone is a dynamic organ that undergoes continuous remodeling by the coordinated, and balanced, resorption and formation activities of, osteoclasts and osteoblasts, respectively. The balance between these processes shifts at menopause and women typically undergo a rapid phase of bone loss that begins approximately 2–3 years before the cessation of menses and continues for up to 5 years postmenopause. In addition, factors influencing peak bone mass and loss, range from nutrition, to lifestyle, to certain medical disorders.[1]
Osteoclasts are bone-resorbing multinucleated cells which are derived from macrophage-monocyte lineage progenitors. Osteoclast differentiation is dependent upon the interaction of myeloid preosteoclast precursors with either osteoblasts or stromal cells and it is influenced by a wide range of local factors including local hypoxia.[2] Hypoxia is prominent in the microenvironment in both bony and soft tissue injury. Recently, it was reported that anoxia inhibits the osteogenic differentiation of pluripotent mesenchymal precursors. In addition, it has been reported that hypoxia is a major stimulator of osteoclast formation and bone resorption.[3]
Oxidative stress (OS) is defined as the imbalance between the production of free radicals, in particular reactive oxygen species (ROS), and the capacity of disposing them through antioxidants. It leads to progressive accumulation of OS related damage in cells. This process is inevitably linked to aging and appears to be involved in the onset of several diseases affecting the elderly.[4] In the female population, postmenopausal individuals are regarded as more vulnerable to OS than those in reproductive age, because their oxidative balance is deranged not only by the generally older age but also by a lower level of 17 β–estradiol (E2), believed to act as an antioxidant.[5] Consistently, a considerable amount of in vitro evidence suggests that ROS could be involved in the pathogenesis of postmenopausal osteoporosis (PO), which is characterized by increased bone loss and consequent higher risk of fractures.[6] In spite of the remarkable progresses achieved in the understanding of how estrogen deficiency induces PO, the underlying pathogenic mechanisms have been found to be complex and multifaceted. One of the most intriguing hypotheses at this regard considers the ability of these sexual hormones to protect bone against OS by acting as antioxidant. Another mechanism suggested takes into account the ability of E2 to regulate receptor activator of Nuclear Factor-kappa B (NF-κB) ligand (RANKL)-RANK osteoprotegerin pathway. RANK along with OS are, in turn, potent activators of NF-κB, the osteclastogenic factor that regulates osteoclast differentiation and thus bone resorption and remodeling.[6]
In this perspective, estrogen deprivation would seem to act as the main trigger for the apparent bone erosive effect of increasing systemic oxidative challenge in our postmenopausal women. Further in vitro experiments on human osteoclast-like cell lines and animal experiments showing that E2 enhances the intracellular antioxidant defenses. It suggests that estrogenic skeletal protection occurring in premenopausal period of female life might be ascribed, at least partly, to the opposing activity of these hormones toward OS development.[7] This challenging body of evidence prompted us to investigate whether, also in vivo, OS might be an influencing factor for the bone turnover impairment underlying PO development. The research work undertaken in this thesis focuses on correlation between bone mineral density (BMD) and OS, in women experiencing two phases of life, reproductive age and postmenopause, characterized by different physiological level of endogenous 17β-E2. For better understanding of these relationships, the following sections include a discussion of bone biology, PO and OS.
MATERIAL AND METHODS
Study design
Cross-sectional, prospective study.
Sample size
Totally, 95 healthy females were included comprising both reproductive and postmenopausal phase. The study was done, January 2013 to October 2014 at Jawaharlal Nehru Medical College Hospital (JNMCH), Aligarh Muslim University (AMU), Aligarh. Younger subjects were enrolled among employees of the Hospital/University and their family members. The sample subjects were recruited among women undergoing bone densitometry test at the Rajiv Gandhi Centre for Diabetes and Endocrinology, JNMCH, AMU, Aligarh.
According to recent ReSTAGE's modification of stages of reproductive aging workshop staging criteria.[8] Women with regular menstrual cycle were grouped as reproductive age group healthy reproductive women (HRA). Women with periods of amenorrhoea >12 months were grouped as postmenopausal women.
Inclusion criteria
Eligible participants were apparently healthy women aged between 21 and 65 years. Initial evaluations were made by detailed questioner that included demographic information, medical and reproductive history, main life style habits, hormone therapy and diet. All subjects were divided in three groups; osteoporotic postmenopausal females (OPOST), healthy postmenopausal females (HPOST), and healthy females of reproductive age group HRA.
Exclusion criteria
Pregnancy, lactation, perimenopausal status, pharmacological and hormone therapies, alcohol abuse, supplementation with nutritional antioxidants, chronic pathologies (e.g. diabetes, hypertension, malabsorption, etc.) and secondary osteoporosis (e.g. hyperparathyroidism) or other bone diseases.
The study was approved by institutional ethics and research advisory committee, faculty of Medicine, AMU, Aligarh.
17 β-estradiol was measured by radioimmune assay. It is a competition assay. Samples and calibrators are incubated 3 h with I-labeled E2, as tracer, in antibody coated tubes. After incubation, the content of tubes is aspirated and bound radioactivity is measured. A calibration curve is established and unknown values are determined by interpolation from the curve.
Follicle stimulating hormone (FSH) determination was determined by immunoradiometric assay. It is a sandwich type assay. In the kit (FSH IRMA KIT, Beckman Coulter), mouse monoclonal antibodies directed against two different epitopes of FSH and hence not competing are used. Samples or calibrators are incubated in tubes coated with the first monoclonal antibody in presence of the second monoclonal antibody labeled with iodine 125. After incubation, the contents of tubes are rinsed so as to remove unbound I-125 labeled antibody. The bound radioactivity is then determined in a gamma counter. The FSH concentrations in samples are obtained by interpolation from the standard curve. The concentration of FSH in sample is directly proportional to radioactivity.
Bone densitometry assessment
Areal bone density was assessed at lumbar spine and femur neck by Discovery Dual Energy X-ray Absorptiometry Scanner (GE Lunar DEXA by Wipro GE Healthcare Pvt Ltd, USA supplied by M/s. Wipro GE Healthcare, 1st Floor, Block-C, Vipul's Tech Square, Golf Course Road, Sector 43, Gurgaon, Haryana, India). PO was diagnosed when BMD T-score (the number of standard deviations below the average for a young adult at peak bone density) was lower than 2.5 standard deviation from BMD peak at either femoral neck or lumbar spine, according to WHO guidelines. In accordance with these criteria, women with T-score at either skeleton area between −2.5 and −1.0 were classified as osteopenic and those with a value higher than −1.0 as normal.
Endogenous antioxidant markers
Estimation of catalase[9]
This enzyme catalyzes following reactions

The decomposition of H2 O2 can be followed directly by the decrease in extinction at 240 nm (E240 = 40 cm2 /U moles). The difference in extinction per unit time is the measure of the catalase activity/mg of protein (227). Reagents used were 50 mM potassium phosphate buffer (pH = 7.0) and 30 mM H2 O2 (prepared in potassium phosphate buffer).[7]
Glutathione peroxidase assay
The activity of glutathione peroxidase (GPx) was measured as described elsewhere (Mohandas et al., 1984; Mates et al., 1999; Hasan et al., 2006).[10,11,12] The oxidized glutathione (GSSG) produced during GPx reaction was immediately reduced by nicotinamide adenine dinucleotide phosphate (NADPH) and glutathione reductase. Therefore, the rate of NADPH consumption was regarded as the rate of GSSG formation during the GPx reaction. The reaction mixture (1.0 ml) containing 50 mM potassium phosphate (pH 7.0), 1 mM sodium azide, 2 mM GSH, 0.2 mM NADPH, 1 unit/ml glutathione reductase, 1.5 mM cumene hydroperoxide, and 20-100 μl of samples were incubated at 25°C for 5 min. The reaction was initiated by the addition of cumene hydroperoxide. The kinetic change was spectrophotometrically recorded at 340 nm (37°C) for 3 min. GPx activity was calculated after subtraction of the blank value, as μmol of NADPH oxidized/min/mg protein (U/mg protein).[8,9,10]
Determination of superoxide dismutase levels
Superoxide dismutase (SOD) activities were evaluated by spectrophotometer as described by Splitz and Oberley, 1989. In this competitive inhibition assay, superoxide generated by xanthine–xanthine oxidase is detected by monitoring the reduction of nitroblue tetrazolium at 505 nm. Total SOD activity was measured at pH 7.8 in Tris–HCl 0.2 M and Cu2+ Zn2+ SOD activity was measured at pH 10.2 in Tris-HCl 0.2 M. Standard curve was performed using human SOD (Sigma) at different concentrations (0.1, 0.25, 0.5, 1, 2 U/ml). One unit of activity was defined as the amount of protein that yields 50% of maximal inhibition of nitroblue tetrazolium reduction by superoxide. The results were reported as units of SOD per mg of proteins or units of SOD per mg of protein.[13]
Determination of total antioxidant power
The measurement of the ferric reducing ability of plasma (FRAP) was done by the assay based on the method of Jansen and Ruskovska (2013) as well as of Benzie and Strain (1996), which was slightly modified. The method is based on the principle of the reduction of the ferric-tripyridyltriazine complex to the ferrous form, upon which an intense blue color develops, and the change of absorbance is measured at 593 nm (kinetic method). FRAP assay was measured in a microplate format in microtiter plates, by the end-point approach. Briefly, 10 μL of sample and 40 μL of water were pipetted in the microplate in duplicate. After that, 200 μL of working reagent were added in each well (а: Acetate buffer pH 3, 6; b: FeCl3 solution; c: 2, 4, 6,-tripyridyl-s-triazine solution; 10:1:1), and the reaction mixture was incubated for exactly 8 min at 37°C. As the assay was carried out on microplate format as said above, thus the absorbance was measured on a enzyme-linked immunosorbent assay reader at 600 nm, against a reagent blank. Standards of 500, 1,000 and 2,000 μmol/L FeSO4 were used for calibration of the assay. The results of the test are expressed as μmol/L FeSO4.[14,15]
Statistical analysis
Data were analyzed using SPSS 18.0 for Windows (IBM, Chicago, IL, USA). Continuous variables were first analyzed for the normal distribution, because the distribution of lumbar spine and neck BMD and serum antioxidants level were highly skewed, we used One-way analysis of variance and of covariance for unequal variances (implemented with Bonferroni post-hoc test) to compare more than two groups at a time. These were used to evaluate the difference between sample groups before and after adjustment for confounding factors, respectively. Preliminary multiple regression analyses were performed to evaluate the possibility of collinearity problem among variables to include as covariates in multivariate analysis. Finally, univariate (by Pearson's correlation test) and multivariate (by partial correlation or multiple regression) analyses were performed to check the associations between continuous variables. A two-tailed P < 0.05 was considered statistically significant.
OBSERVATIONS AND RESULTS
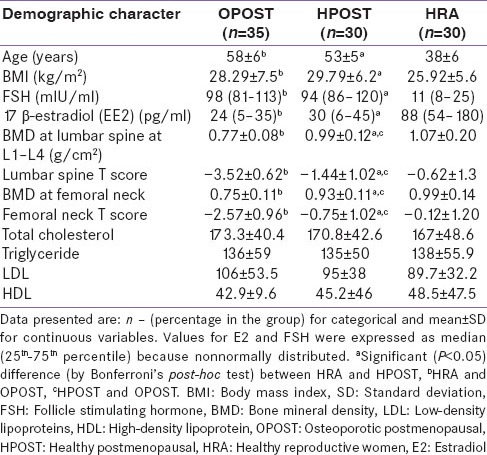
Women in OPOST and HPOST were older than HRA women (P < 0.001). Women in older age group OPOST and HPOST have significantly (P < 0.001) more body mass index (BMI) than women in reproductive age group. Women in HPOST group have significantly more BMI than in OPOST (P < 0.01). Serum level 17β-E2 and FSH were expressed as median (25th–75th percentile). E2 and FSH were higher and lower, respectively, in HRA with respect to the other two groups (P < 0.001 for both) [Table 1]. BMD and correspondent T score were significantly decreased at lumbar spine and at femur neck in OPOST women with respect to the values detected in other two groups (P < 0.001), with means significantly lower in HPOST women compared to healthy reproductive females HRA (P < 0.01) [Table 1]. Difference in mean total cholesterol, mean triglyceride, mean low-density lipoproteins (LDL), and mean high-density lipoproteins level in postmenopausal women (OPOST and HPOST) and women in healthy reproductive age group was not statistically significant (P > 0.05) [Table 1].
Table 1.
Demographic characters of study population (n=95)
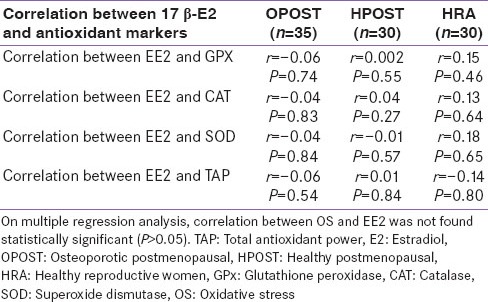
Serum level of GPx, Catalase, SOD and total antioxidant power were significantly lower in OPOST group as compared to other two groups (P < 0.001) and significantly higher in HPOST than HRA group (P < 0.01) [Table 2]. Serum level of endogenous antioxidant markers in OPOST, HPOST, and HRA groups showed no significant correlation with BMD [Table 3]. The role of antioxidant markers as predictor of BMD in all the groups were also assessed by considering age and BMI as confounding factors. No correlation was found on multiple regression analysis [Table 4]. Serum levels of 17β-E2 and antioxidant markers were also assessed in all the three groups and no correlation between OS and E2 was found [Table 5].
Table 2.
Serum level of endogenous antioxidant markers in OPOST, HPOST and HRA
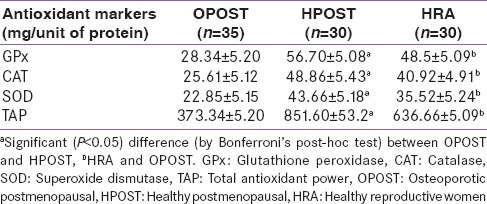
Table 3.
Correlation between BMD and serum antioxidant level in study subjects (n=95)
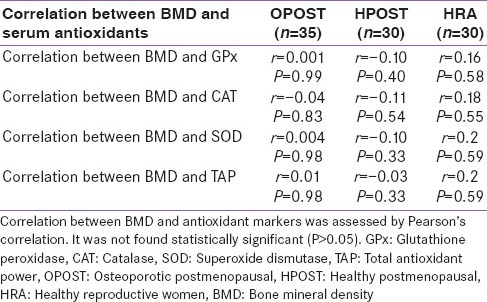
Table 4.
Role of antioxidant markers as predictor of BMD in study subjects (n=95) (By considering age and BMI as confounding factors)
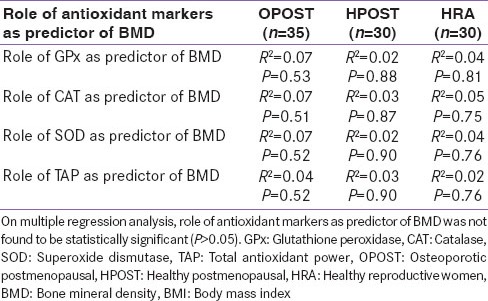
Table 5.
Correlation between 17 β-E2 and antioxidant markers in study subjects (n=95)
DISCUSSION
We observed that women in postmenopausal osteoporotic group have significantly lower BMD as compared to women in healthy reproductive age group HRA (P < 001). Furthermore, women in the OPOST group and HPOST group being older than HRA group have significantly more BMI (P < 0.001). The 17β-E2 level in the serum expressed as median was higher in HRA than in HPOST and OPOST groups [Table 1]. The OS was assessed by antioxidant enzymes: GPx, SOD and catalase in the serum. The serum levels of these antioxidant enzymes were significantly lower in OPOST group as compared to HPOST and HRA groups of patients (P < 0.001) [Table 2]. However, the correlation between BMD and serum antioxidant levels [Table 3] and total antioxidant power (TAP) was not found to be statistically significant [Table 4]. We also did not observed any significant correlation of 17β-E2 and serum antioxidant levels [Table 5]. In this preliminary observation we have measured all major endogenous antioxidants level. The failure to detect the association between BMD and serum levels of antioxidants does not preclude the role of OS in osteoporosis because it is a complex and dynamic process and there are several confounding factors like nutritional status, life-style and exercise, markers of oxidative damage (lipid peroxidation marker), which were not accessed in this study.
Bone is metabolically active, and undergoes continuous remodeling by the coordinated and balanced, resorption and formation activities of osteoclasts and osteoblasts, respectively.[14] The estrogen decline occurring in women after menopause leads to derangement of this homeostasis, with an increase of bone turnover rate and a state where resorption exceeds formation.[15] These metabolic changes underlie the onset of PO characterized by low BMD and predisposing to postmenopausal women to increased skeleton fragility and risk of fracture.[15] The underline pathogenesis mechanism responsible for PO to estrogen deficiency is complex and poorly understood. One of the most intriguing hypotheses in this regard considers the ability of these sex hormones to protect bone against OS by acting as antioxidant.[16] OS is a biochemical disequilibrium propitiated by excessive production of free radicals and ROS, which provoke oxidative damage to biomolecules which cannot be counteracted by antioxidative systems. ROS are oxygen-containing molecules that are produced during normal metabolism.[17] This process is inevitably linked to aging and appears to be involved in the onset of several diseases affecting the elderly. In the female population, postmenopausal individuals are regarded as more vulnerable to OS than those in reproductive age, because their oxidative balance is deranged not only by the generally older age but also by a lower level of 17β-E2, believed to act as an antioxidant.[18] There is evidence that ROS are involved in bone resorption, with a direct contribution of osteoclast-generated superoxide to bone degradation. In addition, it has been demonstrated that osteoblasts produce antioxidants like GPx to protect against ROS.[19]
In the frame of recent interest regarding the effects of OS on the onset of menopause-related disturbances,[5] we observed that women in postmenopausal osteoporotic group have significantly lower BMD as compared to healthy women (P < 0.001, Table 1). This was consistent with the results of Cervellati et al., 2012.[7]
Aging can be regarded as a factor influencing the bone susceptibility to OS challenge, since it is involved in most menopause-related metabolic modifications and diseases,[18] and it affects antioxidants effectiveness.[20] However, a determinant role of aging in triggering the interaction between BMD and OS does not clearly emerge from our data, since the multivariate analysis [Table 4] showed that this interaction was independent of postmenopausal women's age. This was consistent with finding of Carlo Cervellati et al., 2012.[7] On the other hand, these statistical outcomes do not allow us to definitely rule out that aging might contribute to promoting OS related bone loss, because of its strict implication in mechanisms underlying the accumulation of oxidative damages in all body tissues.[21,23]
Women in HPOST group have BMI higher than in OPOST group. This was consistent with finding of Carlo Cervellati et al., 2012.[7] Overweight (BMI ≥ 27) has been postulated as a protective factor for osteoporosis,[23] due to increase in the amount of biologically available estrogens. This is due to the conversion of androstenedione into estrone in adipose cells, and decrease in the concentration of sex hormone-binding globulins.[24] But, a determinant role of BMI in triggering the interaction between BMD and OS does not clearly emerge from our data, since the multivariate analysis showed that this interaction was independent of BMI (P > 0.05) [Table 4].
The estrogen deprivation would seem to act as the main trigger for the apparent bone erosive effect of increasing systemic oxidative challenge in our postmenopausal women, possibly independently of the antioxidant proprieties of E2 consistent with Cervellati et al., 2012,[7] Pansini et al.,(2008),[5] Ozgocmen et al., (2007).[25] Indeed, if 17β-E2 has an antioxidant action against systemic oxidative damage (as in the in vitro inhibition of LDL oxidation), this is usually obtained only at supra-physiological levels of hormone.
Oxidative stress was assessed by serum level of antioxidant enzymes, the markers of antioxidant defense mechanism for bone resorption. The low serum GPx level found in OPOST as compared to HPOST is consistent with previous studies Martha A Sanchez–Rodriguez et al. 2007,[20] Sontakke et al.,[21] and Maggio et al.,[22] They have reported that the activity of GPx in plasma was significantly lower in postmenopausal osteoporotic women. Dreher et al.[23] claimed that missing or decreased expression of GPx could result in impaired osteoblast function and consecutive development of bone diseases such as osteoporosis. One of our important results was that the correlation between BMD and GPx was not found to be statistically significant (P > 0.05). This was not consistent with results of Hahn et al., 2008.[24] The disparity in the correlation between BMD and GPx observed in the present study can be explained by the dynamic nature of the OS which is a complex process and several confounding factors are implicated and were not accessed in this study.
The observed lower serum levels of SOD in the OPOST as compared to HPOST and HRA groups is in accordance with previous studies Sontakke et al.,[21] and Maggio et al.,[22] but not with Martha A Sanchez-Rodriguez et al., 2007,[20] in which no significant difference was seen in its level in osteoporotic and HPOST. No significant correlation was found between BMD and SOD level which is consistent with Martha A Sanchez-Rodriguez et al., 2007.[20]
We found lower serum levels of catalase in OPOST as compared to HPOST and HRA which is consistent with result of Ozgocmen et al.,2007.[25] No significant correlation was found between BMD and catalase level which is not consistent with results of Ozgocmen et al. 2007.[25] There are only few studies in which catalase has been assessed as marker for antioxidant status.
Total serum Antioxidant Power which was lower in OPOST as compared to HPOST and HRA is consistent with results of Carlo Cervellati et al., 2012[7] and Sanchez- Rodriguez et al.,2007.[20] These are the only two studies in which TAP was analyzed to assess the role of OS in osteoporosis.
CONCLUSION
This study concludes that women in OPOST group have significantly more OS compensated by endogenous antioxidants leading to significantly low level of these antioxidants. The observation supports that OS play an important role in the pathogenesis of postmenopausal osteoporosis. We proposed that markers of oxidative damage including lipid peroxidation marker, BMD, OS marker, antioxidant enzyme levels, and various confounding factors must be considered to evaluate definite role of OS in postmenopausal osteoporosis.
LIMITATION
Some important limitations of the study must be acknowledged. The design of the study was cross-sectional. It precludes our ability to establish any temporal relationship between OS and BMD. In future longitudinal study, involving middle-aged healthy reproductive HRA women, should be done in which subjects should be followed up until 5 years after occurrence of menopause, with periodic estimations of oxidative, (E2 and FSH) and bone status through assessment of BMD and blood markers of bone turnover useful to monitor alterations in rates of bone resorption and formation. Lack of full nutritional assessment of the study subjects makes a correct evaluation of TAP results difficult, which, in turn, depends on ROS-dependent depletion or scarce dietary intake of antioxidants. In future studies, full nutritional assessment along with calcium and Vitamin D intake may also be taken in consideration.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
REFERENCES
- 1.Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem. 2004;279:40007–16. doi: 10.1074/jbc.M403715200. [DOI] [PubMed] [Google Scholar]
- 2.Gur A, Sarac AJ, Nas K, Cevik R. The relationship between educational level and bone mineral density in postmenopausal women. BMC Fam Pract. 2004;5:18. doi: 10.1186/1471-2296-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, et al. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989;125:1805–13. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- 4.Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des. 2004;10:1611–26. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- 5.Pansini F, Mollica G, Bergamini CM. Management of the menopausal disturbances and oxidative stress. Curr Pharm Des. 2005;11:2063–73. doi: 10.2174/1381612054065819. [DOI] [PubMed] [Google Scholar]
- 6.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: An inflammatory tale. J Clin Invest. 2006;116:1186–94. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervellati C, Bonaccorsi G, Cremonini E, Bergamini CM, Patella A, Castaldini C, et al. Bone mass density selectively correlates with serum markers of oxidative damage in post-menopausal women. Clin Chem Lab Med. 2013;51:333–8. doi: 10.1515/cclm-2012-0095. [DOI] [PubMed] [Google Scholar]
- 8.Harlow SD, Crawford S, Dennerstein L, Burger HG, Mitchell ES, Sowers MF, et al. Recommendations from a multi-study evaluation of proposed criteria for staging reproductive aging. Climacteric. 2007;10:112–9. doi: 10.1080/13697130701258838. [DOI] [PubMed] [Google Scholar]
- 9.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. London: Academic Press; 1974. pp. 671–84. [Google Scholar]
- 10.Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res. 1984;44:5086–91. [PubMed] [Google Scholar]
- 11.Matés JM, Segura JM, Pérez-Gómez C, Rosado R, Olalla L, Blanca M, et al. Antioxidant enzymatic activities in human blood cells after an allergic reaction to pollen or house dust mite. Blood Cells Mol Dis. 1999;25:103–9. doi: 10.1006/bcmd.1999.0234. [DOI] [PubMed] [Google Scholar]
- 12.Hasan N, Yusuf N, Toossi Z, Islam N. Suppression of Mycobacterium tuberculosis induced reactive oxygen species (ROS) and TNF-alpha mRNA expression in human monocytes by allicin. FEBS Lett. 2006;580:2517–22. doi: 10.1016/j.febslet.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 13.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 14.Jansen EH, Ruskovska T. Comparative analysis of serum (Anti) oxidative Status Par?meters in Healthy Persons. Int J Mol Sci. 2013;14:6106–15. doi: 10.3390/ijms14036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 16.Masi L, Brandi ML. Physiopathological basis of bone turnover. Q J Nucl Med. 2001;45:2–6. [PubMed] [Google Scholar]
- 17.Lindsay R. Estrogen deficiency. In: Riggs BL, Melton LJ, editors. Osteoporosis: Etiology, Diagnosis, and Management. 2nd ed. Philadelphia, Pa, USA: Lippincott-Raven; 1996. pp. 133–60. [Google Scholar]
- 18.Cervellati C, Bonaccorsi G, Cremonini E, Bergamini CM, Patella A, Castaldini C, et al. Bone mass density selectively correlates with serum markers of oxidative damage in post-menopausal women. Clin Chem Lab Med. 2013;51:333–8. doi: 10.1515/cclm-2012-0095. [DOI] [PubMed] [Google Scholar]
- 19.Galli F, Piroddi M, Annetti C, Aisa C, Floridi E, Floridi A. Oxidative stress and reactive oxygen species. Contrib Nephrol. 2005;149:240–60. doi: 10.1159/000085686. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Rodriguez MA, Ruiz-Ramos M, Correa-Munoz E, Mendoza-Nunez VM. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet Disord. 2007;8:124. doi: 10.1186/1471-2474-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sontakke AN, Tare RS. A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin Chim Acta. 2002;318:145–8. doi: 10.1016/s0009-8981(01)00766-5. [DOI] [PubMed] [Google Scholar]
- 22.Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88:1523–7. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- 23.Dreher I, Schütze N, Baur A, Hesse K, Schneider D, Köhrle J, et al. Selenoproteins are expressed in fetal human osteoblast-like cells. Biochem Biophys Res Commun. 1998;245:101–7. doi: 10.1006/bbrc.1998.8393. [DOI] [PubMed] [Google Scholar]
- 24.Hahn M, Conterato GM, Frizzo CP, Augusti PR, da Silva JC, Unfer TC, et al. Effects of bone disease and calcium supplementation on antioxidant enzymes in postmenopausal women. Clin Biochem. 2008;41:69–74. doi: 10.1016/j.clinbiochem.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Ozgocmen S, Kaya H, Fadillioglu E, Aydogan R, Yilmaz Z. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem. 2007;295:45–52. doi: 10.1007/s11010-006-9270-z. [DOI] [PubMed] [Google Scholar]


