Abstract
Context:
Gestational diabetes mellitus (GDM) should be diagnosed early, as untreated maternal hyperglycemia leads to adverse materno-fetal outcome.
Subjects and Methods:
We studied prevalence of gestational diabetes by a house to house survey of a rural population in western India. A cluster of remote villages with little access to health care were chosen.
Results:
A total of 989 women participated in the study out of which 9.5% (n = 94) were diagnosed as GDM. In a stepwise multivariate regression analysis a higher body mass index during pregnancy was a predictor of gestational diabetes. The prevalence of diabetes was similar in women with gestational age of >24 weeks and <24 weeks, suggesting the need for early screening.
Conclusions:
Our results suggest the need for implementing health programs to diagnose and treat gestational diabetes in this population.
Keywords: Fetus, hyperglycemia, mother, pregnancy, prevalence
INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as carbohydrate intolerance of varying severity with onset or first recognition during pregnancy.[1] The diagnosis of gestational diabetes is important, because of the increased risk of adverse maternal and feto-neonatal outcomes. In addition, GDM also confers a future risk of type 2 diabetes to mothers and their fetuses.[2]
Gestational diabetes mellitus affects about 7% of all pregnancies worldwide and recent studies have reported an increase in the prevalence in last two decades.[3,4,5,6,7] In India, the prevalence ranges from 6% to 9% in rural and 12-21% in urban areas, with most studies being done in either South or North India.[8,9,10,11]
A vast number of people with diabetes in India live in rural areas with inadequate access to health care, lack of awareness, lack of a balanced nutrition and limited opportunities for exercise despite increasing economic growth. Increasing mechanization and urbanization of rural populations has been associated with an increasing occurrence of type 2 diabetes in rural areas of India too.[12] Understanding prevalence of GDM in this particular population will help in directing the appropriate resources for care of mothers and children affected by this disorder. In this study, we evaluated the prevalence of GDM in rural areas of Pune district in Maharashtra. We specifically selected remote areas, with little access to health care or screening facilities.
SUBJECTS AND METHODS
Identification of the population
The program was carried out during September 2012 to June 2014. We identified remote villages of Pune district, Maharashtra with a population ranging from 1,500 to 4,500 each. The farthest village from the Pune city (Junnar) was identified and villages in its proximity were included in succession. The program extended to include villages from different blocks of Pune district, at least at a distance of 70 km or more from Pune city. Since villages closer to the city have access to secondary and tertiary care medical facilities only those villages that lacked adequate healthcare facilities were chosen. Lack of an adequate healthcare facility was defined as the nearest health care facility for diabetes care (including a glucometer) being >25 km away. With the collaboration of District Public Health Department, the Accredited Social Health Activist (ASHA) of the each village were contacted. After a day-long structured training (which was organized at the nearest primary health center), ASHA helped to identify all the pregnant women in the Villages via a door to door survey. A total of 349 villages were screened as a part of a larger ongoing population based program with a cumulative population of 8,71,588. A total of 5,200 pregnant women (defined as missed menstrual period followed by positive urine human chorionic gonadotropin test for pregnancy) were invited for oral glucose tolerance testing (OGTT) which was performed under supervision of a team of health care providers.
Diagnosis of gestational diabetes mellitus
Medical and obstetric history, family history, anthropometric measurements and blood pressure were recorded using standard protocols. One step OGTT with a single glycemic value suggested by Diabetes in Pregnancy Study Group (DIPSI) to screen as well as confirm GDM was used to categorize the subjects.[13,14] The test involved estimating capillary glucose value 2 h after 75 g glucose load irrespective of the gestational age and the time from the last meal. This procedure assumes that glucose concentrations are little affected by the time since the last meal in a normal glucose tolerant woman due to brisk and adequate insulin response, whereas the glucose concentrations would rise in women with gestational diabetes.[13,14]
The women were categorized as follows depending on the values of the 2 h post 75 g glucose capillary OGTT: Normal (<120 mg/dl), gestational glucose intolerance (120-139 mg/dl), GDM (140-199 mg/dl) and overt diabetes first detected during gestation (≥200 mg/dl), as per the previously published DIPSI Criteria.[14] For purposes of defining gestational diabetes in this study, therefore, a cut-off of 140 mg/dl or above was applied. Gestational hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg in a pregnant woman.[15]
Statistical analysis
The statistical analysis was carried out using Statistical Package for Social Studies (SPSS, Inc, Chicago, IL, USA) for Windows version 18.0. Categorical data are presented as n (%) Normally distributed data are presented as means and standard deviation or 95% confidence interval (CI). Pearson′s Chi-square test was used to evaluate differences between groups for categorical variables. Independent sample t-test was used to test the differences of quantitative variables across two study groups after confirming the underlying normality assumption. The P < 0.05 was considered to be statistically significant. Multivariate logistic regression analysis (stepwise method) was used to evaluate the independent determinants of prevalence of GDM.
RESULTS
A total of 989 pregnant women participated in the study. The overall prevalence of GDM in this population was 9.5% (94/989). The characteristics of the population are presented in Table 1.
Table 1.
Characteristics of the study population
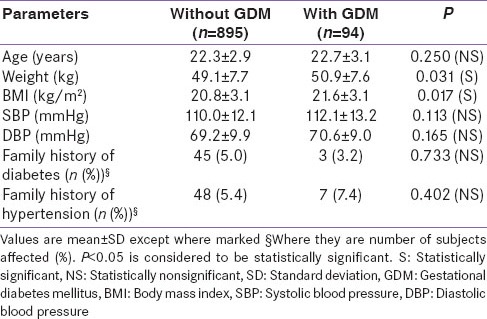
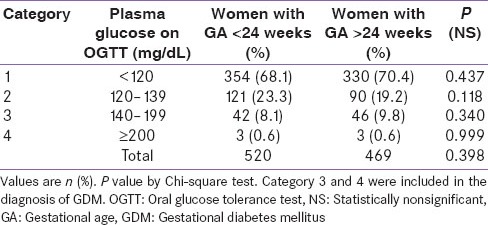
The mean plasma glucose after OGTT was 107.3 ± 16.1 mg/dL in women without GDM and 164.3 ± 42.7 mg/dL in women with GDM. There was no statistically significant difference in the maternal age, family history of diabetes or hypertension and prevalence of gestation hypertension between women with GDM and women without GDM. The prevalence of GDM prior to 24 weeks of gestation (See Category 3 and 4 in Table 2) was 8.6% (45 out of 520 women) and that after 24 weeks of gestation was 10.4% (49 out of 469 women). The weight and body mass index (BMI) was higher in pregnant women with GDM as compared to those without GDM. The proportional of subjects with an abnormal glucose value was similar throughout the pregnancy in both groups whether screened before and after 24 weeks of gestational period [Table 2].
Table 2.
Prevalence of an abnormal glucose value with reference to the GA
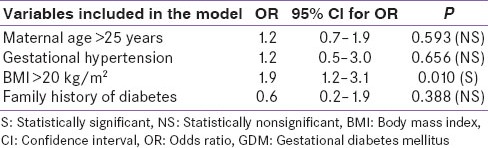
After stepwise multivariate logistic regression analysis for combined population, none of the parameters (maternal age, gestational hypertension, family history of diabetes) except BMI (P < 0.05) were found to have significant association with prevalence of GDM [Table 3].
Table 3.
OR for risk factors of GDM based on multivariate logistic regression analysis
DISCUSSION
We present the results of our community based survey of the prevalence of gestational diabetes from Western India. Importantly, it focuses on prevalence in remote areas where no medical care is available. Our study was spread over several villages, often in hilly terrains, and involved door to door visits by field workers to bring subjects for screening. Therefore we believe that our study is especially representative of the rural Indian community residing in remote areas.
Our results suggest that about 9.5% of pregnant women have gestational diabetes. The value of 9.5% is similar to the rural subgroup of pregnant women in a study by Seshiah et al. in South India.[9] The percentages of pregnant women with abnormal glucose tolerance in our study were similar irrespective of whether women were screened before or after 24 weeks of gestation. This suggests that pregnant women of India need to be screened routinely at the first anti-natal visit for presence of GDM. This high proportion of patients with gestational diabetes living in remote, rural parts of India, suggests the need for public health strategies to diagnose and manage GDM in this area.
On multiple logistic regression analysis, age, family history or gestational hypertension did not associate with the diagnosis of gestational diabetes. Given the lack of predictive value of common parameters like age and family history in our study, it seems reasonable to advise universal screening of all pregnant women, as is currently done. Pregnant women with gestational diabetes had significantly more weight and BMI than those without gestational diabetes. While this could have been attributed either to higher body weight before pregnancy, more weight gain during pregnancy or more macrosomy during pregnancy-our study was not designed to answer these questions. In addition to this, our study has several limitations, and a major one is that it did not record details like pre-pregnancy body weight (due to lack of available information on this by the subjects) or fetal weight estimation to answer this question. The relevance of the BMI in pregnancy is, by itself, a subject for debate.[16] An additional limitation was a very low rate of women turning up for OGTT compared to the number of subjects who were invited. This may be explained by poor socio-economic status, illiteracy, difficulties in reaching health-care team (some women needed to travel around 10 km to reach our health-care team) and lack of awareness about GDM in-spite of education by ASHA.
Another limitation of our study is the lack of follow up details available, in order to ascertain pregnancy outcome. Finally, a limitation of the study is the DIPSI criteria, which suggests the use of an OGTT irrespective of the last meal. The limitations of this criterion are well known.[11] However, given the difficulty of implementing the other fasting based protocols in the very remote locales of our study, we did not simultaneously perform and compare different diagnostic criteria for screening of GDM. Notably, many women had to walk long distances to reach our screening facility, and thus expecting a true fasting sample in these remote areas would have been nearly impossible.
Equally important but lacking in this study is the follow up of these women. It has been shown from a study of 597 rural women from Pune (Pune Maternal Nutrition Study) that women with a higher weight gain in pregnancy had a higher chance of eventually developing post-pregnancy hyperglycaemia.[17] Hence, in addition to diagnosing, treating and managing labor in gestational diabetes, it is equally important to institute lifestyle modifications, and periodic monitoring for these women.
To summarize, the high prevalence of gestational diabetes in our study suggests that women from remote, rural villages of India have a high prevalence of gestational diabetes. The result of our study calls for a targeted effort to bring proper diagnostic and therapeutic care to improve the lives of these women at risk of diabetes during pregnancy.
ACKNOWLEDGEMENT
We acknowledge the contribution from Mr. Rahul Bangude towards acquisition of the data.
Financial support and sponsorship
This survey is a part of an outreach diabetes program funded by the Chellaram Foundation. For more details log on to http://www.cdi.org.in/index.php/outreach.
Conflict of interest
There are no conflicts of interest.
REFERENCES
- 1.Metzger BE, Coustan DR. Summary and Recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–7. [PubMed] [Google Scholar]
- 2.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet. 2009;373:1773–9. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991-2000. Obstet Gynecol. 2004;103:526–33. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- 4.Ishak M, Petocz P. Gestational diabetes among Aboriginal Australians: Prevalence, time trend, and comparisons with non-Aboriginal Australians. Ethn Dis. 2003;13:55–60. [PubMed] [Google Scholar]
- 5.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28:579–84. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 6.Montana Department of Public Health and Human Services Chronic Disease Prevention and Health Promotion Program Trends in diabetes in pregnancy among American Indian and White mothers in Montana 1989-2003: An update. 2005;2:1–8. [Google Scholar]
- 7.Thorpe LE, Berger D, Ellis JA, Bettegowda VR, Brown G, Matte T, et al. Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990-2001. Am J Public Health. 2005;95:1536–9. doi: 10.2105/AJPH.2005.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zargar AH, Sheikh MI, Bashir MI, Masoodi SR, Laway BA, Wani AI, et al. Prevalence of gestational diabetes mellitus in Kashmiri women from the Indian subcontinent. Diabetes Res Clin Pract. 2004;66:139–45. doi: 10.1016/j.diabres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu) – A community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 10.Verma A, Singh B, Mengi V. Gestational diabetes in rural women of Jammu. Indian J Community Med. 2008;33:54–5. doi: 10.4103/0970-0218.39247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohan V, Mahalakshmi MM, Bhavadharini B, Maheswari K, Kalaiyarasi G, Anjana RM, et al. Comparison of screening for gestational diabetes mellitus by oral glucose tolerance tests done in the non-fasting (random) and fasting states. Acta Diabetol. 2014;51:1007–13. doi: 10.1007/s00592-014-0660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramachandran A, Snehalatha C. Current scenario of diabetes in India. J Diabetes. 2009;1:18–28. doi: 10.1111/j.1753-0407.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 13.Anjalakshi C, Balaji V, Balaji MS, Ashalata S, Suganthi S, Arthi T, et al. A single test procedure to diagnose gestational diabetes mellitus. Acta Diabetol. 2009;46:51–4. doi: 10.1007/s00592-008-0060-9. [DOI] [PubMed] [Google Scholar]
- 14.Seshiah V. Diabetes in Pregnancy Study Group. Fifth national conference of diabetes in pregnancy study group, India. J Assoc Physicians India. 2010;58:329–30. [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 16.Davies GA, Maxwell C, McLeod L, Gagnon R, Basso M, Bos H, et al. Obesity in pregnancy. J Obstet Gynaecol Can. 2010;32:165–73. doi: 10.1016/S1701-2163(16)34432-2. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni SR, Fall CH, Joshi NV, Lubree HG, Deshpande VU, Pasarkar RV, et al. Determinants of incident hyperglycemia 6 years after delivery in young rural Indian mothers: The Pune Maternal Nutrition Study (PMNS) Diabetes Care. 2007;30:2542–7. doi: 10.2337/dc07-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]


