Abstract
Sodium glucose transporter 2 (SGLT2) inhibitors are a recently developed class of drug that have been approved for use in type 2 diabetes. Their unique extra-pancreatic glucuretic mode of action has encouraged their usage in type 1 diabetes as well. At the same time, reports of pseudo ketoacidosis and ketoacidosis related to their use have been published. No clear mechanism for this phenomenon has been demonstrated so far. This communication delves into the biochemical effects of SGLT2 inhibition, discusses the utility of these drugs and proposes steps to maximize safe usage of the molecules.
Keywords: Canagliflozin, dapagliflozin, empagliflozin, good clinical sense, ketoacidosis, ketonuria, type 1 diabetes, type 2 diabetes
INTRODUCTION
Sodium glucose transporter 2 (SGLT2) inhibitors are a recently developed class of drug that have been approved for use in type 2 diabetes. Their unique extra-pancreatic glucuretic mode of action has encouraged their usage in type 1 diabetes as well. Many case reports and proof-of-concept studies have been published on the use of this class of drugs in type 1 diabetes. The following paragraphs describe some of the published results. At the same time, SGLT2 inhibitors are thought to promote ketogenesis and precipitate ketoacidosis. This article reviews the cases reported in the literature and assesses the possible etiopathogenesis of the metabolic derangement. The authors conclude with suggestions for pragmatic and safe use of SGLT2i in practice.
SAFETY IN TYPE 1 DIABETES
In a 2-week long, proof-of-concept study, doses of dapagliflozin, ranging from 1 to 10 mg/day were compared with placebo in 70 adults with type 1 diabetes, on treatment with stable doses of insulin. Sixty-two subjects completed the study, without experiencing any episode of diabetic ketoacidosis (DKA). One event of major hypoglycemia was reported. Average blood glucose, mean amplitude of glycemic excursion, and total daily insulin dose all declined in the dapagliflozin groups, though statistical significance could not be achieved.[1] In a similar, 8-week long study, using a standard dose of empagliflozin (25 mg/day), 40 subjects reported statistically significant reduction in HbA1C, fasting glucose, daily insulin dose, weight, and waist circumference. The incidence of symptomatic hypoglycemia increased with empagliflozin treatment. No episode of ketoacidosis was reported.[2]
In a study carried out on 40 type 1 diabetes adults, designed to assess renal hemodynamic effects, Cherney et al. reported a reduction(−33 ml/min/1.73 m2) glomerular filtration rate (GFR) in previously hyper filtrating individuals. This study supports the hypothesis that SGLT2i has a nephroprotective effect in persons with early nephropathy, which is characterized by an increase in GFR.[3]
Published case reports also support the use of SGLT2i in type 1 diabetes, observing better control, less hypoglycemia, weight loss, and lower insulin requirement with this adjunctive therapy.[4] Various authors have described pleiotropic, nonglycemic effects, such as on arterial stiffness and heart rate variability, in patients with type 1 diabetes.[5] The nephroprotective effects of SGLT2i are also been explored in current literature, and open up exciting vistas for research.[6,7,8] This has led many experts to suggest the potential of SGLT2i in type 1 diabetes.[9]
KETOACIDOSIS WITH SODIUM GLUCOSE TRANSPORTER 2 INHIBITION
In contradiction to studies on the efficacy and safety of SGLT2i in type 1 diabetes, case reports have been published about the occurrence of ketoacidosis and pseudo-ketoacidosis with SGLT2i use.
A Japanese report of ketoacidosis in a patient of Prader-Willi syndrome treated with ipragliflozin highlights interesting aspects of diabetes care.[10] This 32-year-old female, with a history of diabetes for 22 years, on a low-carbohydrate diet for 11 years, was being sub optimally treated with triple oral therapy: Glimepiride, metformin, and linagliptin. She was shifted from this treatment to monotherapy with ipragliflozin, perhaps not a prudent decision. The authors note the relatively low glycated albumin of their case, in spite of a high HbA1C, suggesting that SGLT2 inhibition had helped achieve normoglycemia over the preceding fortnight. While the total caloric intake was adequate (1860 cals/day), only14.3% of this was contributed by carbohydrates. The estimated carbohydrate consumption was 66 g/day (264 cal/day), further compromised by reduced appetite and water intake for 2 days.
The phenomenon of starvation ketosis is well-known. Ehrstrom, in fact, used the term “acetonuria of dipsophobes” nearly a century ago.[11,12] The authors’ observation regarding occurrence of ketosis in spite of a high calorie (but low carbohydrate) diet is syncretic with earlier evidence, which has discarded the calorie deficiency hypothesis of ketogenesis.[13] Ketogenesis is clearly known to be due to carbohydrate insufficiency at the cellular level, leading to forced utilization of fats as an energy substrate. Severe starvation ketoacidosis superimposed on diabetes mellitus is also reported in the literature.[14] Thus, the argument that starvation ketosis is not accompanied by acidosis is not valid.
Yet another case report, from USA, describes a 50-year-old woman with poor glycemic control, in whom canagliflozin 300 mg/day was added to a regime of glipizide and metformin.[15] This patient had reported current, severe gastrointestinal symptoms and 65 lbs weight loss, over 6 months, for which seemingly no action was taken. Starting a high dose of canagliflozin, instead of reducing metformin, initiating insulin, and stopping sulfonylurea, was perhaps not good clinical judgment.
Though ketonuria is not routinely assessed in most clinics, the symptoms that this case presented with suggest that she may have had ketonemia or ketonuria (through not ketoacidosis) prior to starting of SGL2i therapy, The abdominal symptoms persisted even after resolution of ketosis, leading one to consider local factors, like gastroparesis, rather than systemic ones. The authors of this case, too, suggest starvation as a cause of ketosis.
The authors of this case study observed persistent glycosuria till 11 days after cessation of canagliflozin therapy. The reason for delayed reversibility of SGL2i in this patient is uncertain. It is pertinent, however, to consider that relative to ambient glucose concentrations in circulation (which increased gradually, with parenteral supplementation), the glucose concentrations in urine did not increase over time. This means that relative to glycemia, glycosuria probably declined, albeit a bit more slowly than expected, over the course of the event. It was also reported nearly a century ago that correction of starvation ketosis by glucose is associated with abnormal glucose tolerance: There is an exaggerated rise and delayed return to normal, of blood sugar values, along with glycosuria.[11] This may be due to a temporary physiological rest enjoyed by the β-cell, as postulated a century ago.[11] Starvation affects glycogen metabolism, and it may take time for glycogenolysis to stop, after a period of prolonged starvation.[16]
An Indian case report uses the term “pseudo ketoacidosis,” to define an asymptomatic person presenting with glycosuria and ketonuria while on SGLT2i therapy. In this communication, the authors warn against the possibility of wrongly diagnosing ketoacidosis in SGLT2i treated patients, who will have significant glycosuria, due to the mode of action of their drug, and may develop ketonuria because of dehydration.[17] In Cherney's study on hemodynamic effects of empagliflozin, two subjects (out of 40) developed DKA and discontinued participation: These episodes were related to insulin pump failure and acute gastroenteritis (one each), and were not thought to be due to SGLT2 inhibition.[3]
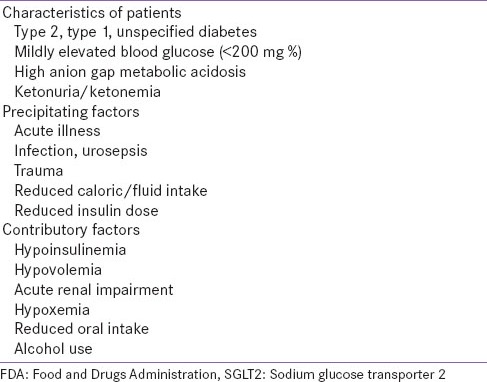
Recently, the Food and Drugs Administration (FDA) has reported 20 cases of ketosis or ketoacidosis with SGLT2i use.[18] While most of the patients enumerated in this list have type 2 diabetes, there are some with type 1 diabetes, or unspecified type of diabetes, who have been treated “off-label” with SGLT2 inhibitors. It is possible that some of these patients may have had late-onset autoimmune diabetes, but were wrongly diagnosed as [and treated as] type 2 diabetes. The FDA communiqué lists various precipitating and contributory factors which have been associated with reported episodes of acidosis or ketosis [Table 1]. These pointers serve as warning symptoms and signs of impending DKA, and should prompt initiation of insulin therapy. While most of these clinical situations warrant discontinuation of ongoing, or postponement of planned SGLT2i therapy, they also warrant the same action for almost all oral glucose-lowering medication.
Table 1.
FDA communication regarding ketoacidosis with SGLT2 inhibitor therapy
KETOGENIC MECHANISM
The foregoing discussion is not to suggest that SGLT2i do not have any ketogenic potential. Phlorizin, a combined SGLT2 and SGLT1 inhibitor, has been demonstrated to induce ketogenesis[19] SGLT2i acts to reduce the glucose substrate available to the body for energy utilization. In the event of any extra demand for glucose (e.g., pregnancy, starvation), or a sudden cessation of glucose supply (e.g., low carbohydrate diet, fasting), or lack of nutrient absorption (gastrointestinal upset), the SGLT2 inhibited body may not be able to maintain its homeostasis. After exhausting hepatic and muscular glycogen reserves, the body will have to shift to gluconeogenesis and adopt a ketogenic metabolic pathway. A minimum of 100 g carbohydrates are required daily to prevent ketosis.[20] Thus, SGLT2i should be avoided in persons who are unable to consume this amount of carbohydrates.
Sodium glucose transporter 2i also alters the insulin glucagon ratio.[21] The increase in glucagon levels results in relative insulinopenia which manifests as ketonuria when challenged with starvation and/or dehydration. High glucagon levels are associated with nausea, which may precipitate or worsen loss of appetite. It is pertinent to state here that dopamine antagonism, using metoclopramide, has been shown to suppress ketogenesis,[22] and may be a potential adjuvant therapy for starvation ketosis.
PRAGMATIC GUIDANCE
An assessment of all the publications reviewed above, suggests the following “good clinical sense”:
Place in therapy
Sodium glucose transporter 2i are an approved therapy for the management of type 2 diabetes. SGLT2i are being studied in type 1 diabetes with encouraging results.
Sodium glucose transporter 2i have a “catabolic” action upon the body, as evidenced by the reduction in body weight, which may be modulated by loss of calories through glucuresis, or by a decrease of the insulin glucagon ratio with their use.
Thus, SGLT2i should be a preferred therapy for diabetes with “maladaptive anabolism”, that is, obesity/overweight. It can comfortably be used in healthy, lean individuals as well. SGLT2i, however, may not be appropriate choices for patients with catabolic features, e.g. h/o extreme weight loss, or with symptoms suggestive of ketonuria, e.g. recurrent abdominal pain.
Use as monotherapy
Sodium glucose transporter 2i may be used as alternatives if metformin is contraindicated or not tolerated. There would be few clinical situations in which SGLT2i are allowed, while metformin is not. However, in case of gastrointestinal intolerance to metformin, SGLT2i do represent a viable oral option. Whenever SGLT2i therapy is initiated, a lower dose may be considered, especially if the patient complains of gastrointestinal side effects.
Use as 2nd/3rd line drug
When used as substitution therapy, it would be prudent to change one molecule for one at a time, e.g. substituting pioglitazone (perhaps), with SGLT2i. Substitution is indicated only if there is suboptimal tolerability, or a safety concern, with existing therapy. Lack of efficacy should prompt intensification of therapy, rather than the substitution of one oral glucose-lowering drug for another. It is not good clinical practice to substitute three ongoing drugs with an SGLT2i and expect to achieve good results.
When used as intensification or add-on therapy, the patients’ glucophenotype should be taken into consideration. At best, SGLT2i can be said to have an insulin dose-lowering effect. SGLT2i are not an alternative to insulin, especially not in a person with obvious catabolic features. Current guidelines clearly recommend that insulin can be used as initial therapy in such patients.
Counseling
Counseling regarding self-management of diabetes is important, and this should be explained to all patients. The contributory factors listed in the FDA communique, viz, infection, trauma, alcohol intake, reduced oral or liquid intake, are situations where medical advice must be sought. One must maintain regular fluid and nutrient intake, not only while on SGLT2i, but also while on any glucose-lowering therapy. The effect of SGLT2i on urine glucose levels should also be informed to patients (and other health care providers) so that unnecessary distress can be avoided.
SUMMARY
The publication of cases of starvation ketosis with SGLT2i therapy highlights the need for comprehensive education and awareness campaigns, targeting both physicians and people with diabetes. While the latter should be counseled to maintain regular healthy fluid and food intake, physicians must be informed about the importance of appropriate case selection, stepwise initiation of new therapy, gradual de-intensification of ongoing treatment, and recognition of symptoms/signs of ketosis.
The key to effective use of all glucose-lowering medication is appropriate patient selection, counseling, and follow-up. The availability of SGLT2i, a novel and useful class of drugs, has highlighted the need for the medical profession to introspect regarding the direction in which diabetes care is moving. In the current era of evidence-based medicine, we must not forget the need for “good clinical sense.” There is no algorithm or guideline or investigation which can replace common sense. It is good clinical sense which will ensure that we are able to translate pharmaceutical advances into clinical benefits for the people with diabetes who seek our advice.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
REFERENCES
- 1.Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: A randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38:412–9. doi: 10.2337/dc13-2955. [DOI] [PubMed] [Google Scholar]
- 2.Perkins BA, Cherney DZ, Partridge H, Soleymanlou N, Tschirhart H, Zinman B, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: Results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37:1480–3. doi: 10.2337/dc13-2338. [DOI] [PubMed] [Google Scholar]
- 3.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–97. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 4.Bell DS. Case reports that illustrate the efficacy of SGLT2 inhibitors in the type 1 diabetic patient. Case Rep Endocrinol. 2015;2015:676191. doi: 10.1155/2015/676191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. doi: 10.1186/1475-2840-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina-Jijón E, Rodríguez-Muñoz R, Namorado Mdel C, Pedraza-Chaverri J, Reyes JL. Oxidative stress induces claudin-2 nitration in experimental type 1 diabetic nephropathy. Free Radic Biol Med. 2014;72:162–75. doi: 10.1016/j.freeradbiomed.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Cherney DZ, Perkins BA. Sodium-glucose cotransporter 2 inhibition in type 1 diabetes: Simultaneous glucose lowering and renal protection? Can J Diabetes. 2014;38:356–63. doi: 10.1016/j.jcjd.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Skrtic M, Yang GK, Perkins BA, Soleymanlou N, Lytvyn Y, von Eynatten M, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia. 2014;57:2599–602. doi: 10.1007/s00125-014-3396-4. [DOI] [PubMed] [Google Scholar]
- 9.Dutta D, Kalra S. Sodium glucose transporter 2 (sglt2) inhibitors: Current status in clinical practice. J Pak Med Assoc. 2014;64:1203–6. [PubMed] [Google Scholar]
- 10.Hayami T, Kato Y, Kamiya H, Kondo M, Naito E, Sugiura Y, et al. A case of ketoacidosis by a SGLT2 inhibitor in a diabetic patient with low carbohydrate diet. [Last accessed on 2015 Feb 20];J Diabetes Investig. 2015 doi: 10.1111/jdi.12330. DOI: 10.1111/jdi.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldblatt MW. Observations on the effect of various carbohydrates on the ketosis of starvation in human subjects. Biochem J. 1925;19:948–57. doi: 10.1042/bj0190948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EHRSTRöM R. Wassermangel der Gewebe und Ausscheidung von Azetonkörpern. Acta Medica Scandinavica. 1922;56:507–9. doi: 10.1111/j.0954-6820.1922.tb18501.x [article in German] [Google Scholar]
- 13.Freund G. The calorie deficiency hypothesis of ketogenesis tested in man. Metabolism. 1965;14:985–90. doi: 10.1016/0026-0495(65)90114-9. [DOI] [PubMed] [Google Scholar]
- 14.Mahoney CA. Extreme gestational starvation ketoacidosis: Case report and review of pathophysiology. Am J Kidney Dis. 1992;20:276–80. doi: 10.1016/s0272-6386(12)80701-3. [DOI] [PubMed] [Google Scholar]
- 15.Burr K, Nguyen AT, Rasouli N. SAT-595: A Case Report of Ketoacidosis Associated with Canagliflozin (Invokana) [Last accessed on 2015 May 17]. Available at: http://press.endocrine.org/doi/abs/10.1210/endo-meetings.2015.DGM.5.SAT-595 .
- 16.Peters JP. Starvation diabetes, the reason for the use of glucose in the treatment of diabetic acidosis. The Yale journal of biology and medicine. 1945;17:705. [PMC free article] [PubMed] [Google Scholar]
- 17.Kelwade J, Sethi BK, Nagesh SV, Vaseem A. A case of “pseudo-ketoacidosis”. Indian J Endocrinol Metab. 2014;18:743. doi: 10.4103/2230-8210.139228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills SE, Lyle RR, Beitz DC, Young JW. In vitro hepatic gluconeogenesis during experimental ketosis produced in steers by 1,3-butanediol and phlorizin. J Dairy Sci. 1984;67:2265–73. doi: 10.3168/jds.S0022-0302(84)81574-X. [DOI] [PubMed] [Google Scholar]
- 19.FDA Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. [Last accessed on 2015 May 17]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm .
- 20.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 21.Kalra S, Gupta Y, Patil S. Sodium-glucose cotransporter-2 inhibition and the insulin: Glucagon ratio: Unexplored dimensions. Indian J Endocrinol Metab. 2015;19:426–9. doi: 10.4103/2230-8210.152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston DG, Blesa-Malpica G, Burrin JM, Waugh C, Cook D, Orskov H, et al. Dopamine blockade inhibits starvation ketosis in man. Clin Endocrinol (Oxf) 1983;19:389–96. doi: 10.1111/j.1365-2265.1983.tb00012.x. [DOI] [PubMed] [Google Scholar]


