Abstract
Heat shock proteins (HSPs) are molecular chaperones with roles in longevity and muscular preservation. We aimed to show elevating HSP70 improves indices of health span. Aged C57/BL6 mice acclimated to a western diet were randomized into: geranylgeranylacetone (GGA)-treated (100mg/kg/d), biweekly heat therapy (HT), or control. The GGA and HT are well-known pharmacological and environmental inducers of HSP70, respectively. Assessments before and after 8 weeks of treatment included glycemic endpoints, body composition, and muscular endurance, power, and perfusion. An HT mice had more than threefold, and GGA mice had a twofold greater HSP70 compared with control. Despite comparable body compositions, both treatment groups had significantly better insulin sensitivity and insulin signaling capacity. Compared with baseline, HT mice ran 23% longer than at study start, which was significantly more than GGA or control. Hanging ability (muscular endurance) also tended to be best preserved in HT mice. Muscle power, contractile force, capillary perfusion, and innervation were not different. Heat treatment has a clear benefit on muscular endurance, whereas HT and GGA both improved insulin sensitivity. Different effects may relate to muscle HSP70 levels. An HSP induction could be a promising approach for improving health span in the aged mice.
Key Words: Heat shock proteins, Insulin resistance, Heat therapy, Sarcopenia.
Heat shock proteins (HSPs), named after the observed up-regulation following heat shock, are a family of protective chaperone proteins that maintain normal cellular function when cells are under various stressors. During response to stress, the transcription factor heat shock factor 1 (HSF1) binds to the promoter region of the HSP genes. The HSP70 mRNA transcripts are the most abundant (1), and both HSF1 activation and expression of HSP70 have been shown to decrease with age (2). Aging is associated with generally reduced levels of heat shock protein 70 (HSP70), which plays a conserved role in cellular homeostasis in all species (3). Genetic manipulation to increase generalized HSP70 levels has improved lifespan in invertebrate models (4), and thus it is the focus of our studies.
It has been demonstrated that aged muscle tissue does not increase HSP production in adaptation to normal exercise (5); however, increases in muscle mass and function can be generated by pharmacological induction or overexpression of HSP70 (6,7). Specifically, increasing HSP70 with the pharmacological agent, geranylgeranylacetone (GGA) has significantly increased muscle mass in young rats and improved their obese, insulin resistant phenotype (8). Preclinical experiments have demonstrated that systemically administered HSP70 localizes to skeletal muscle (9), and that overexpression of muscle HSP70 inhibits forkhead box O which is involved in disuse atrophy (10). Sarcopenia, defined as the loss of both skeletal muscle mass and function, and affects up to 45% of older adults with prevalence increasing with age (11). Sarcopenia, endothelial dysfunction, and insulin resistance are all common comorbidities seen with aging, and they set the clinical stage for diabetes, cardiovascular disease, and fall risk which are all conditions of great public health significance. The generalized process of aging is characterized by a systemic decline in function and the inability to maintain homeostasis. The HSPs are critical for cellular homeostasis; however, neither none of the effects of interventions to increase HSP70 protein levels have been evaluated with respect to age-related muscle function or mass, nor is the mechanism known by which observed improvements in muscle mass or function occur.
Reduced muscle HSP70 protein and gene expression has also been noted in younger insulin-resistant and hyperglycemic people, monkeys, and rodents (12–14). Insulin-like signaling influences lifespan in lower species and insulin resistance contributes to unhealthy aging in humans (15). Recent studies have shown that systemic insulin sensitivity can be improved in rodent and nonhuman primate models through pharmacological and heat induction of HSPs (12,16). Improved insulin sensitivity of skeletal muscle has been demonstrated following HSP induction (17). Improved insulin action at the level of the myocyte results from increased capillary recruitment and nutritive delivery, and increased anabolic signaling pathways within the cell (18,19). The purpose of the current study was to investigate whether increases in HSP70 may improve health span by attenuating indices of sarcopenia and insulin resistance in aged mice.
Methods
Animals
Aged (20-month old) female C57BL/6 mice sourced from the NIH NIA rodent colony were used for this study. Females were used as sex differences in mouse sarcopenia are not consistently reported (20). Mice were housed 2 to 5/tub in 11.5 × 7.25 × 5 in tubs in accordance to treatment group. Lights were on 600–1800 hours. Experimental diet that was constructed in-house was supplied at 7 g/mouse/d and water was available ad libitum. Diet intake was estimated by weekly measurement of the difference between diet fed and that remaining at the end of 24 hours. All animal manipulations were performed according to the guidelines of state and federal laws, the U.S. Department of Health and Human Services, and the Animal Care and Use Committee, Wake Forest School of Medicine. Wake Forest University is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Study Design
All animals consumed a western diet consisting of 36% of calories as fat, 48% calories as carbohydrate, and 16% of calories as protein. This diet was chosen to accentuate age-related deficits in glucose tolerance and ensure the study was conducted under a nutritionally relevant context for the human population.
Twelve mice were used to evaluate the time course of the heat shock response after a single hyperthermia session. Three mice were randomized to be assessed as control (CTL), 24, 48, or 72 hours postheat treatment (HT). The HT mice received dry-heat therapy (HT) by a radiant heat lamp placed over a closed container, which was kept at 41 ± 2°C for 20–30 minutes. Mice were sacrificed with collection of their gastrocnemius muscle for HSP70 concentration determinations.
Forty-two aged mice were assessed after chronic, repeated, HSP induction by pharmacological or by hyperthermia as described earlier. Mice underwent a 6-week acclimation period consuming the western diet before being randomized by body weight into one of three treatment groups (n = 12–15/group) for an 8-week study: GGA-treated, heat-treated (HT), or CTL. Mice were nearly 24 months of age at study end. During the study, mice were maintained on the western diet; GGA mice received 100 mg/kg/day compounded into their diet. Estimates of dose delivered was made by calculating average diet intake per mouse. The HT mice were exposed twice weekly. Body temperature data was recorded using implantable temperature transponder chips inserted subcutaneously on the dorsum between the shoulder blades (Bio Medic Data Systems, Seaford, DE). All mice were scanned twice weekly, prior to heat treatment, to obtain body temperature measurements. All mice were moved and handled along with the HT mice on treatment days to ensure comparable stress exposure. Bodyweight data was recorded once weekly. Assessments were done at 96 hours after the final heat treatment.
Skeletal Muscle HSP70
Tissue levels at study end were quantified by immunoblotting. Gastrocnemius muscle was homogenized (Tissue-Tearor BioSpec Products Inc., Bartlesville, OK) using optimized buffers (Bioscource, Invitrogen Inc., Carlsbad, CA) with added reducing agents and proteinase inhibitors (all reagents Sigma, St. Louis, MO). Following protein concentration quantification, equal amounts (40 μg) were resolved by SDS-PAGE and transferred to nitrocellulose membranes, using the Novex Mini-Cell Electrophoresis system (Invitrogen). Membranes were blocked overnight and probed with primary antibodies for HSP70 (StressMarq Biosciences, Victoria, CA) and appropriate secondary antibodies conjugated to fluorescent signals. The signal was detected, quantified (Odyssey CLx, Li-Cor Biosciences, Lincoln, NE), and normalized for GAPDH levels (Imgenex Corp., San Diego, CA).
Glucose Utilization
At week 8, glycated hemoglobin chain A1c was measured from whole blood to indicate long term glycemic control and a glucose tolerance test was performed as described previously (21). Glycated hemoglobin (A1c%) levels were measured through high-performance liquid chromatography (Primus PDQ; Primus Diagnostics, Kansas City, MO). Fasting insulin was also measured by mouse-specific ELISA assay (Mercodia, Uppsala, Sweden) and glucose by the glucose oxidase assay. Unstimulated gastrocnemius muscle tissue was assayed for total and tyrosine phosphorylated insulin receptor substrate 1 (IRS1) and Akt (also known as protein kinase B) by ELISAs (Invitrogen, Camarillo, CA). Results are expressed as total and percent activated for each receptor.
Skeletal Muscle Performance
Treadmill endurance.
During the initial acclimation period mice were introduced to the treadmill by placing the mice on the unmoving belt for 15 minutes. Acclimation continued for 4 days with speeds between 5 and 10 m/min and an incline set to 5°. Treadmill endurance was recorded at baseline and week 8. The belt was started at a slow speed (6 m/min) and accelerated by 1⅓ m/min every 3 minutes with the incline set to 10°. Mice were run until exhaustion, defined as when the mouse was willing to sustain 2 seconds or more of sitting on hard bristles placed at each lane end, rather than return to the treadmill for a third time. Total run time was recorded for each mouse.
2-Limb and 4-limb hang time.
Muscular endurance was evaluated at baseline and week 8 by 2-limb and 4-limb hang times. For 2-limb testing mice were suspended by the forelimbs from a high wire with padding below. Time was recorded from when the mouse initially gripped the wire with both forelimbs until the animal fell onto the padding below. If a mouse’s hind limb made contact with the wire, time was stopped, the mouse was repositioned, and the timer restarted. The 4-limb test was similar with the exception that mice were suspended by all four limbs onto wire mesh. The animal was placed onto the mesh, the timer started when the mesh was inverted, and was stopped when the mouse fell onto the padding below.
Grip strength.
Muscle power was evaluated at baseline and week 8 by forelimb grip strength (Columbus Instruments Grip Strength Meter DFIS 10, Columbus, OH). Mice gripped a force plate by both forelimbs and were pulled away from the plate by their tail. Mice were tested 15 times, and the nine highest force values were averaged. All tests were administered by the same technician.
Muscle contractility.
Maximum muscle force, maximal fatigue, and time to reach 50% of maximum contractile response were induced by in situ peroneal nerve stimulation protocols. Force measurements were made with an in vivo force transducer (Aurora Scientific, Aurora, Ontario, Canada). In brief, this apparatus measured force production by dorsiflexion of a foot plate to which the mouse’s hind foot was attached. Animals were anesthetized with an isoflurane chamber and subsequent isoflurane vaporizer (1%–3% isoflurane) and given analgesia with ketoprofen. Fur was removed on the limb of interest, the common peroneal nerve was located, and an electrode placed over the nerve. Resting tension, muscle length, and stimulation current were iteratively adjusted for each muscle to obtain optimal twitch force. To obtain force–frequency curves, the nerve was stimulated with 250ms trains of pulses at increasing frequencies every 2 minutes. Following a 5-minute rest period, the muscle was stimulated to fatigue by delivering tetanic trains (60 Hz for 100ms) every 2 seconds for 5 minutes (22). Force recordings were normalized by lower limb muscle cross-sectional area determined from computed tomography scans and bodyweight.
Body Composition
Computed tomography scans were used to measure fat and lean tissue volume for the entire mouse and hind limb sections at week 8. Scans were performed on a Toshiba 32-slice Aquilion scanner (Toshiba America Medical Systems, Tustin, CA). The images were reconstructed with TeraRecon Aquarius Intuition software (TeraRecon, Foster City, CA), and then converted into a dicom format for analysis. Animals that exhibited observable tumors on computed tomography imaging (n = 5) were excluded from analysis. Thresholds of −140 to −40 Hounsfield units (HU) were applied to isolate the fat-containing voxels and screening thresholds of −5 to 135 HU were used to isolate lean tissue. Fat mass was calculated from volume results (corrected for fat density of 0.918g/cm3) and expressed as a percentage of the animal’s total body weight. Lean tissue was similarly calculated from volume results (corrected for muscle density of 1.055g/cm3). Additionally, hind limb muscle mass, composition, and attenuation values were separately estimated by applying the aforementioned thresholds, and regions of interest for attenuation values, for muscle by selecting only image slices distal to the pubic symphysis. The maximal cross-sectional area of the musculature midtibia was measured by manually tracing the outline of the muscle-fat interface and the total area and average attenuation was recorded (23). At termination, muscles were dissected out and weighed.
Muscle Perfusion by Microsphere Dispersion
As a terminal procedure, 15 μm dark-colored microspheres diluted to 100 μL volume in 0.9% sterile saline were injected slowly over 10 seconds into the jugular vein (200,000 spheres/mouse; Stason Pharmaceuticals, Irvine, CA) (24). Injections occured after blood sampling of 100 μL to normalize blood volumes. After 10 minutes, the mouse was euthanized and hind limb muscle tissue removed. Muscle tissue was ethanolic potassium hydroxide digested in glass tubes for 4 hours at 75°C prior to filtering. Spheres were manually counted using a hemocytometer and expressed as number of spheres/g muscle tissue. A minimum of 400 microspheres was counted to ensure accuracy. Nitrite and nitrate levels were measured in plasma samples, using an Eicom NOx analyzer (eNO-20) as per the manufacturer instructions. eNO is a sensitive instrument that can detect nitrite/nitrate up to 10nM × 10 µl (0.1 pmol) concentration in the injected samples. Data are reported as the sum of nitrite and nitrate measured in plasma (NOx).
Innervation to Skeletal Muscle
Muscle performance is in part dictated by neurological activation of the myofibers and so neuromuscular junctions were quantified in three mice from each group by immunohistochemistry at study end. Gastrocnemius, soleus, and anterior tibial muscles were dissected out after 2% paraformaldehyde perfusion and fixation overnight at 4°C. Tissue was rinsed twice with PBS and placed in 30% sucrose for at least 72 hours at 4°C. The muscles were then sucrose embedded and cut at 40 μm. Antigen retrieval was achieved using an SDS pretreatment and the sections then stained for the vesicular acetylcholine transporter protein (Santa Cruz Biotechnology, Santa Cruz, CA) and α-bungarotoxin (Invitrogen, Eugene, OR). The total endplates and percentage of innervated neuromuscular junctions was determined using previously established counting criteria (17).
Data Analysis
Data was log transformed if required to achieve statistical assumptions of normality. Data are expressed throughout as means ± standard error of the mean. Group differences were analyzed using one-way analysis of variance with alpha level set at .05 for statistical significance. Post hoc analyses were conducted using Tukey’s honest significant differences testing. Correlation coefficients were determined by Pearson’s r statistics for association. All statistical testing was performed using Statistica V10 (StatSoft Inc., Carlsbad, CA).
Results
Heat treatment increased muscle HSP70 levels (Figure 1A) more than twofold at all time points measured in aged mice. Levels remained significantly elevated 3 days after the single heat exposure (p = .03) and thus twice weekly HT was pursued in the subsequent study. After 8 weeks of study, the HT mice had more than threefold the levels of HSP70 compared with CTL (p = .02; Figure 1B). The GGA treatment induced higher HSP70 levels compared with CTL (p = .03), but was significantly less than HT mice (p = .03).
Figure 1.
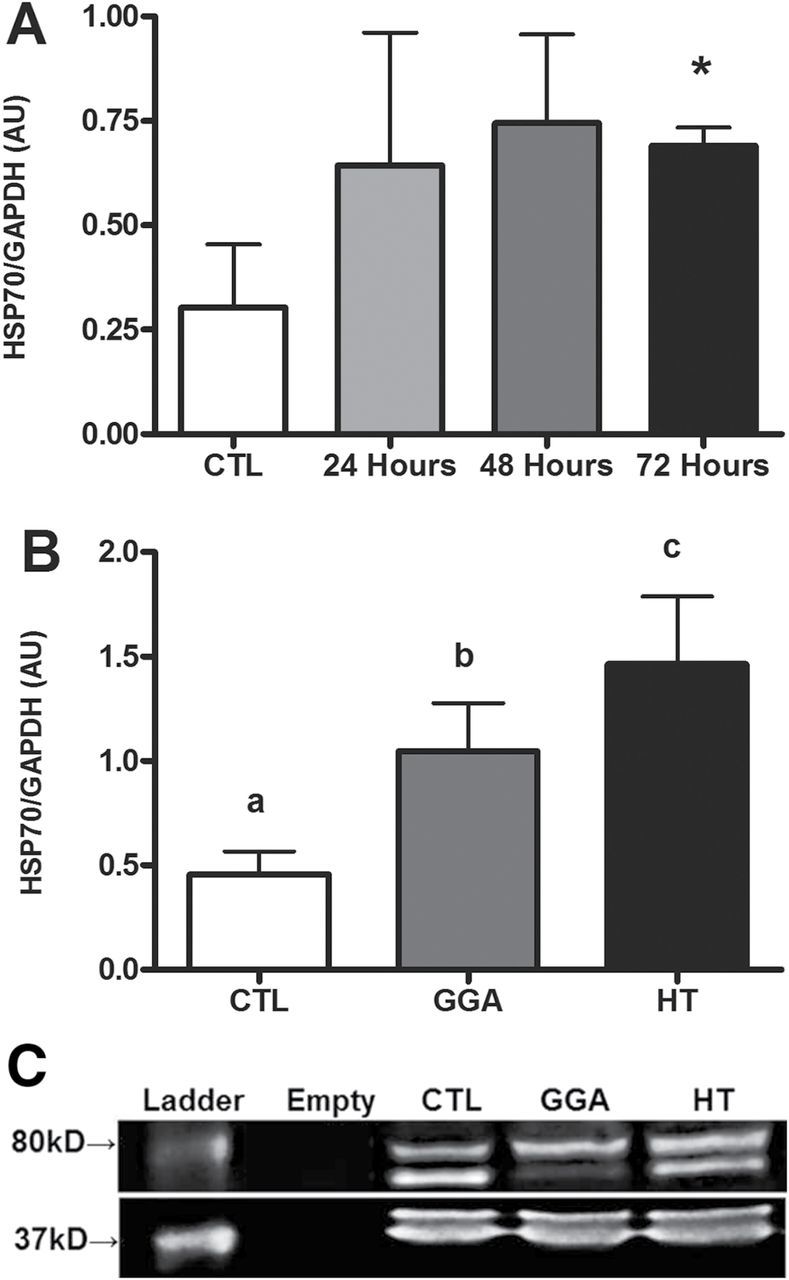
(A) Heat shock protein 70 (HSP70) levels in gastrocnemius muscle from aged mice (n = 3/group) without heat (control [CTL]) or 24, 48, or 72 hours after a single dry heat session of 30 minutes duration. Levels increased within 24 hours and remained elevated throughout 72 hours (*p = .03 vs CTL). (B) HSP70 levels in gastrocnemius muscle from aged mice consuming a western diet (n = 7–12/group) at the completion of study. CTL mice had the lowest HSP70 levels with geranylgeranylacetone therapy having approximately twofold higher, and HT having threefold higher levels after 8 weeks of treatment. Unlike letters denotes significance between groups. (C) Representative Western blot from data shown in panel B.
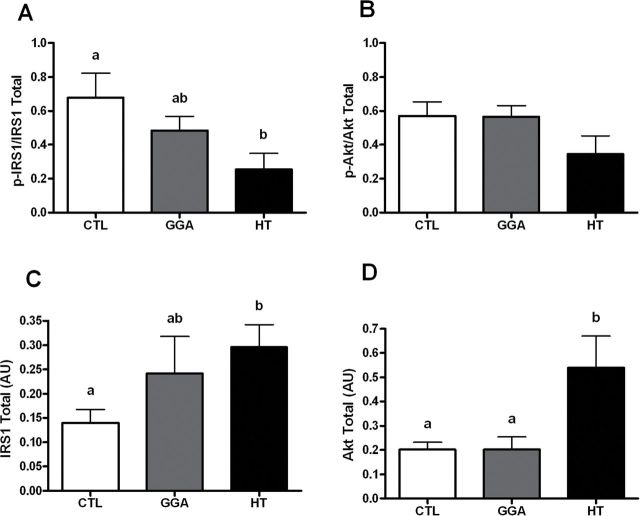
The HT significantly improved insulin sensitivity as evidenced by improved average glycemic control (Figure 2A) compared with both GGA and CTL (p = .006 and .02, respectively). No differences were seen between group baseline A1c% levels (data not shown, p = .51). The level of reduction in A1c% seen in the HT group ranged between 0.4% and 0.6%, which has high clinical significance. The improvement in glycemic control was seen secondary to improved insulin sensitivity as evidenced by fasting insulin levels in HT mice being approximately half that of CTL. The GGA mice also had reduced insulin levels required to maintain normoglycemia (Figure 2B). Response to glucose challenge was equivalent between groups (Table 1). The glucose tolerance test result suggests postglucose load responses are similar, and therefore the reduction in A1c values is either due to reduced fasting glucose values that we were unable to detect or due to improved disposal of lower glycemic loads typical of the postprandial state. We examined basal insulin signaling proteins in muscle tissue and HT mice had significantly lower activated IRS1 (p = .03) and 46% the amount of activated Akt (p = .13) compared with the CTL which is consistent with lower fasting insulin concentrations (Figure 3A and B). The coordinated insulin signaling pathway is supported by a significant correlation between activated IRS1 and Akt (r = .48, p = .02). We observed that despite lower basal insulin and insulin signaling, the capacity for insulin receptor activation and glucose uptake appears to be increased with HT, as total IRS1 and Akt present in the cytoplasm is significantly increased compared with CTL (p = .02 for both; Figure 3C and D). In all cases, the potency of HT was highest for insulin sensitizing effects, with GGA having similar or intermediate effects, which is in line with the HSP70 protein levels observed (Figure 1B).
Figure 2.
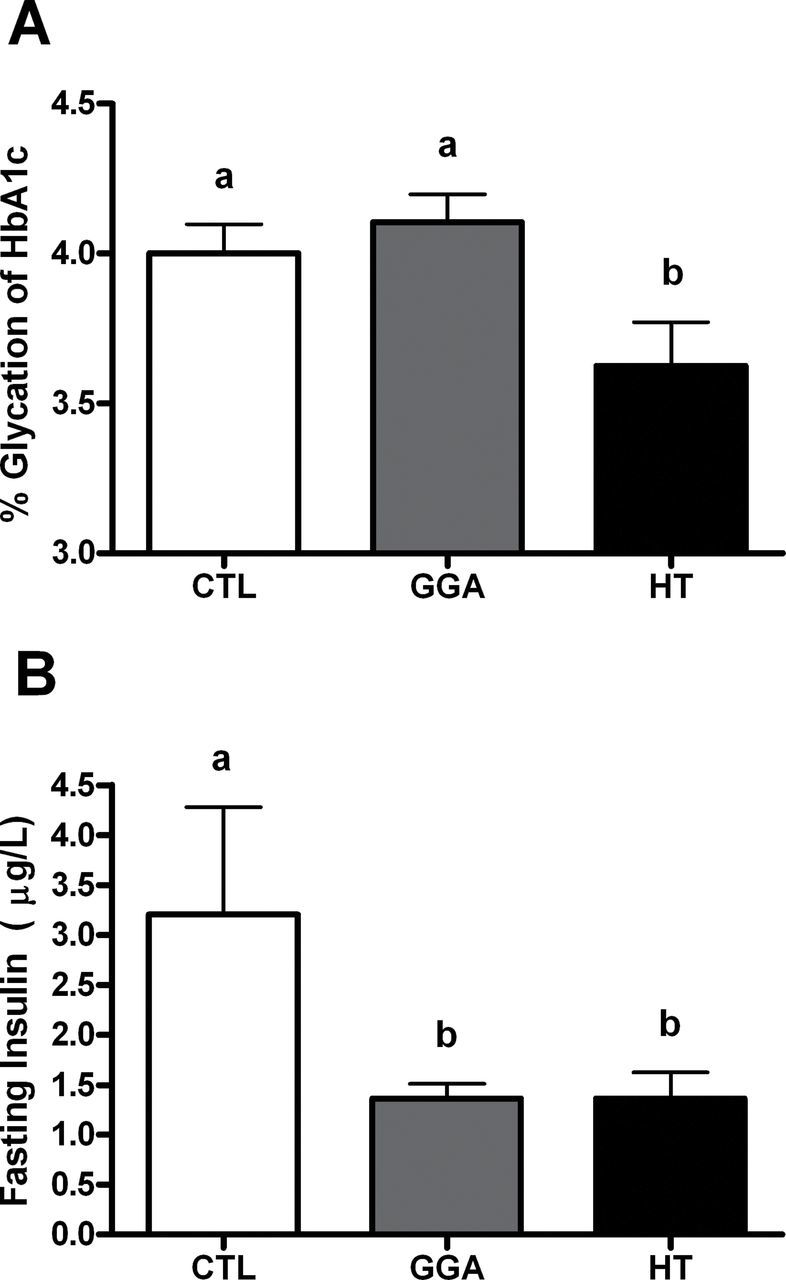
(A) Average glycemic control (CTL) as measured by percent glycation of hemoglobin chain A1c after 8 weeks of study in aged mice (n = 8–10/group). Only HT mice showed significantly better glucose CTL compared with CTL mice (p = .02) and geranylgeranylacetone (GGA; p = .006). Unlike letters denotes significance between groups. (B) Fasting insulin concentrations at study end. Both GGA and HT mice had significantly lower insulin levels compared with CTL mice (p = .03 for both; n = 6–9/group) which suggest improved insulin sensitivity. Unlike letters denotes significance between groups.
Table 1.
Body Composition, Core Temperature, and GTT Area Under the Curve (AUC) in Aged CTL, GGA, and HT Mice at Study End
| BW (g) | Body Fat (%BW) | Body Temp (°C) | Glucose AUC | Hind Limb Lean Mass (%BW) | Gastrocnemius Area (mm 2 ) | Muscle Density (HU) | |
|---|---|---|---|---|---|---|---|
| n/group | 8–10 | 5–9 | 8–10 | 8–10 | 5–9 | 5–9 | 5–9 |
| CTL | 39.8 (1.61) | 22.2 (0.79) | 36.7 (0.25) | 15761 | 2.58 (0.15) | 2.63 (0.11) | 75 (1.6) |
| GGA | 39.4 (1.70) | 20.9 (0.36) | 36.8 (0.11) | 16177 | 2.66 (0.13) | 2.65 (0.13) | 79 (1.4) |
| HT | 41.6 (1.80) | 22.1 (0.89) | 36.4 (0.18) | 15838 | 2.29 (0.10) | 2.59 (0.20) | 78 (2.3) |
| p value | .64 | .32 | .40 | .93 | .24 | .96 | .22 |
Notes: CTL = control; GGA = geranylgeranylacetone; GTT = glucose tolerance test; HU = Hounsfield units.
Figure 3.
(A) Basal activated insulin receptor substrate 1 (IRS1) levels in skeletal muscle of aged mice after 8 weeks of study. The HT had significantly lower activation (p = .03; n = 8/group) compared with control (CTL). (B) Basal activated Akt levels in skeletal muscle of aged mice at study end (p = .13; n = 8/group). The HT mice had 40% lower levels than CTL. (C) Total IRS1 levels in skeletal muscle of aged mice indicate HT mice have greater capacity for insulin receptor activation with more than double the levels seen in CTL mice (p = .02; n = 8/group). (D) Total Akt levels in skeletal muscle of aged HT mice are more than twice that of CTL and geranylgeranylacetone mice at study end (p = .04 and .02, respectively; n = 8/group).
Improvements in insulin sensitivity were seen in the absence of changes in body composition (Table 1). Fasting glucose was significantly associated with the amount of lean mass (R = −.84, p = .002) suggesting greater musculature is associated with the capacity for glucose disposal. Muscle attenuation reflects muscular density and fat content. Both treatment groups had nonsignificantly higher muscular density which may relate to insulin sensitivity, as density will be positively affected by lower fat content.
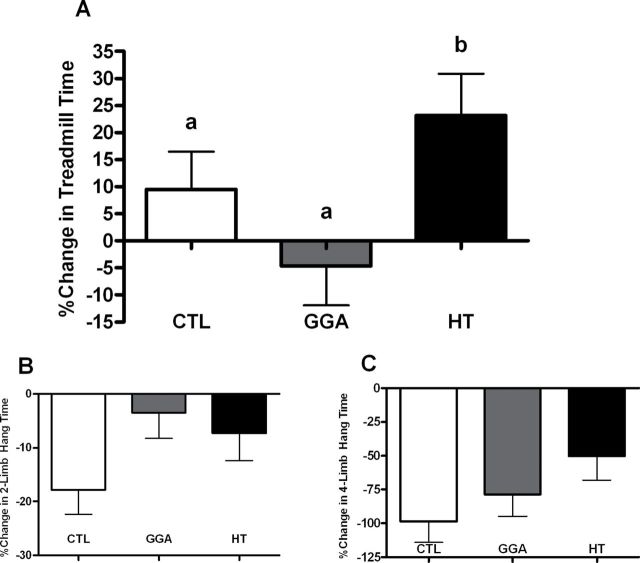
Muscular endurance was evaluated by treadmill running and duration of hanging both before and after study, as performance was expected to decline with aging over the course of the 2-month study duration. We observed that HT mice significantly improved their running times (p = .03; Figure 4A) by 23% from their baseline. This physical ability was reflected in a trend for preserved capacity for hanging as compared with CTL (Figure 4B and C). Improvements in treadmill running and hanging were not due to improved blood flow to the musculature, as microsphere perfusion of the hind limbs was not different between groups (Table 2). Further, there was a lack of difference in plasma nitrite and nitrate levels that contribute to vasodilatory effects. As heat stress causes an increase in heart rate and cardiac output, heart weight was examined to see if cardiovascular preconditioning may have contributed to treadmill performance and no differences were observed. Treadmill running was positively associated with lean mass (r = .70, p = .02), and change in four-limb hanging times was negatively associated with A1c% (r = −.47, p = .02) such that better glycemic control over study was seen with preservation of muscular endurance.
Figure 4.
(A) Percent change from baseline of running time on a treadmill in aged control (CTL), geranylgeranylacetone (GGA), and HT mice (n = 8–10/group). The HT performance did not decline with age but significantly (p = .03) increased by 23% from study start. (B) Percent change from baseline in forelimb hanging ability in CTL, GGA, and HT mice (n = 8–10/group). No significant differences were observed (p = .10); however, a trend for both treated groups to preserve ability was seen as compared with CTL. (C) Percent change from baseline in all four-limb hanging ability in CTL, GGA, and HT mice (n = 8–10/group). No significant differences were observed (p = .10); however, the decline seen in HT mice was half that seen in CTL mice.
Table 2.
Endpoints Relating to Perfusion From Aged CTL, GGA, and HT Mice at Study End
| Heart Weight (%BW) | Muscle Perfusion (microspheres/g) | Plasma NOx (μM) | |
|---|---|---|---|
| n/group | 5–7 | 4–6 | 6–9 |
| CTL | 0.58 (0.04) | 707 (227) | 16.6 (2.36) |
| GGA | 0.51 (0.05) | 406 (45) | 15.9 (1.24) |
| HT | 0.46 (0.05) | 339 (114) | 18.2 (3.13) |
| p value | .19 | .25 | .79 |
Notes: CTL = control; GGA = geranylgeranylacetone.
Treatment-related improvements in HSP70 levels, insulin sensitivity, and muscular endurance did not extend to measures of muscular power, as measured by grip strength or nerve-stimulated contraction (Table 3). Furthermore, any improvements in running ability did not appear to be mediated by neuromuscular adaptation as abundance of innervated motor endplates were not different between groups (data for gastrocnemius muscle shown, with similar results were seen for other muscle tissue examined).
Table 3.
Isolated Performance Measures and Innervations of Muscle From Aged CTL, GGA, and HT Mice at Study End
| Maximal Force (g/mm 2 ) | Time to 50% Fatigue (s) | Maximal Fatigue (g/mm 2 ) | Grip Strength % Change (N) | Innervated NMJs (% count) | |
|---|---|---|---|---|---|
| n/group | 8–10 | 8–10 | 8–10 | 8–10 | 3 |
| CTL | 9.97 (1.35) | 33.6 (4.07) | 13.9 (1.66) | −19 (4.2) | 16.7 (9.70) |
| GGA | 9.66 (0.86) | 31.7 (2.73) | 13.8 (1.31) | −16 (5.4) | 18.0 (6.93) |
| HT | 10.5 (2.18) | 34.5 (5.52) | 16.0 (3.53) | −23 (3.4) | 23.8 (5.57) |
| p value | .88 | .89 | .75 | .33 | .79 |
Notes: CTL = control; GGA = geranylgeranylacetone; NMJs = neuromuscular junctions.
Discussion
Induction of HSP70 preserved insulin sensitivity and running endurance in a relevant mouse model of insulin resistance and aging. By using either a heat treatment regimen or GGA, a well-tolerated clinical antiulcer drug, we were able to produce a two- to threefold increase in muscle HSP70 which were associated with lower A1c%, reduced fasting insulin levels, improved treadmill performance, and trends towards preserved hanging ability. Heat treatment was superior to pharmacological induction of HSP70 and endurance effects were independent of muscle mass or composition, as neither hind limb muscle mass nor muscle density differed among treatment groups. Performance differences may reflect the threefold increase in HSP70 levels observed in muscles from HT mice, versus a twofold increase in GGA mice, compared with CTL mice. These levels of HSP70 were similar to, or exceeded, increases in HSP70 seen with chronic exercise training in aged rodents (25), but were significantly lower than mice manipulated to overexpress HSP70 in muscle (6,26).
Improvements in insulin sensitivity and glucose handling following HT have been previously observed in rodents (both in vivo and in vitro (12,17,27)), and in human patients (28). Improvements in glycemic control are likely to reflect preservation of muscle metabolic function (26) as muscle takes up ~90% of circulating glucose (29). Prior studies utilizing GGA to induce HSP70 have demonstrated increases in muscle mass and glycemic control in rodents when dosed at levels twice that used in our study (8,12). The use of higher GGA doses may have augmented the HSP70 protein levels in muscle to be closer to the levels we observed with heat treatment, and thus associated with functional and metabolic improvements. Our study may support dose-related improvements based on cytoplasmic HSP70 abundance, similar to the positive relationship observed across species between tissue HSP70 levels and life span (30).
Insulin sensitivity, and its role in longevity, has been studied in a number of animal model systems. Caloric restriction in nonhuman primates improves life span and notably results in the reduction of incident diabetes with aging (31–33). This finding is recapitulated in centenarian human populations, and their offspring, which have a low prevalence of diabetes (34–36), and points to the importance of insulin resistance as a driver of age-related comorbidities. Insulin sensitivity as a central aging mechanism is supported by the longest lived mouse models, which have high insulin sensitivity through genetic modification of growth hormone biology (37). The decrease in insulin sensitivity with age in people is associated with increased morbidity and mortality (15) and has been correlated to decreased cytoplasmic HSP70 (38). Correspondingly, cytoplasmic HSP70 levels have been examined across many different species, and higher levels confer longer maximum life spans (15,30). For example, in invertebrates, overexpression of HSP70 confers >40% extension in lifespan (4). Modulating cytoplasmic HSP70 to improve insulin sensitivity appears to be a possible therapeutic modality and our current study supports regular HT as an intervention to increase HSP70 and improve health span in aged individuals.
The level of insulin resistance is a modifier of the relationship between obesity and sarcopenia (39), being a contributor to continued low-grade inflammation, and impairing anabolic signaling in muscle tissue (19). Muscle anabolism and regeneration are both impaired in the elderly (18,40,41), setting the stage for loss of muscle mass. This relationship between skeletal muscle index and insulin sensitivity is also seen in midlife individuals from the Third National Health and Nutrition Examination Survey, even after adjusting for obesity measures and age (42).
The HSP70 levels in muscle with aging are variable, with some reports indicating higher or comparable values to younger animals (17,43–45). However, rodent studies that specifically increase HSP70 levels in muscle tissue have consistently resulted in protections from atrophy or reduction in function (6,7,10,46). Young high-fat diet fed mice that overexpress HSP70 confirm that very high levels of muscular HSP70 have the ability to preserve whole-body insulin sensitivity, increased number of mitochondria per unit muscle, and superior treadmill running ability (26). Preservation of muscle may also result of HSP70’s ability to inhibit transcription of atrogin-1 and MuRF-1 which promote muscle degradation (10,47).
Our heat treatment results diverge from that of previous studies in young and aged rodents, which both demonstrated increased hind limb muscle mass (44,48). However, these studies differ from ours in that we treated mice biweekly for 20–30 minutes, as compared with a single 60-minute session and we measured muscle mass through both computed tomography and as wet weight after eight weeks of repeated treatment, as compared with only measuring wet weight of dissected muscle after the single heat session. It is, therefore, possible that changes in muscle mass are a short-lived effect of heat treatment, whereas changes in HSP levels, improved metabolism, and physiologic performance are more persistent and dominant effects.
The present study is the first to demonstrate preservation of insulin sensitivity and skeletal muscle endurance in aged rodents following the induction of HSP70. Recent results, using a newer pharmacological agent in diabetic rats (BGP-15) to induce muscle HSP70 to similar levels as in our study, demonstrate greater insulin sensitivity and muscle mitochondria (26) that supports a primary mechanism of improved muscle metabolic capacity with HSP70 induction. A strength of the current study was the comprehensive physiological endpoints evaluated relevant to skeletal muscle function. Innervation levels of the gastrocnemius muscle were lower than previously reported for the tibialis anterior muscle of aged mice, which may reflect the difference between muscle groups evaluated, insulin resistance levels, and/or the lack of exposure to a running wheel (49). Although our study cannot directly compare mechanisms between the GGA and HT groups, there may be a potential relationship between different HSP70 levels induced by them, and subsequent muscle performance and glycemic control. The GGA group had intermediate levels of HSP70 and partial improvements in insulin sensitivity, and muscular endurance. Our study had enhanced translational value as compared with other rodent studies because we used aged mice fed a western diet to accentuate age-related deficits in glucose tolerance and ensure study conditions were relevant to geriatric citizens of developed nations. However, the use of aged animals was also a study limitation. Loss of animals due to age-related illness, mainly neoplasia, resulted in small group sizes and decreased statistical power. We saw no group differences in tumor burden or type. In addition, we did not evaluate other possible heat-mediated effects, such as cardiovascular function, which may affect study outcomes. However, heart weights and perfusion was comparable across groups.
We believe the use of heat as an intervention was a strength of our study because of its clinical applicability. Few specific clinical interventions exist to combat sarcopenia in the elderly. Dietary protein supplementation and resistance exercise are often recommended; however, compliance issues are common. The opportunity to explore HSP inducers as an effective therapy for both insulin resistance and sarcopenia is exciting as demonstrated by our study findings. Furthermore, implementation of HSP70 inducing therapeutics late in life is likely to be beneficial. Either HT or currently existing pharmaceuticals could be evaluated for HSP70 induction and improvements in muscle function and insulin sensitivity, which together should improve health span in the elderly.
Funding
Funding for this study came from National Institutes of HealthK01 AG033641 (K.K.), P30 AG021332 (Wake Forest University Claude D. Pepper Older Americans Independence Center), and T32 OD10946 (M.S.).
References
- 1. Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heydari AR, You S, Takahashi R, Gutsmann-Conrad A, Sarge KD, Richardson A. Age-related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Exp Cell Res. 2000;256:83–93. [DOI] [PubMed] [Google Scholar]
- 3. Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. [DOI] [PubMed] [Google Scholar]
- 4. Yokoyama K, Fukumoto K, Murakami T., et al. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 2002;516:53–57. [DOI] [PubMed] [Google Scholar]
- 5. Vasilaki A, Iwanejko LM, McArdle F, Broome CS, Jackson MJ, McArdle A. Skeletal muscles of aged male mice fail to adapt following contractile activity. Biochem Soc Trans. 2003;31:455–456. [DOI] [PubMed] [Google Scholar]
- 6. Broome CS, Kayani AC, Palomero J., et al. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J. 2006;20:1549–1551. [DOI] [PubMed] [Google Scholar]
- 7. Dodd S, Hain B, Judge A. Hsp70 prevents disuse muscle atrophy in senescent rats. Biogerontology. 2009;10:605–611. [DOI] [PubMed] [Google Scholar]
- 8. Adachi H, Kondo T, Ogawa R., et al. An acylic polyisoprenoid derivative, geranylgeranylacetone protects against visceral adiposity and insulin resistance in high-fat-fed mice. Am J Physiol Endocrinol Metab. 2010;299:E764–E771. [DOI] [PubMed] [Google Scholar]
- 9. Gifondorwa DJ, Robinson MB, Hayes CD., et al. Exogenous delivery of heat shock protein 70 increases lifespan in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2007;27:13173–13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Senf SM, Dodd SL, Judge AR. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am J Physiol Cell Physiol. 2010;298:C38–C45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63:556–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung J, Nguyen AK, Henstridge DC., et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105:1739–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones. 2009;14:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurucz I, Morva A, Vaag A., et al. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51:1102–1109. [DOI] [PubMed] [Google Scholar]
- 15. Rincon M, Rudin E, Barzilai N. The insulin/IGF-1 signaling in mammals and its relevance to human longevity. Exp Gerontol. 2005;40:873–877. [DOI] [PubMed] [Google Scholar]
- 16. Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300:E894–E901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol (1985). 2011;110:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fry CS, Drummond MJ, Glynn EL., et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Timmerman KL, Lee JL, Dreyer HC., et al. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab. 2010;95:3848–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheard PW, Anderson RD. Age-related loss of muscle fibres is highly variable amongst mouse skeletal muscles. Biogerontology. 2012;13:157–167. [DOI] [PubMed] [Google Scholar]
- 21. Kavanagh K, Sajadian S, Jenkins KA., et al. Neonatal and fetal exposure to trans-fatty acids retards early growth and adiposity while adversely affecting glucose in mice. Nutr Res. 2010;30:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27:43–59. [DOI] [PubMed] [Google Scholar]
- 23. Hansen RD, Williamson DA, Finnegan TP., et al. Estimation of thigh muscle cross-sectional area by dual-energy X-ray absorptiometry in frail elderly patients. Am J Clin Nutr. 2007;86:952–958. [DOI] [PubMed] [Google Scholar]
- 24. Prinzen FW, Bassingthwaighte JB. Blood flow distributions by microsphere deposition methods. Cardiovasc Res. 2000;45:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas MM, Vigna C, Betik AC, Tupling AR, Hepple RT. Initiating treadmill training in late middle age offers modest adaptations in Ca2+ handling but enhances oxidative damage in senescent rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1269–R1278. [DOI] [PubMed] [Google Scholar]
- 26. Henstridge DC, Bruce CR, Drew BG., et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63:1881–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bathaie SZ, Jafarnejad A, Hosseinkhani S, Nakhjavani M. The effect of hot-tub therapy on serum Hsp70 level and its benefit on diabetic rats: a preliminary report. Int J Hyperthermia. 2010;26:577–585. [DOI] [PubMed] [Google Scholar]
- 28. Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341:924–925. [DOI] [PubMed] [Google Scholar]
- 29. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl. 2):S157–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salway KD, Gallagher EJ, Page MM, Stuart JA. Higher levels of heat shock proteins in longer-lived mammals and birds. Mech Ageing Dev. 2011;132:287–297. [DOI] [PubMed] [Google Scholar]
- 31. Colman RJ, Anderson RM, Johnson SC., et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mattison JA, Roth GS, Beasley TM., et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Atzmon G, Schechter C, Greiner W, Davidson D, Rennert G, Barzilai N. Clinical phenotype of families with longevity. J Am Geriatr Soc. 2004;52:274–277. [DOI] [PubMed] [Google Scholar]
- 35. Collino S, Montoliu I, Martin FP., et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One. 2013;8:e56564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adams ER, Nolan VG, Andersen SL, Perls TT, Terry DF. Centenarian offspring: start healthier and stay healthier. J Am Geriatr Soc. 2008;56:2089–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brown-Borg HM, Bartke A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012;67:652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodrigues-Krause J, Krause M, O’Hagan C., et al. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levine ME, Crimmins EM. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity (Silver Spring). 2012;20:2101–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vasilaki A, Jackson MJ. Role of reactive oxygen species in the defective regeneration seen in aging muscle. Free Radic Biol Med. 2013;65:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol (1985). 2009;106:2040–2048. [DOI] [PubMed] [Google Scholar]
- 42. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96:2898–2903. [DOI] [PubMed] [Google Scholar]
- 43. Cobley JN, Sakellariou GK, Owens DJ., et al. Lifelong training preserves some redox-regulated adaptive responses after an acute exercise stimulus in aged human skeletal muscle. Free Radic Biol Med. 2014;70:23–32. [DOI] [PubMed] [Google Scholar]
- 44. Ohno Y, Yamada S, Goto A., et al. Effects of heat stress on muscle mass and the expression levels of heat shock proteins and lysosomal cathepsin L in soleus muscle of young and aged mice. Mol Cell Biochem. 2012;369:45–53. [DOI] [PubMed] [Google Scholar]
- 45. Kavanagh K, Wylie AT, Chavanne TJ., et al. Aging does not reduce heat shock protein 70 in the absence of chronic insulin resistance. J Gerontol A Biol Sci Med Sci. 2012;67:1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gehrig SM, van der Poel C, Sayer TA., et al. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature. 2012;484:394–398. [DOI] [PubMed] [Google Scholar]
- 47. Egawa T, Ohno Y, Goto A., et al. AICAR-induced activation of AMPK negatively regulates myotube hypertrophy through the HSP72-mediated pathway in C2C12 skeletal muscle cells. Am J Physiol Endocrinol Metab. 2014;306:E344–E354. [DOI] [PubMed] [Google Scholar]
- 48. Kobayashi T, Goto K, Kojima A., et al. Possible role of calcineurin in heating-related increase of rat muscle mass. Biochem Biophys Res Commun. 2005;331:1301–1309. [DOI] [PubMed] [Google Scholar]
- 49. Cheng A, Morsch M, Murata Y, Ghazanfari N, Reddel SW, Phillips WD. Sequence of age-associated changes to the mouse neuromuscular junction and the protective effects of voluntary exercise. PLoS One. 2013;8:e67970. [DOI] [PMC free article] [PubMed] [Google Scholar]