Abstract
Females of the northern house mosquito, Culex pipiens L., are capable of entering an adult overwintering diapause characterized by arrested ovarian development, enhanced stress tolerance, and elevated lipid stores. In contrast, the southern house mosquito, Culex quinquefasciatus Say, lacks this capacity and is therefore unable to survive the harsh winters found in northern regions of North America. These two species are capable of forming fertile hybrids in the United States, yet the diapause characteristics of these hybrids have not been extensively investigated. We crossed Cx. pipiens from Columbus, OH, with Cx. quinquefasciatus from Vero Beach, FL, and reared F1 hybrids from all mothers separately under diapause-inducing, short-day conditions (a photoperiod of 8:16 [L:D] h) at 18°C. Egg follicle length and lipid content were used to assess the diapause status of hybrids. Diapause incidence of hybrids varied widely for progeny from different mothers of the same species, but hybrids with Cx. pipiens mothers were consistently more prone to enter diapause than hybrids that had Cx. quinquefasciatus mothers. Our results suggest a strong maternal influence on the diapause phenotype and that a high percentage (45–75%) of Cx. pipiens–Cx. quinquefasciatus hybrids are capable of entering diapause. This implies that many hybrids can successfully overwinter, leading to a possible widening of the hybrid zone of these two species in North America.
Keywords: northern house mosquito, southern house mosquito, egg follicle length, fat content
Introduction
Mosquitoes in the Culex pipiens complex are major vectors of important diseases such as West Nile virus and St. Louis encephalitis. Yet, this complex remains among the most challenging for mosquito biologists because the species vary widely in behavioral and physiological traits, but are nearly indistinguishable morphologically (Harbach et al. 1985, Vinogradova 2000). For example, northern house mosquitoes, Culex pipiens L., are capable of entering an overwintering adult reproductive diapause in response to the short day lengths of late summer and early fall (Eldridge 1968). This diapause, characterized by failure of the corpora allata to produce juvenile hormone, results in a suite of characteristics including arrested ovarian development, enhanced stress tolerance, a behavioral switch from blood feeding to nectar feeding, and fat hypertrophy (Spielman and Wong 1973, Mitchell and Briegel 1989, Robich and Denlinger 2005, Rinehart et al. 2006, Benoit and Denlinger 2007, Sim and Denlinger 2008). Diapause has allowed Cx. pipiens to survive harsh winters, and this in turn has facilitated their distribution throughout the Holoarctic and select regions of temperate zones in the Southern Hemisphere (Barr 1957). In contrast, southern house mosquitoes, Culex quinquefasciatus Say, lack the capacity for an overwintering diapause (Wilton and Smith 1985); thus, distribution of this species is restricted to the tropics, subtropics, and lower latitudes of the temperate zone (Barr 1957).
Despite these differences in diapause capabilities, Cx. pipiens and Cx. quinquefasciatus can be morphologically distinguished only by examining fine structural details of the male genitalia (Sundararaman 1949, Barr and Kartman 1951). More recently, less time-intensive and more accurate polymerase chain reaction (PCR)-based assays have been developed to distinguish Cx. pipiens, Cx. quinquefasciatus, their hybrids and other members of the Culex pipiens complex, including Culex pipiens pallens (L.) and Culex australicus Dobrotworsky & Drummond (Aspen and Savage 2003, Smith and Fonseca 2004).
Cx. pipiens and Cx. quinquefasciatus are currently considered to be separate species as they do not interbreed in Europe and South Africa, where their ranges overlap (Cornel et al. 2003), and hence are considered to be true species under the biological species concept (Mayr 1957). However, in many regions of the United States, the two species do interbreed and produce fertile hybrids (Barr 1957, Jakob et al. 1979, Tabashnik and Powell 1983, Pryor and Daly 1991, Cornel et al. 2003, Savage et al. 2006). While this hybrid zone was formerly thought to be fairly narrow, between 36 and 39°N latitude (Barr 1957), a more recent study using microsatellites suggests that the hybrid zone is much broader than originally perceived (Kothera et al. 2009). Why these mosquitoes are capable of hybridizing in North America but rarely do so elsewhere is unclear. Fonseca et al. (2004) propose that Cx. pipiens in the northern United States are actually hybrids of two European subspecies: Cx. pipiens sensu stricto which enters diapause, breeds above ground, is anautogenous (requiring a blood meal for egg production), and zoophilic (preferring to feed on avian hosts), and Culex pipiens molestus (Forskal), which does not enter diapause, breeds in confined spaces (stenogamy) such as underground subways in northern European cities, is autogenous (capable of producing their first batch of eggs without taking a blood meal), and anthropophilic (preferring to bite people). This may explain why Cx. pipiens in the United States will readily feed on both avian and human hosts, thereby acting as an efficient bridge vector of West Nile virus (Fonseca et al. 2004). The possibility that Cx. pipiens in North America is a hybrid of two European subspecies may also help explain why these mosquitoes more readily interbreed with sympatric Cx. quinquefasciatus.
Although several studies have documented the presence and temporal changes in abundance of Cx. pipiens–Cx. quinquefasciatus intermediates throughout the hybrid zone in the United States (Barr 1957, Jakob et al. 1979, Tabachnick and Powell 1983, Pryor and Daly 1991, Cornel et al. 2003, Savage et al. 2006, Kothera et al. 2009), few studies have systematically examined the diapause characteristics of these hybrids. Wilton and Smith (1985) performed several crosses and backcrosses of Cx. pipiens mosquitoes from Fort Collins, CO, with Cx. quinquefasciatus mosquitoes from Houston, TX, and evaluated the diapause capacity of the hybrids. They were thus able to rank mosquitoes according to the proportion of their genome that contained a Cx. quinquefasciatus background, and, not surprisingly, they reported a negative correlation between the ability of hybrids to enter diapause and the proportion of their genome that came from the Houston stock.
More recently Mori et al. (2007) used quantitative trait loci (QTL) analyses to identify regions of the genome associated with diapause by crossing Cx. pipiens from South Bend, IN, and Gosse, Nara, Japan, with Cx. quinquefasciatus mosquitoes from Vero Beach, FL. Several QTLs were associated not only with diapause but also with body size and developmental timing, indicating that certain genes likely have pleiotropic effects on multiple life history traits. Mori et al. (2007) did not report the diapause incidence in the hybrids that they examined, and therefore it remains unclear whether both parents contribute equally in determining the ability of hybrid progeny to enter diapause.
In our study, we systematically examined two diapause characteristics (egg follicle length and lipid content) in >500 female hybrids from 27 pairs of Cx. pipiens–Cx quinquefasciatus mosquitoes. Specifically, we examined at least 20 offspring each from 12 pairs of female Cx. pipiens crossed with male Cx. quinquefasciatus and at least 20 offspring each from 15 separate pairs of female Cx. quinquefasciatus mosquitoes crossed with male Cx. pipiens. Our results indicate that hybrid daughters were more likely to enter diapause if their mother was Cx. pipiens. This maternal influence on the diapause potential of progeny from Cx. pipiens–Cx. quinquefasciatus crosses has important ramifications for the overwintering capabilities of these hybrids and population dynamics within the hybrid zone.
Materials and Methods
Insect Rearing
Parental Generation
A colony of Cx. pipiens (Buckeye strain) from Columbus, OH, was maintained as described previously (Robich and Denlinger 2005). This colony was established in September 2000 from larvae collected in Columbus, OH, and additional field-collected mosquitoes were added to the laboratory colony in 2009. Larvae were reared under either diapause-averting long-day conditions (a photoperiod of 16:8 [L:D] h) at 25°C, or diapause-inducing short-day conditions (a photoperiod of 8:16 [L:D] h) at 18°C. The diapause incidence among short-day-reared Cx. pipiens females was routinely monitored by measuring the average length of previtellogenic egg follicles (see below; Spielman and Wong 1973, Sim and Denlinger 2008). After females had been in diapause for ∼3 mo, diapause was terminated by moving the females to long-day conditions (16:8) at 25°C for 1–2 wk. Postdiapause females were blood fed and their offspring were used to establish the next generation of Cx. pipiens mosquitoes. In this way, a high diapause incidence (90–100%) was maintained in the parental colony.
The colony of Cx. quinquefasciatus was obtained from the laboratory of Chelsea Smartt at the Florida Medical Entomology Laboratory, Vero Beach, University of Florida. This colony was established in the fall of 2008 from larvae collected in Vero Beach, and had been reared in the laboratory for ∼2 yr before the start of these experiments. Cx. quinquefasciatus were similarly reared under a photoperiod of 16:8 L:D h at 25°C but in a separate environmental chamber from the colony of Cx. pipiens.
Prior to crossing the mosquitoes, a subset of each species was reared under diapause-inducing short-day conditions (a photoperiod of 8:16 [L:D] h) at 18°C. One and two weeks after adult emergence, short-day-reared Cx. pipiens and Cx. quinquefasciatus females were collected, and their diapause status was assessed by measuring egg follicle length and fat content, two widely used indicators of diapause (Spielman and Wong 1973, Sim and Denlinger 2008; Kang et al. 2014).
Hybridization
In August 2010, Cx. pipiens and Cx. quinquefasciatus pupae were separated into putative males and females as males develop earlier and are slightly smaller than females. Pupae of each species were placed in separate cages that were monitored daily, and newly eclosed adults of the inappropriate sex were removed. Two days after peak adult emergence, ∼100 virgin females of Cx. pipiens and ∼100 males of Cx. quinquefasciatus were placed together in a cage. Similarly, 100 virgin females of Cx. quinquefasciatus and 100 males of Cx. pipiens were placed in a separate cage. The mosquitoes were kept under identical long-day conditions at 25°C and were offered a 10% sucrose solution ad libitum. Ten days after peak adult emergence, both cages were provided a live chicken for 30 min to blood feed. Fourteen days after adult emergence, oviposition water was placed in each cage.
F1 Generation
Fifteen egg rafts (each containing 75–200 eggs) from each cross were placed in separate containers and used to generate the F1 generation. Larvae were placed under diapause-inducing, short-day conditions (a photoperiod of 8:16 [L:D] h; 18°C). Three egg rafts from the female Cx. pipiens × male Cx. quinquefasciatus cross failed to develop, but otherwise all hybrid offspring hatched and developed normally. All larval containers were provisioned with the same amounts of ground fish food (Tetramin, Tetra, Mulle, Germany), and pupae and adults from each female parent were placed in separate cages (27 cages in total). One and two weeks after adult emergence, ∼15 F1 hybrids were collected from each cage, and diapause status was assessed by measuring egg follicle length and fat content.
Confirming the Genetic Background of Parents and F1 Progeny
To ensure the pure genetic background of each parental colony, as well as to verify hybridization in the F1 generation, we used the PCR assay designed by Smith and Fonseca (2004) to distinguish Cx. pipiens, Cx. quinquefasciatus, and their hybrids. This assay specifically amplifies regions of an intron within the acetylcholine esterase2 gene and produces fragments that are specific to Cx. pipiens and Cx. quinquefasciatus, as well as two bands that are specific to hybrids. To conduct this assay, genomic DNA (gDNA) was isolated from 10 males of each parental colony, as well as 10 males from the female Cx. quinquefasciatus × male Cx. pipiens cross using the insect protocol for the DNeasy Kit (Qiagen, Valencia, CA). PCR reactions contained 12.5 µl of Apex TaqRed DNA Polymerase Master Mix (Genessee Scientific, San Diego, CA), 500 nm ACEpip primer, 1,000 nm ACEquin primer, and 1,000 nm B1246s reverse primer, 4.0 µl of molecular-grade water, and 1.0 µl of gDNA. PCR consisted of a 5-min incubation at 94°C and 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, followed by an additional 7 min at 72°C. PCR products were separated and visualized using gel electrophoresis with a 1% agarose gel. As expected, PCR reactions from Cx. pipiens parental gDNA produced a single 610-bp fragment, Cx. quinquefasciatus parental gDNA produced a single 273-bp fragment, while hybrid gDNA contained both fragments (data not shown)
Egg Follicle Measurements
One and two weeks after adult eclosion, ovaries were dissected from parental and hybrid female mosquitoes in 0.9% saline solution, egg follicles were separated using a dissection needle, and the lengths of 10 egg follicles were measured for each female at 200-fold magnification (Zeiss Axioskop, Thornwood, NY).
Lipid Assays
Lipid content was measured in 5 female progeny from 5 female parents in each cross (i.e., 25 females per cross; 50 females in total) and from 10 females each of Cx. pipiens and Cx. quinquefasciatus. All mosquitoes were reared under diapause-inducing conditions (a photoperiod of 8:16 [L:D] h; 18°C), and adults were sampled 1 wk after adult eclosion. Lipid content was measured as described previously (Van Handel 1985) with slight modifications that enabled rapid measurement of lipid content in multiple samples using a plate reader.
Briefly, serially diluted lipid standards were prepared as described in the Van Handel protocol. Dry weight of each mosquito was recorded, the mosquito was then placed in a 1.5-ml microcentrifuge tube and homogenized in a 500-µl solution of 1:1 chloroform: methanol (vol:vol). Tubes were centrifuged for 5 min at 17,949 g, and 125 µl of supernatant from each sample was transferred to a clean 0.5-ml microcentrifuge tube. Samples and lipid standards were placed into a 90°C heating block for 20 min to evaporate the solvent. Then, 100 µl of 95% sulfuric acid was added to each tube, tubes were vortexed for 5 s and briefly centrifuged. Tubes were then heated for an additional 10 min at 90°C; samples and standards were allowed to cool 5 min before adding 100 µl vanillin–phosphoric acid to each tube. Samples and standards were then again vortexed and centrifuged. Color was allowed to develop in each tube for 20 min; 60 µl of samples and standards were transferred to each well of a 96-well Falcon Microtest plate (three wells for each sample and standard), and absorbance was measured at 525 nm using a FLUOstar Omega plate reader (BMG laboratory; Cary, NC). A linear curve, generated by using absorbance values and known concentrations of lipid standards, was used to calculate lipid content.
Results
Status of Egg Follicles in Hybrids
The common criterion used for determining diapause status is to dissect the females and determine the length of egg follicles 1 wk after adult eclosion (Spielman and Wong 1973, Sim and Denlinger 2008, Kang et al. 2014). Females were considered to be in diapause if the mean egg follicle length was <75 µm. An egg follicle length >90 µm was indicative of nondiapause. Values between 76 and 89 µm were classified as intermediate. Samples of 10 egg follicles per female were collected from 15 parental Cx. pipiens and Cx. quinquefasciatus mosquitoes, ∼15 females F1 hybrids from 12 Cx. pipiens mothers that had mated with male Cx. quinquefasciatus (176 hybrids), and ∼15 female F1 hybrids from 15 Cx. quinquefasciatus mothers that had mated with male Cx. pipiens (217 hybrids).
At 1 wk, all parental female Cx. pipiens were in a clear diapause state, with a mean egg follicle length for all dissected females of 58.0 ± 1.0 µm SE. At this time, 60% of female parental Cx. quinquefasciatus were classified as nondiapause, and the remainder were either in an intermediate state (33%) or appeared to be in diapause (6.7%); mean egg follicle length for all dissected females was 93.1 ± 2.5 µm SE. Similarly, several female F1 hybrids from both crosses were classified in all three states, but significantly more female F1 hybrids with Cx. pipiens mothers were in a diapause state (74.9 ± 5.9%) versus hybrids with Cx. quinquefasciatus mothers (53.4 ± 9.0%; χ2 = 35.46; df = 1; P < 0.0001).
As we did not expect any Cx. quinquefasciatus mosquitoes to enter diapause, we suspected that individuals that had average egg follicles lengths in intermediate and diapause categories were not truly in diapause but perhaps required more time to develop their egg follicles. We thus repeated the dissections 2 wk after adult emergence (Fig. 1). At 2 wk, all parental Cx. pipiens retained their diapause status (mean egg follicle length = 57.8 ± 0.8 µm SE; n = 15), and all parental Cx. quinquefasciatus females were clearly classified as being in a nondiapause state (mean egg follicle length = 110 ± 1.6 µm SE; n = 15), thus indicating that a 1-wk time interval was not sufficient to adequately determine the developmental status of Cx. quinquefasciatus. Among F1 hybrids, we again observed a significantly higher diapause incidence among mosquitoes whose female parent was Cx. pipiens (74.9 ± 5.7%) relative to hybrids whose female parent was Cx. quinquefasciatus (46.1 ± 9.6%; χ2 = 76.12, df = 1; P < 0.0001; Fig. 1A).
Fig. 1.
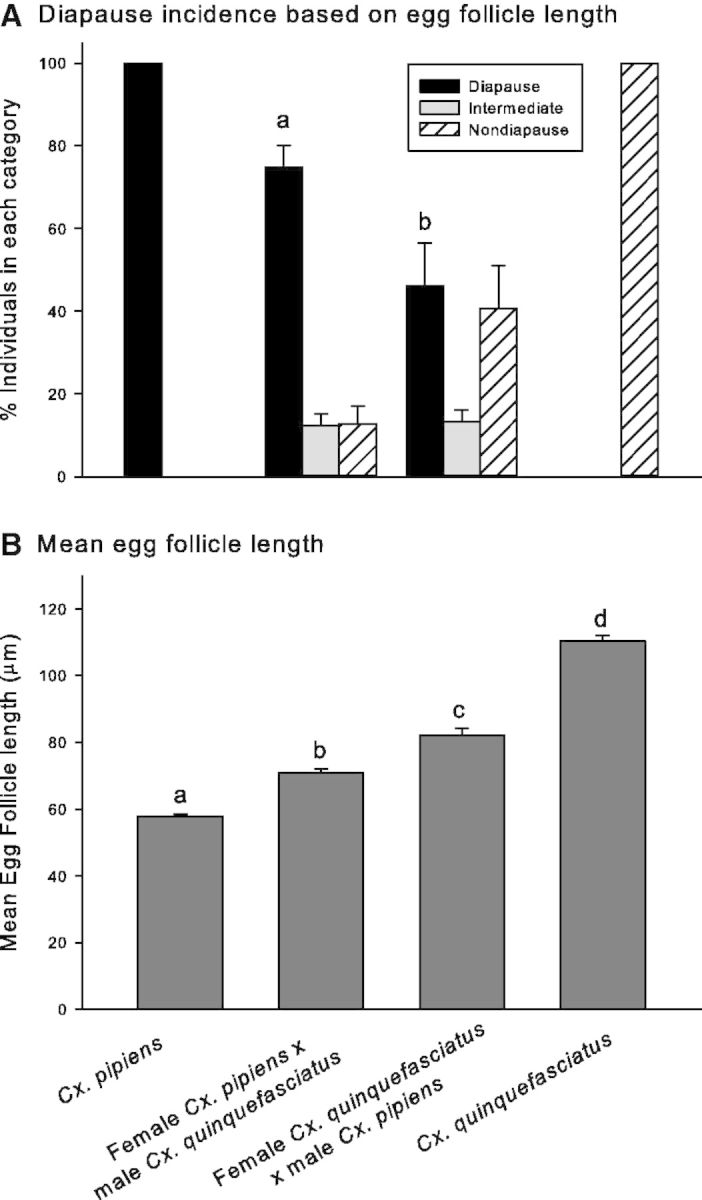
Egg follicle data in Culex females. (A) Percent of Culex females categorized as being in a diapause, intermediate, or nondiapause state based on egg follicle length. All individuals were reared under diapause-inducing conditions, a photoperiod of 8:16 (L:D) h at 18°C. Two-week-old females were defined as being in a diapause state if mean length of 10 egg follicles was <75 µm, a nondiapause state if mean follicle length was >90 µm, and an intermediate state if mean follicle length was 76–89 µm. Cx. pipiens n = 15; female Cx. pipiens × male Cx. quinquefasciatus n = 148; female Cx. quinquefasciatus × male Cx. pipiens n = 178; Cx. quinquefasciatus n = 15. Bars represent SEs, and different letters indicate significant differences in diapause status between the two sets of hybrid mosquitoes. (B) Mean ± SE egg follicle length of 2-wk-old female mosquitoes reared under diapause-inducing conditions (a photoperiod of 8:16 [L:D] h; 18°C). Different letters indicate significant differences. Cx. pipiens n = 15; female Cx. pipiens × male Cx. quinquefasciatus n = 148; female Cx. quinquefasciatus ×male Cx. pipiens n = 178; Cx. quinquefasciatus n = 15. One-way ANOVA F = 20.47; df = 355; P < 0.0001.
Variation in the diapause incidence was pronounced among the hybrids. For example, all offspring from one female Cx. pipiens parent that had been crossed with a male Cx. quinquefasciatus mosquito entered diapause, while only 33% of the offspring from another female from the same cross entered diapause. Likewise, one female Cx. quinquefasciatus mosquito that had been crossed with a male Cx. pipiens mosquito produced hybrid offspring that were all in diapause 2 wk after adult emergence, while four other similarly mated Cx. quinquefasciatus females produced no diapausing offspring. Therefore, we observed a significant effect of female parent among F1 hybrids within the same cross (Kruskal–Wallis one-way analysis of variance [ANOVA] on ranks between the egg follicle lengths of each female parent within the female Cx. pipiens × male Cx. quinquefasciatus cross; H = 34.797; df = 10; P < 0.0001; and the female Cx. quinquefasciatus × male Cx. pipiens cross; H = 112.724; df = 12; P < 0.001). A significant effect of maternal history on mean follicle length in progeny generated from the two crosses was also evident when mean values were pooled as shown in Fig. 1B: hybrids with Cx. pipiens mothers had significantly smaller egg follicles (70.9 ± 1.1 µm SE; n = 148) than hybrids with Cx. quinquefasciatus mothers (82.1 ±2.2 µm SE; n = 178; two-tailed Student’s t-test; t = 4.2134; df = 324; P < 0.0001).
Lipid Accumulation in Hybrids
Like egg follicle length, lipid accumulation is a good marker for diapause in Cx. pipiens (Sim and Denlinger 2008, Kang et al. 2014). One week after adult eclosion, females programmed for diapause usually have accumulated twice the amount of lipids as are present in nondiapausing females. For these experiments, we categorized females as being in diapause if their lipid content was >160 µg per female, those with a lipid content of <80 µg were considered to be nondiapause, and those containing 81–159 µg were classified as intermediate. Lipid content in 10 parental Cx. pipiens and Cx. quinquefasciatus mosquitoes was measured 1 wk after eclosion, and by this time all Cx. pipiens mosquitoes had accumulated substantial lipid stores while Cx. quinquefasciatus females failed to do so (Fig. 2A and B). Because of the variation we noted for hybrids from the above egg follicle results, we attempted to also capture variation in lipid content by measuring lipid levels in offspring from five different female parents whose progeny showed high, low, or intermediate diapause incidences in each cross based on the above egg follicle data.
Fig. 2.
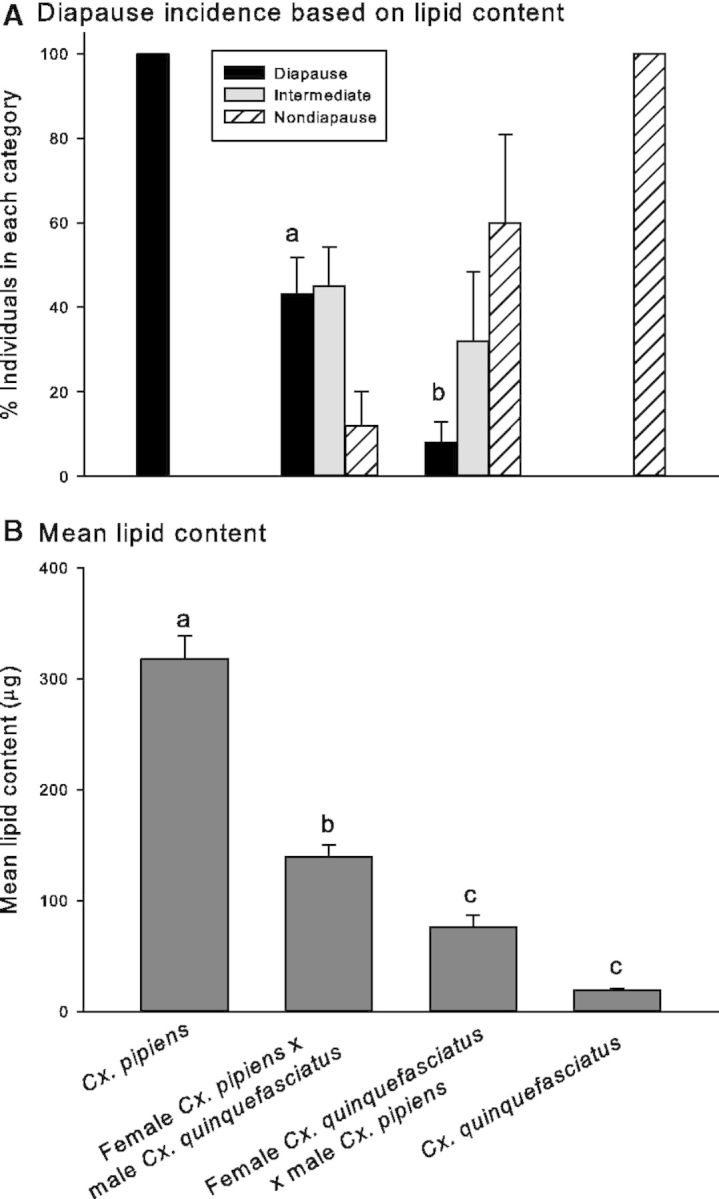
Lipid content data in Culex females. (A) One-week-old females were defined as being in a diapause state if they contained >150 µg of lipid, a nondiapause state if they had <80 µg lipid, and an intermediate state if they had 81–149 µg lipid. Cx. pipiens n = 10; female Cx. pipiens ×male Cx. quinquefasciatus n = 25; female Cx. quinquefasciatus ×male Cx. pipiens n = 25; Cx. quinquefasciatus n = 10. Bars represent SEs, and different letters indicate significant differences in diapause status between the two sets of hybrid mosquitoes. (B) Mean ± SE lipid content in 1-wk-old female mosquitoes reared under diapause-inducing conditions (a photoperiod of 8:16 [L:D] h; 18°C). Different letters indicate significant differences. Cx. pipiens n = 10; female Cx. pipiens × male Cx. quinquefasciatus n = 25; female Cx. quinquefasciatus × male Cx. pipiens n = 25; Cx. quinquefasciatus n = 10. Kruskal–Wallis one-way ANOVA on ranks H = 45.225; df = 3; P < 0.0001 with Dunn multiple comparison test for significance among groups.
All parental Cx. pipiens females had lipid levels consistent with diapause, and all parental Cx. quinquefasciatus females had low lipid levels that corresponded to nondiapause (Fig. 2A and B). Consistent with the egg follicle results, a higher percentage of F1 hybrids with Cx. pipiens mothers (43.0 ± 8.9%) had lipid levels indicative of diapause versus F1 hybrids with Cx. quinquefasciatus mothers (8.0 ± 4.9%; χ2 = 10.97; df = 1; P = 0.0009). As with the egg follicle results, significant variation in lipid content was evident in progeny from the five mothers within each cross (one-way ANOVA of lipid content among hybrids of female Cx. pipiens × male Cx. quinquefasciatus cross; F = 3.166; df = 23; P = 0.038; Kruskal–Wallis one-way ANOVA on ranks of lipid content among hybrids of female Cx. quinquefasciatus × male Cx. pipiens cross; H = 19.220; df = 4; P < 0.0001). Despite this variation among mothers within a cross, mean values for lipid content were significantly different: highest levels of lipids were present in parental Cx. pipiens, and F1 hybrids with Cx. pipiens mothers had significantly higher lipid content than hybrids with Cx. quinquefasciatus mothers, while Cx. quinquefasciatus mosquitoes had the lowest lipid levels (Fig. 2B; Kruskal–Wallis one-way ANOVA on ranks; H = 45.225; df = 3; P < 0.0001).
Discussion
Our results show that many Cx. pipiens × Cx. quinquefasciatus hybrids clearly have the capacity for diapause and that hybrids with Cx. pipiens mothers generally have a much higher diapause incidence. Yet, diapause varies greatly among mothers within the same cross, indicating that male parents and other factors influence the ability of hybrid offspring to enter diapause. The results we obtained differ from a previous observation in which a population of Cx. pipiens from Fort Collins, CO, was crossed with a population of Cx. quinquefasciatus from Houston, TX (Wilton and Smith 1985). Based on egg follicle lengths, Wilton and Smith report that only 7% of the hybrids entered diapause, while we observed diapause incidences of 45–75% in our hybrids. Wilton and Smith found no evidence for a maternal effect on the diapause incidence of the hybrids, but the low diapause incidence they observed would have likely precluded its detection. Differences between their study and ours include not only differences in the populations used for the crosses, and hence possible differences in the level of introgression between Cx. pipiens and Cx. quinquefasciatus colonies that were used by us and Wilton and Smith, but also differences in the “diapause-inducing conditions” used in the experiments: mosquitoes reared by Wilton and Smith were exposed to a photoperiod of 9:15 (L:D) at 22°C, while we reared our mosquitoes at a photoperiod of 8:16 (L:D) at 18°C. Regardless of the basis for these distinctions, it is evident from our work that a large proportion of hybrids are fully capable of entering diapause.
As demonstrated by Mori et al. (2007), numerous traits are impacted by hybridization between these two closely related mosquitoes. It is also abundantly evident that diapausing mosquitoes are physiologically, behaviorally, and molecularly distinct from nondiapausing mosquitoes (Denlinger and Armbruster 2014). The features we examined here, egg follicle length and fat content, are thus not the only characteristics of diapause, but they are among the most widely used indicators of diapause status in Cx. pipiens (Spielman and Wong 1973, Sim and Denlinger 2008, Kang et al. 2014). The fact that results of lipid content paralleled the results based on egg follicle length indicates the reliability of both features as indicators of diapause and also that these two characteristics of diapause are likely controlled by a common upstream regulator such as the insulin and juvenile hormone signaling pathways (Sim and Denlinger 2008, Kang et al. 2014). Other intriguing features of diapause in Cx. pipiens, such as their failure to blood feed (Mitchell and Briegel 1989, Wilton and Smith 1985, Robich and Denlinger 2005) and elevated desiccation and cold resistance (Rinehart et al. 2006, Benoit and Denlinger 2007), have not been examined in hybrids, but the whole collection of traits that characterize the diapause syndrome are normally linked (Denlinger and Armbruster 2014).
While the diapause or nondiapause features of the parents were quite distinct, as was the case for many of the hybrids, there was considerable variability in the traits of egg follicle length and lipid content among the hybrid progeny of different females such that numerous individuals were classified as having an intermediate diapause status, a category never observed in the parents. The fate of hybrid individuals with intermediate egg follicle length and lipid content in the wild is unknown. Diapause is costly from an energetics perspective (Hahn and Denlinger 2011), and a female mosquito that has not accumulated sufficient energy reserves is unlikely to be able to bridge the winter and survive until new nectar or other resources become available in the spring. Although a hybrid with intermediate lipid stores may be able to survive in southern portions of the hybrid zone and during a mild northern winter, it is unlikely that a female mosquito that has not garnered the full complement of lipids would be able to survive a harsh winter in northern regions of the hybrid zone. As winters vary in intensity and duration from year to year, the distribution and proportion of Cx. pipiens–Cx. quinquefasciatus hybrids throughout North America may change not only seasonally, as several previous studies demonstrate (Jakob et al. 1979, Pryor and Daly 1991, Savage et al. 2006), but also annually. The variation that we note in the diapause response could offer a bet-hedging strategy, allowing nondiapausing hybrid progeny to thrive and reproduce in mild winters, while only diapausing hybrids survive when the winter is more severe. Annual variation in the intensity of winter could thus offer advantages to specific hybrids in certain years and thus shape the temporal profile of the population.
The narrow hybrid zone proposed by Barr (1957) suggests that the diapause capacity of hybrids is limited, and this was supported by the experiments of Wilton and Smith (1985), indicating that few hybrids are capable of diapause. More recent microsatellite data indicate that the hybrid zone is much wider than earlier envisioned; rather than being limited to 36–39°N latitudes in the United States, as proposed by Barr (1957), the microsatellite results suggest that the hybrid zone extends from 30 to 40°N (Kothera et al. 2009). It is evident from our study that many hybrids do indeed have the capacity for diapause, especially those that have a Cx. pipiens mother. The diapause potential we observed in hybrids is consistent with the idea that hybrids could survive across the wide hybrid zone noted by Kothera et al. (2009), and perhaps even further north where winters are more severe. These authors proposed that the inability of hybrids to enter diapause was likely the sole mechanism preventing Cx. pipiens and Cx. quinquefasciatus mosquitoes in North America from becoming completely introgressed. The high diapause incidence we observed among hybrids suggests that the presence or absence of diapause may not be the only contributing factor that is preventing the introgression of Cx. pipiens and Cx. quinquefasciatus, yet the capacity for diapause in hybrids can be expected to impact the population structure and composition within the hybrid zone and contribute to both seasonal and annual variation in the population dynamics of these two vector species and their hybrid progeny.
Acknowledgments
We thank Chelsea Smartt for providing the Vero Beach colony of Cx. quinquefasciatus. We also thank Nicholas Teets for helping us optimize the lipid assay protocol to be used with a plate reader, and Justin Peyton for his advice on statistical analyses. This research was supported in part by National Institutes of Health Grant 2R56-AI058279; Megan Meuti was supported by an the National Science Foundation Graduate Research Fellowship. The chicken used for blood feeding the mosquitoes was handled according to an Institutional Animal Care and Use Committee protocol approved by The Ohio State University.
References Cited
- Aspen S., Savage H. M. 2003. Polymerase chain reaction assay identifies North American members of the Culex pipiens complex based on nucleotide sequence differences in the acetylcholinesterase gene Ace. 2 . J. Am. Mosq. Control 19:323–328. [PubMed] [Google Scholar]
- Barr A. R. 1957. The distribution of Culex p. pipiens and C. p. quinquefasciatus in North America. Am. J. Trop. Med. Hyg. 6: 153–165. [DOI] [PubMed] [Google Scholar]
- Barr A. R., Kartman L. 1951. Biometrical notes on the hybridization of Culex pipiens L. and C. quinquefasciatus Say. J. Parasitiol. 37: 419–420. [PubMed] [Google Scholar]
- Benoit J. B., Denlinger D. L. 2007. Suppression of water loss during adult diapause in the Northern house mosquito, Culex pipiens. J. Exp. Biol. 210: 217–226. [DOI] [PubMed] [Google Scholar]
- Cornel A. J., Mcabee R. D., Rasgon J., Stanich M. A., Scott T. W., Coetzee M. 2003. Differences in extent of genetic introgression between sympatric Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in California and South Africa. J. Med. Entomol. 40: 36–51. [DOI] [PubMed] [Google Scholar]
- Denlinger D. L., Armbruster P. A. 2014. Mosquito diapause. Ann. Rev. Entomol. 59: 73–83. [DOI] [PubMed] [Google Scholar]
- Eldridge B. F. 1968. The effect of temperature and photoperiod on blood-feeding and ovarian development in mosquitoes of the Culex pipiens complex. Science 151: 826–828. [DOI] [PubMed] [Google Scholar]
- Fonseca D. M., Keyghobadi N., Malcolm C. A., Mehmet C., Schaffner F., Mogi M., Fleischer R. C., Wilkerson R. C. 2004. Emerging vectors in the Culex pipiens complex. Science 303: 1535–1538. [DOI] [PubMed] [Google Scholar]
- Hahn D. A., Denlinger D. L. 2011. Energetics of diapause. Ann. Rev. Entomol. 56: 103–121. [DOI] [PubMed] [Google Scholar]
- Harbach R. E., Dahl C., White G. B. 1985. Culex (Culex) pipiens Linnaeus (Diptera, Culicidae)-concepts, type designations, and description. Proc. Entomol. Soc. Wash. 87: 1–24. [Google Scholar]
- Jakob W. L., Daggers S. A., Francy D. B., Mullenix J., Moseley K. 1979. The Culex pipiens complex in Memphis, Tennessee. Mosq. Syst. 11: 179–186. [Google Scholar]
- Kang D. S., Denlinger D. L., Sim C. 2014. Suppression of allatotropin stimulates reproductive diapause in the mosquito Culex pipiens. J. Insect Phys. 64: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothera L., Zimmerman E. M., Richards C. M., Savage H. M. 2009. Microsatellite characterization of subspecies and their hybrids in Culex pipiens complex (Diptera: Culicidae) mosquitoes along a North-South transect in the central United States. J. Med. Entomol. 46: 236–248. [DOI] [PubMed] [Google Scholar]
- Mayr E. 1957. Species concepts and definitions, pp. 1-22. In Mayr E. (ed.), The species problem, vol. 50. American Association for the Advancement of Science Publication, Washington, D.C., USA. [Google Scholar]
- Mitchell C. J., Briegel H. 1989. Fate of the blood meal in force-fed, diapausing Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 26: 332–341. [DOI] [PubMed] [Google Scholar]
- Mori A., Romero-Severson J., Severson D. W. 2007. Genetic basis for reproductive diapause is correlated with life history traits within the Culex pipiens complex. Insect Mol. Biol. 16: 515–524. [DOI] [PubMed] [Google Scholar]
- Pryor S. C., Daly J. 1991. Temporal variation in morphological and genetic characteristics within a hybrid population of Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 28: 481–486. [DOI] [PubMed] [Google Scholar]
- Rinehart J. P., Robich R. M., Denlinger D. L. 2006. Enhanced cold and desiccation tolerance in diapausing adults of Culex pipiens, and a role for Hsp70 in response to cold shock but not as a component of the diapause program. J. Med. Entomol. 43: 713–722. [DOI] [PubMed] [Google Scholar]
- Robich R. M., Denlinger D. L. 2005. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proc. Natl Acad. Sci. USA 102: 15912–15917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage H. M., Anderson M., Gordon E., McMillen L., Colton L., Charnetzky D., Delorey M., Aspen S., Burkhalter K., Biggerstaf B. J., Godsey M. 2006. Oviposition activity patterns and West Nile virus infection rates for members of the Culex pipiens complex at different habitat types within the hybrid zone, Shelby County, TN, 2002 (Diptera: Culicidae). J. Med. Entomol. 43: 1227–1238. [DOI] [PubMed] [Google Scholar]
- Sim C., Denlinger D. L. 2008. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl Acad. Sci. USA 105: 6777–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Fonseca D. M. 2004. Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae). Am. J. Trop. Med. Hyg. 70: 339–345. [PubMed] [Google Scholar]
- Spielman A., Wong J. 1973. Environmental control of ovarian diapause in Culex pipiens. Ann. Entomol. Soc. Am. 66: 905–907. [Google Scholar]
- Sundararaman S. 1949. Biometrical studies on integradation in the genitalia of certain populations of Culex pipiens and Culex quinquefasiatus in the United States. Am. J. Hyg. 50: 307–314. [DOI] [PubMed] [Google Scholar]
- Tabachnick W. J., Powell J. R. 1983. Genetic analysis of Culex pipiens populations in the central valley of California. Ann. Entomol. Soc. Am. 76: 715–720. [Google Scholar]
- Van Handel E. 1985. Rapid determination of total lipid in mosquitoes. J. Am. Mosq. Control Assoc. 1: 302–304. [PubMed] [Google Scholar]
- Vinogradova E. B. 2000. Culex pipiens pipiens mosquitoes: Taxonomy, distribution, ecology, physiology, genetics, applied importance and control. Pensoft, Moscow, Russia. [Google Scholar]
- Wilton D. R., Smith G. C. 1985. Ovarian diapause in three geographic strains of Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 22: 524–528. [DOI] [PubMed] [Google Scholar]


