Abstract
Background. The mechanism underlying the ability of virulent Salmonella organisms to escape clearance by macrophages is incompletely understood. Here, we report a novel mechanism by which Salmonella escapes macrophages.
Methods. Microarray and quantitative real-time polymerase chain reaction analyses were used to screen key microRNAs regulating Salmonella–host cell interactions. Target gene was tested using luciferase reporter and Western blot assays. The role of microRNA 128 (miR-128) was assayed using intestinal epithelial cells and a mouse infection model.
Results. The miR-128 level in human intestinal epithelial HT29 cells was strongly increased by infection with strain SE2472, and the elevation in miR-128 levels in mouse intestine and colon tissues correlated with the level of Salmonella infection in mice. Macrophage colony-stimulating factor (M-CSF) was identified as a target of miR-128, and increased miR-128 levels in epithelial cells due to infection with strain SE2472 significantly decreased the level of cell-secreted M-CSF, leading to impaired M-CSF–mediated macrophage recruitment. The secreted proteins from Salmonella were identified as possible effectors to induce miR-128 expression via the p53 signaling pathway. Moreover, intragastric delivery of anti–miR-128 antagomir into mice significantly increased M-CSF–mediated macrophage recruitment and suppressed Salmonella infection.
Conclusions. Salmonella can upregulate intestinal epithelial miR-128 expression, which, in turn, decreases levels of epithelial cell–secreted M-CSF and M-CSF–induced macrophage recruitment.
Keywords: Salmonella, miR-128, M-CSF, macrophage, infection
Salmonella is a facultative intracellular pathogen that causes a diverse spectrum of diseases, ranging from mild self-limiting gastroenteritis to fatal systemic typhoid fever. The 3 main serovars of Salmonella are Typhi, Typhimurium, and Enteritidis. S. Enteritidis has recently become the most common cause of food poisoning, with a significant number of case reports [1, 2]. Salmonella have evolved sophisticated machinery to alter host cell function that is essential for its virulence capabilities and survival [3]. Therefore, understanding the host-bacterial interactions is central in developing prevention and treatment strategies for the diseases associated with Salmonella infection. To infect the host, Salmonella must not only pass through the upper gastrointestinal tract and the intestinal epithelial barrier, but also overcome attack from both the innate and adaptive immune systems [4].
As one of major components of host innate immunity, macrophages play an essential role not only in host defense against infection by many pathogens, using a compartmentalized dual detection system, but also in the regulation of immune responses and inflammation [5]. The recruitment and activation of macrophages serves as a major mechanism of defense against infection by different intracellular pathogens [6]. Macrophages can be activated by various proinflammatory cytokines. For instance, macrophage colony-stimulating factor (M-CSF; CSF1) can recruit macrophages to the infection site and promote macrophages to phagocytose and kill foreign microorganisms [7]. Thus, regulation of secreted M-CSF levels may contribute to the modulation of macrophage recruitment and activation. Like other intracellular pathogens, Salmonella can use multiple complex and versatile mechanisms, including type III secretion systems (T3SS), to deliver virulence factors into host cells [8]. These virulence factors may re-program host cells and modulate the host immune response. Accumulating evidence suggests that Salmonella species, host cells, and environmental factors compose a complex network that precisely regulates the immune response during persistent Salmonella infection. The regulatory mechanism of this complicate system, however, remains incompletely understood.
MicroRNAs (miRNAs) are approximately 22-nucleotide noncoding RNAs that regulate target cellular messenger RNA (mRNA) expression at the posttranscriptional level [9]. In the context of interplay between the immune system and pathogens, miRNAs have a prominent role in the control of host-pathogen interactions [10]. miR-155, miR-142a, miR-223, miR-146, miR-9, and miR-181a have been recently shown to be crucial regulators of innate immunity and inflammatory responses [11]. The role of miRNAs in microbiota-host interactions has also been reported recently, in which Toll-like receptors (TLRs) serve as essential mediators modulating both gut microbiota and miRNAs/mRNAs in humans [12, 13]. Wang et al [14] further showed that miR-155 expression was induced by TLR signals and that the inducible miR-155 promotes type I interferon signaling in antiviral innate immunity. More recently, Schulte et al [15] analyzed the host miRNA response to Salmonella and uncovered the control of major cytokines by the let-7 family. The discovery of the broad function of miRNAs provides a new layer of molecular control of immune functions during pathogen infection.
The present study is the first to show that virulent Salmonella can actively manipulate the host cell immune response by upregulating miR-128 levels in intestinal epithelial cells, which, in turn, decreases epithelia-secreted M-CSF and impairs M-CSF–mediated macrophage recruitment. Moreover, we found that delivery of anti–miR-128 antisense oligonucleotide (ASO) by polyethylenimine (PEI) significantly reduced the level of Salmonella infection. Our study identified a new miR-128–targeting M-CSF mechanism in modulating the interaction between Salmonella and the intestinal epithelia.
METHODS
Bacteria Strains and Invasion Assay
Two wild-type Salmonella Enteritidis strains were used: virulent strain SE2472 (50% lethal dose [LD50], 4.5 × 103 colony-forming units [CFU] [16]) and the less virulent strain S9802 (LD50 > 108 organisms). A Salmonella invasion assay was performed as previously reported [17]. Bacterial suspensions were diluted to a multiplicity of infection (MOI) of 5–10. CFU on the plates were counted and compared to those of the input bacteria. The invasiveness of Salmonella was measured by determining the survival rate of intracellular bacteria, calculated as [number of intracellular bacteria/number of input bacteria] × 100.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (PCR) of Mature miRNAs
Total cellular RNA was extracted using TRIzol reagent (Invitrogen). The miRNA expression profile was assayed with Taqman Array human microRNA cards (Applied Biosystems). Quantitative real-time PCR was performed using TaqMan miRNA probes (Applied Biosystems). Briefly, 5 µL of total RNA was reverse transcribed to complementary DNA, using AMV reverse transcriptase (TaKaRa) and a stem-loop reverse transcriptase primer (Applied Biosystems). Real-time PCR was performed using a TaqMan PCR kit on an Applied Biosystems 7300 Sequence Detection System. All reactions, including no-template controls, were run in triplicate. After the reaction, the threshold cycle values were determined using default threshold settings.
p53 Small Interfering RNA (siRNA) Construction and Transfection
The p53 siRNA sequence targeting the coding region of p53 mRNA was used. Active siRNA against p53 used in this study had the following sequence: 5′-UGGUUCACUGAAGACCCAGTT-3′ (sense) [18]. The efficacy of p53 siRNA was tested in HT-29 cells expressing endogenous p53 gene. Transfection of siRNA was performed using Lipofectamine 2000 (Invitrogen, CA) according to the manufacturer's instructions. p53 knockdown was determined by Western blot analysis using anti-p53 antibody (Cell Signaling).
Statistical Analysis
Western blot and quantitative real-time PCR data reported here are representative of at least 3 independent experiments. Luciferase reporter and cell migration assays were repeated 5 times, and each experiment was performed in triplicate. The data are presented as mean values ( ± standard error of the mean [SEM]) of at least 3 independent experiments. Differences are considered to be statistically significant at P values of < .05, using nonparametric tests (the Mann–Whitney U test) or 1-way analysis of variance.
RESULTS
Infection by a Virulent Salmonella Strain Upregulates miR-128 Expression in Intestinal Epithelial Cells
Two Salmonella strains, the virulent SE2472 strain and the less virulent S9802 strain, were used in a cell-infection experiment. As shown in Figure 1A, both strains effectively infected cultured HT-29 cells, and no difference in the survival rate of bacteria in the infected cells was observed. However, when we infected mice intragastrically with the 2 Salmonella strains, we found that the CFU in the mouse intestine and colon tissues were markedly increased in the strain SE2472–infected group but not in the strain S9802–infected group (Figure 1B) from days 1–6. Next, we monitored the mouse immune response to Salmonella infection using fluorescence-activated cell sorter analysis. To separate the newly recruited macrophages [19] in mouse intestine from the resident macrophages, we costained the cells with anti-CD14 antibody and anti-F4/80 antibody. As shown in Figure 1C and 1D, the number of newly recruited macrophages in strain S9802-infected mice was significantly increased compared to strain SE2472-infected or mock-infection mice.
Figure 1.
The differential infection of the virulent strain SE2472 and the less virulent strain S9802 in a mouse model. A, Survival rate of bacterial infection in HT-29 cells. B, Differential infection by strain SE2472 and strain S9802 in Balb/c mice (5–6 mice per group). The mice were infected with 5 × 106 colony-forming units (CFU) of strains SE2472 or S9802 intragastrically, and bacteria in the mouse small intestine and colon were detected by CFU count determination on days 1, 3, or 6 after infection. ND, no colony been detected. C and D, Inflammatory response in mouse small intestine and colon after infection. Recruitment of macrophages labeled with rat anti-mouse F4/80 antibody (PE) and rat anti-mouse CD14 antibody (APC) in mouse small intestine and colon tissues were analyzed by flow cytometry. Data are mean values ± standard error of the mean. **P < .01. Abbreviations: E. coli, Escherichia coli; ND, no Salmonella detected; PBS, phosphate-buffered saline.
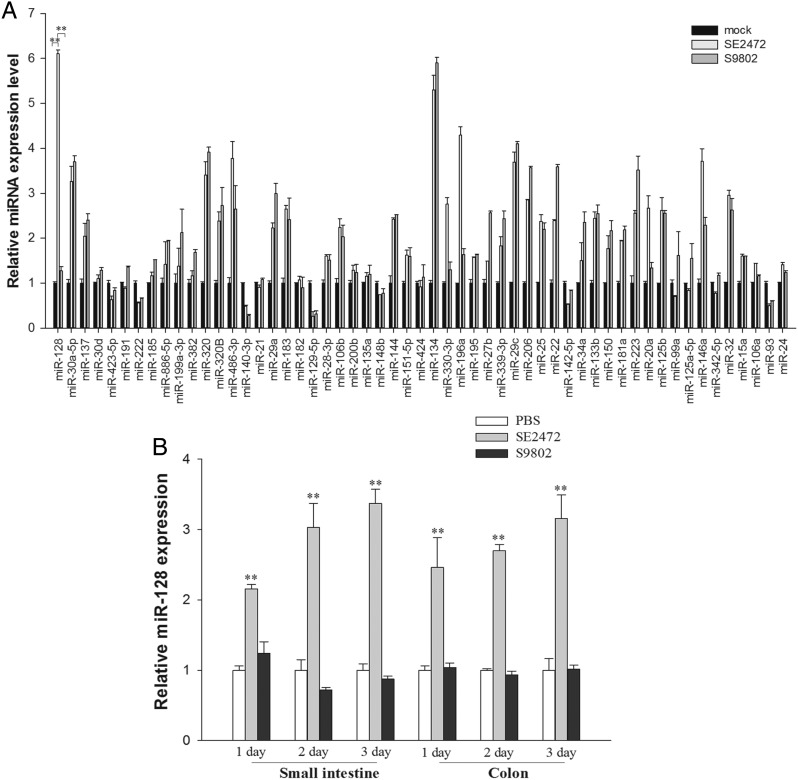
To identify the potential role of miRNAs in Salmonella infection, we determined the miRNA expression profile in HT-29 cells during Salmonella infection, using miRNA microarray analysis (Supplementary Table 1). We further compared the differences in expression of 29 miRNAs between the group infected with strain SE2472, the group infected with strain S9802, and the mock-infected group, using a TaqMan miRNA probe-based quantitative real-time PCR assay. As shown in Figure 2A, compared with the mock group, approximately 17 miRNAs were upregulated and 5 were downregulated following infection by the 2 Salmonella strains. Although the majority of miRNAs shared a similar upregulation and downregulation pattern, miR-128, miR-196a, miR-330-3p, and miR-20a were significantly upregulated in strain SE2472–infected mice but not in strain S9802–treated cells. Among these miRNAs, miR-128 had the greatest upregulation (>6.0-fold) in strain SE2472–infected cells, compared with the mock-infected group, suggesting that miR-128 might be involved in the interplay between the virulent Salmonella strain and host intestinal epithelial cells during infection.
Figure 2.
Differential expression of microRNAs (miRNAs) in human intestinal epithelial cells infected by Salmonella strains SE2472 and S9802. A, miRNA expression profile of human intestinal HT-29 cells with/without Salmonella infection, determined using stem-loop quantitative real-time polymerase chain reaction assays. Among of these miRNAs, the level of miR-128 was elevated nearly 6-fold. B, The expression level of miR-128 in the small intestine and colon from mice infected with various strains for 1, 3, or 6 days. Data are mean values ± standard error of the mean. **P < .01. Abbreviation: PBS, phosphate-buffered saline.
To test whether induction of epithelial miR-128 is a critical part of the host response to infection with the virulent Salmonella strain SE2472, we examined the miR-128 expression in mouse small intestine and colon tissues following infection by the 2 Salmonella strains. As shown in Figure 2B, miR-128 in both the intestine and colon of strain SE2472–infected mice was upregulated approximately 3-fold, compared with levels in control mice, on days 3 or 6 after infection. In contrast, no significant difference in miR-128 expression was observed in the intestine and colon tissues from strain S9802–infected mice and mice from the control group.
Suppression of Epithelial M-CSF Expression and Secretion by miR-128
Targetscan predicted the gene encoding M-CSF to be a target of miR-128 (Figure 3A). To confirm that this gene is a cognate target of miR-128, we inserted the M-CSF 3′ untranslated region (UTR) into a luciferase reporter plasmid (Figure 3B) and introduced the plasmid into HT-29 cells. Compared with the control group, overexpression of pre–miR-128 resulted in a >40% reduction of firefly luciferase reporter activity (normalized against β-galactosidase activity), whereas anti–miR-128 antisense oligonucleotide (ASO) overexpression enhanced firefly luciferase reporter activity by approximately 25% (Figure 3C). In contrast, when the seed sequence of the binding site on the 3′-UTR of M-CSF was mutated, pre–miR-128 and anti–miR-128 failed to affect luciferase reporter activity.
Figure 3.
The identification of the gene encoding macrophage colony-stimulating factor (M-CSF) as a target of microRNA 128 (miR-128). A, A schematic of the M-CSF 3′ untranslated region with the predicted miR-128 binding site. B, Luciferase reporter structure of WT and MUT. C, Relative luciferase activity in HT-29 cells cotransfected with luciferase reporter vectors containing the miR-128–binding site (p-MIR-report-MCSF-Luc) or the mutated region (p-MIR-report-mMCSF-Luc) and pre–miR-128 or anti–miR-128. D, MiR-128 levels in HT-29 cells treated with lipopolysaccharide (LPS), M-CSF–specific small interfering RNA (siRNA), pre–miR-128, or anti–miR-128. E, M-CSF levels in HT-29 cells treated with LPS, M-CSF-specific siRNA, pre–miR-128, or anti–miR-128. F, Secreted M-CSF levels in the culture medium of HT-29 cells treated with LPS, M-CSF siRNA, pre–miR-128, or anti-miR-128. Data are mean values ± standard error of the mean. *P < .05. **P < .01.
Next, we determined the effects of miR-128 on the expression of M-CSF. Because the level of M-CSF was very low in normal cells but significantly increased after lipopolysaccharide (LPS) stimulation [20], we analyzed the effect of miR-128 on M-CSF, using LPS-treated HT-29 cells. As shown in Figure 3D, the level of miR-128 was unaffected by LPS stimulation and treatment with M-CSF siRNA or control oligonucleotide (NTC) but increased or decreased after transfection with pre–miR-128 or anti–miR-128 ASO, respectively. Pre–miR-128 expression decreased LPS-induced M-CSF protein levels to approximately 70%. In agreement with this finding, the introduction of anti–miR-128 increased M-CSF levels (Figure 3E). The specificity of the M-CSF band was confirmed by M-CSF–specific siRNA experiments. The level of M-CSF secreted by LPS-treated epithelial cells was also determined by enzyme-linked immunosorbent assay. The level of secreted M-CSF was significantly increased following LPS stimulation, and this increase was downregulated by M-CSF siRNA and pre–miR-128 (Figure 3F). Interestingly, a moderate elevation in the level of secreted M-CSF was also detected for LPS-treated HT-29 cells after transfection with anti–miR-128.
Infection by Salmonella Inhibited the Migration of Macrophages In Vitro
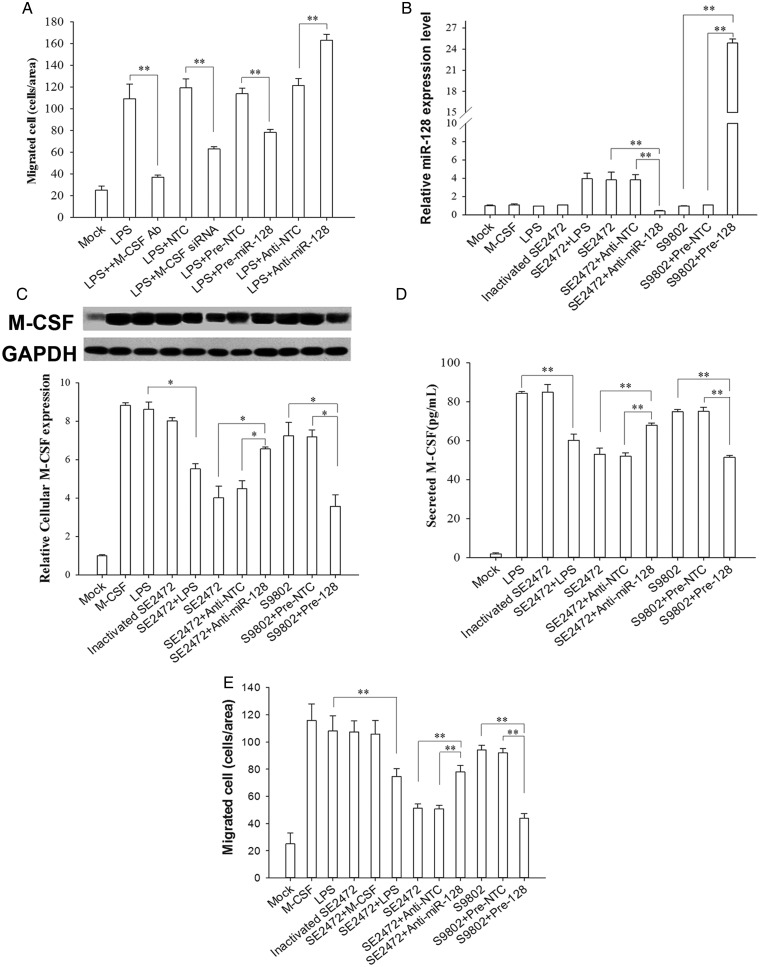
Because M-CSF can promote macrophage chemotaxis during foreign pathogen invasion [21], we studied M-CSF–induced migration of macrophages derived from U937 cells [22]. As shown in Figure 4A, the supernatant from LPS-treated cells strongly enhanced macrophage migration across Transwell filters. This enhancement was inhibited by anti-M-CSF antibody, suggesting that the increased macrophage transmigration was induced by M-CSF. The migration rate of macrophages induced by the supernatant derived from the cells treated with LPS and pre–miR-128 was also significantly decreased. In contrast, anti–miR-128 enhanced macrophage migration initiated by the supernatant from LPS-treated cells. Serving as a positive control, direct knockdown of M-CSF levels via M-CSF siRNA significantly decreased macrophage migration. These results demonstrate that epithelial miR-128 can affect macrophage migration by modulating the secretion of M-CSF.
Figure 4.
The effect of Salmonella infection–induced microRNA 128 (miR-128) on diminishing epithelial secreted macrophage colony-stimulating factor (M-CSF) and M-CSF–mediated macrophage transmigration. A, U937-differentiated macrophage transmigration induced by M-CSF–containing culture medium of lipopolysaccharide (LPS)–treated HT-29 cells. B, MiR-128 levels in HT-29 cells treated with M-CSF, LPS, strain SE2472, heat-inactivated strain SE2472, or strain S9802. C, M-CSF levels in HT-29 cells with different treatments. M-CSF expression is strongly increased in HT-29 cells stimulated with M-CSF, LPS, strain S9802, or heat-inactivated strain SE2472 but not strain SE2472. D, Secreted M-CSF levels in culture supernatants from HT-29 cells with different treatments. E, Macrophage transmigration induced by the supernatants from HT-29 cells with different treatments. Data are mean values ± standard error of the mean. *P < .05, **P < .01.
We next assessed the miR-128 level in HT-29 cells treated with various reagents. M-CSF, LPS, strain S9802, or heat-inactivated strain SE2472 did not affect miR-128 expression in HT-29 cells. miR-128 was upregulated (by approximately 4.0-fold) only when HT-29 cells were infected with live strain SE2472. As expected, SE2472-induced miR-128 elevation in HT-29 cells was reduced by anti–miR-128 ASO, whereas transfection of pre–miR-128 in S9802-infected HT-29 cells further increased miR-128 expression (Figure 4B). Simultaneously, levels of M-CSF in these HT-29 cells were assayed with Western blot. As shown in Figure 4C, compared with M-CSF levels in the mock-infection group, levels were upregulated in HT-29 cells administered with LPS, strain S9802, or heat-inactivated strain SE2472. Interestingly, treatment with live SE2472 did not yield an elevation of M-CSF in HT-29 cells induced by bacterial LPS. Introduction of anti–miR-128 ASO completely reversed the SE2472-mediated M-CSF reduction, whereas transfection with pre–miR-128 in strain S9802–infected cells further decreased M-CSF expression, suggesting that SE2472 infection may reduce host cell M-CSF expression by increasing cellular miR-128 levels. We also detected the level of M-CSF secreted by host cells and found that secreted M-CSF levels were also upregulated in cells exposed to LPS, strain S9802, or heat-inactivated strain SE2472. The level of secreted M-CSF was reduced by live SE2472 compared that in other groups (heat-inactivated strain SE2472 and LPS plus strain SE2472). To test whether the secreted M-CSF levels from HT-29 cells are inversely regulated by miR-128, we further transfected SE2472-infected HT-29 cells with anti–miR-128 ASO and found that the secreted M-CSF levels were increased. In contrast, overexpression of miR-128 in strain S9802–infected cells reduced the levels of secreted M-CSF.
Finally, we assessed macrophage transmigration induced by various conditioned media in HT-29 cell culture. Before the experiments, the supernatants of cultured HT-29 cells were filtered through 0.22-µm-pore filters to remove bacterial cells. Compared with the control group, in which only a few macrophages migrated across the membranes, macrophage transmigration was strongly induced by the supernatants from cultured HT-29 cells exposed to LPS, strain S9802, or heat-inactivated strain SE2472 (Figure 4E). In contrast, a significantly smaller number of transmigrated macrophages was observed in the group treated with the supernatant from live strain SE2472–infected cells. As expected, macrophage transmigration in the group treated with live SE2472-infected cell supernatant was significantly enhanced when HT-29 cells were cotransfected with anti–miR-128 ASO. In contrast, macrophage transmigration in the group treated with strain S9802–infected cell supernatant was strongly decreased when HT-29 cells were cotransfected with pre–miR-128. Taken together, these results suggest that the downregulation of macrophage transmigration is dependent on the upregulation of miR-128 and reduction of M-CSF in epithelial cells.
p53 Is Involved in Salmonella Strain SE2472 Secreted Protein–Mediated miR-128 Upregulation
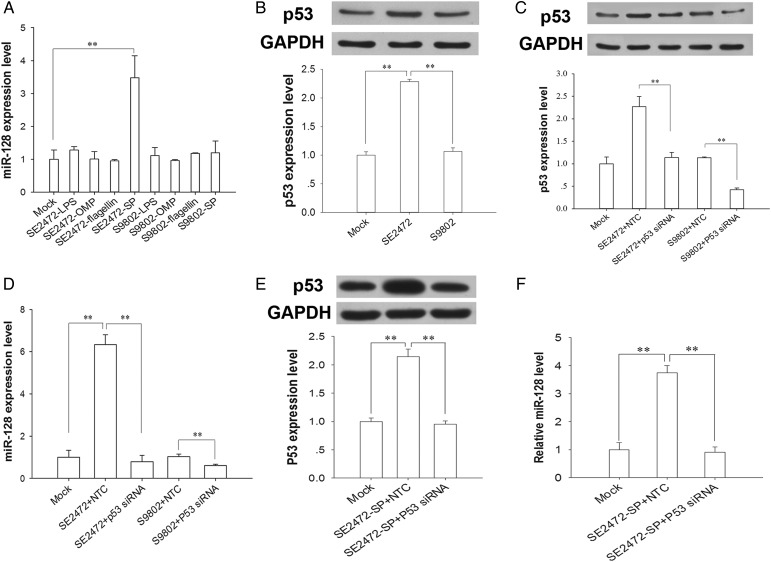
To determine which Salmonella component can stimulate miR-128 expression, we prepared different components from strains SE2472 or S9802 and then treated HT-29 cells with individual components. As shown in Figure 5A, only secreted proteins from strain SE2472 can induce miR-128 upregulation, arguing that secreted proteins from virulent Salmonella strain SE2472 may be the factor to induce miRNA expression in the host cells. As a regulator of miRNAs, p53 has been shown to modulate the processing of many miRNAs [23]. Because bioinformatics analysis also predicted the binding of p53 to the putative promoter of miR-128, the p53 level in HT-29 cells infected with strain SE2472 or strain S9802 was assayed. The results clearly showed that the p53 level was elevated in strain SE2472–treated but not strain S9802–treated cells (Figure 5B). By depleting p53 via p53 siRNA, we found that the decrease in p53 level led to a strong reduction of miR-128 expression in the strain SE2472–infected and strain S9802–infected groups (Figure 5C and 5D), suggesting that endogenous p53 is involved in miR-128 expression during Salmonella infection. Furthermore, we determined the effect of SE2472 secreted proteins on miR-128 expression after p53 knockdown via p53 siRNA. As shown in Figure 5E and 5F, the upregulation of miR-128 by strain SE2472 secreted proteins was also attenuated in p53-knockdown cells.
Figure 5.
The correlation between the p53 level and miR-128 expression in intestinal epithelial cells. A, Expression of microRNA 128 (miR-128) in HT-29 cells with different bacterial component extracts. B, Expression of p53 in HT-29 cells infected by Salmonella strain SE2472 or S9802. C, p53 knockdown via p53 RNA interference in HT-29 cells infected by strains SE2472 or S9802. D, Effect of p53 knockdown on miRNA-128 expression in HT-29 cells infected by strains SE2472 or S9802. E and F, Effect of p53 knockdown on miRNA-128 expression in HT-29 cells treated with Salmonella strain SE2472 secreted proteins. Data are mean values ± standard error of the mean. **P < .01.
Because the NF-κB signal pathway is often activated during bacterial infection and NF-κB has been shown to regulate the expression of many miRNAs [24–26], we also tested whether strain SE2472 secreted protein–mediated epithelial miR-128 upregulation occurs through the NF-κB pathway. As shown in Supplementary Figure 1A and 1B, suppressing NF-κB signal via NF-κB siRNA did not affect the cellular miR-128 level after strain SE2472 infection, suggesting that NF-κB may not play a major role in SE2472 secreted protein–induced miR-128 expression in intestinal epithelial cells. In agreement with this, our results also showed that the M-CSF level was not affected by NF-κB depletion (Supplementary Figure 1C–E).
Preventing Salmonella Infection by Depleting Epithelial miR-128
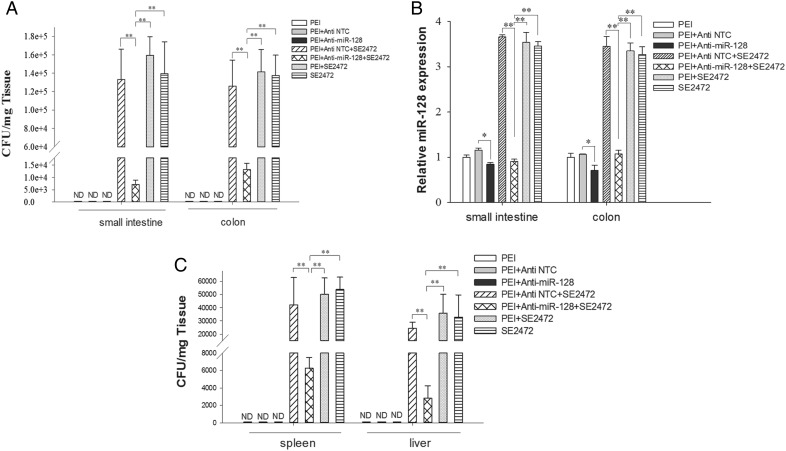
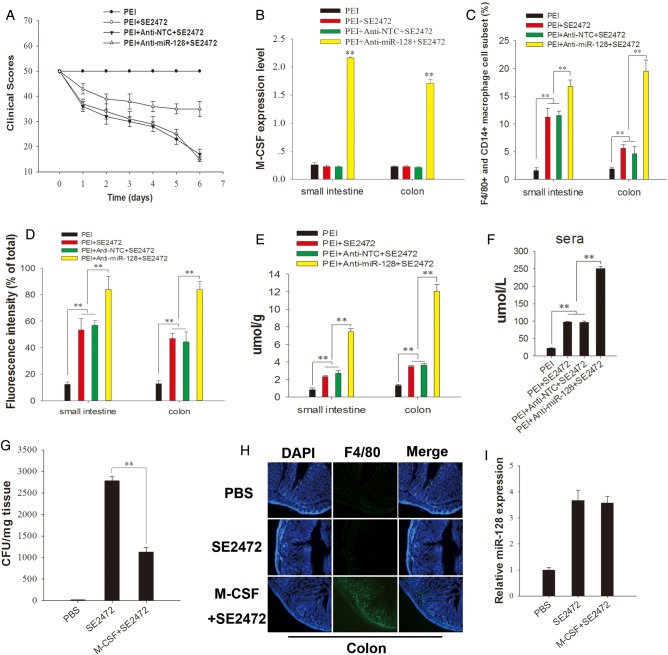
To test the role of miR-128–targeting M-CSF in macrophage recruitment in vivo, we infected female Balb/c mice intragastrically with strain SE2472 (6 × 105 CFU/mL). During infection, anti–miR-128 ASO was delivered into mice intragastrically by PEI as delivery vehicles [27]. On day 6 after infection, the mice were euthanized, and the small intestine, colon, spleen, and liver were collected. As shown in Figure 6, the level of infection by strain SE2472 was largely reduced in the PEI/anti–miR-128 group. The clinical scores of the mice were assessed by visual examination for motility, ruffled fur hunched position, feeding, ataxia, and tremor (Figure 7A). As shown in Figure 7B and Supplementary Figure 2A and 2B, M-CSF levels were strongly increased in the anti–miR-128 ASO-treated group, compared with the groups treated with strain SE2472 plus anti–miR-128 NTC or PEI, suggesting that SE2472 infection decreases M-CSF expression by increasing miR-128 levels. The number of F4/80+ and CD14+ macrophage subsets was dramatically increased in the strain SE2472–infected group treated with anti–miR-128 ASO, compared with that in groups treated with strain SE2472 plus anti–miR-128 NTC or PEI (Figure 7C and Supplementary Figure 2C and 2E). In addition, treatment with anti–miR-128 ASO also significantly increased the production of reactive oxygen species by macrophages in the intestine and colon section of strain SE2472–infected mice (Figure 7D and Supplementary Figure 2D and 2F), compared to groups treated with anti–miR-128 NTC or PEI. Interestingly, by increasing M-CSF level in colon tissue through direct delivery of recombinant M-CSF into mouse colon during strain SE2472 infection, a large number of infiltrated macrophages were detected in the colon, accompanied by an approximately 50% reduction in the number of surviving bacteria (Figure 7G and 7H). As expected, the miR-128 level in mouse colon tissue was not affected by the return of M-CSF (Figure 7I). These results strongly confirm the role of M-CSF in modulating macrophage recruitment and bacterial clearance during Salmonella infection.
Figure 6.
The protective role of PEI delivery of anti–microRNA 128 (miR-128) ASO against Salmonella infection in mice. Mice were inoculated intragastrically with Salmonella and synthetic RNA molecules (5 × 106 colony-forming units/per mice) with or without PEI. The control mouse group received PEI only. The mice were euthanized on day 6 after infection. A, Salmonella counts in mouse small intestine and colon tissues. B, miR-128 levels in the mouse small intestine and colon tissues. C, Salmonella bacteria count in the mouse spleen and liver tissues. Data are mean values ± standard error of the mean. **P < .01. Abbreviation: ND, no Salmonella detected.
Figure 7.
Salmonella infection in mice. A, The clinical scores of mice after Salmonella infection. B, Macrophage colony-stimulating factor (M-CSF) levels in mouse small intestine and colon tissues analyzed through immunolabeling. C, New recruitment of F4/80+ and CD14+ double-positive macrophages analyzed through flow cytometry (Supplementary Figure 2C and 2E). D, Reactive oxygen species production in F4/80+ and CD14+ double-positive macrophages from mouse small intestine and colon (Supplementary Figure 2D and 2F) analyzed with dye (DCFH-DA). E and F, NO production in small intestine and colon (E) or sera (F). G, Salmonella bacteria counts in the mouse colon. H, Recruitment of macrophages in mouse colon analyzed by immunohistochemistry. I, Expression level of microRNA 128 (miR-128) in mouse colon. Mice were infected with strain SE2472, and M-CSF (50 µg) was directly delivered into mouse colon every other day. Data are mean values ± standard error of the mean. **P < .01. Abbreviation: PBS, phosphate-buffered saline.
DISCUSSION
The role of miRNAs in pathogen-host interactions is beginning to be investigated recently [28–30]. Alteration of the host cell miRNA expression profile by Salmonella may serve as an efficient way to modulate the host cell immune response, which allows immune escape of invaded Salmonella. This hypothesis has been supported by many recent studies. Schulte et al [15] identified the let-7 family as the common denominator of Salmonella-regulated miRNAs in macrophages and epithelial cells and that repression of let-7 could relieve cytokine IL-10 mRNAs. Here, we reported that Salmonella infection induced significant alteration of epithelial cell miRNA expression, and the virulence of the Salmonella strain might be dependent on its capacity to induce the expression of miR-128 in the infected epithelium. A regulatory role for miR-128 has been reported in glioma cell proliferation [31], neuroblastoma cell motility and invasiveness [32], and cell motility in ovarian cancer cells [33]. Together with our finding of miR-128 targeting epithelial cell M-CSF, the studies show that miR-128 may have different function under various pathophysiological conditions.
Salmonella has evolved various strategies to subvert host innate or adaptive immune responses through virulence factors [34, 35]. In this study, we reported a novel pathway through which Salmonella could manipulate host miRNA expression to build a suitable environment for bacterial invasion and survival. Through miRNA microarray and quantitative real-time PCR analyses, we found that epithelial miR-128 was strongly upregulated by the virulent strain SE2472 but not the less virulent strain S9802 (Figure 2). Although the mechanism by which SE2472 controls host miRNA expression is unknown, our data suggest that at least specific secreted protein from strain SE2472 (virulent) but not strain S9802 (less virulent) is responsible for epithelial miR-128 upregulation. We tested the effects of various extracts (such as LPS, OMPs, flagellin, and secreted proteins) from both strains (SE2472 and S9802) on epithelial miR-128 expression and found that only secreted proteins from strain SE2472 increased the level of miR-128 (Figure 5A). Furthermore, strain SE2472 affected host P53 expression, whereas strain S9802 did not (Figure 5B). The relationship between p53 and miR-128 during Salmonella infection (Figure 5C and 5D) or treatment with secreted proteins from strain SE2472 (Figure 5E and 5F) further indicated that miR-128 expression is correlated with secreted proteins.
Macrophages are phagocytes with a major role in the clearance of invading bacteria, including Salmonella, and activation and recruitment of macrophages requires various cytokines. Macrophage recruitment to the inflammation site can be induced by various chemoattractants or proinflammatory factors, including M-CSF [36], MCP-1 [37], and MIP-1α/CCL3 [38]. A previous study by Hoi et al [36] showed that epithelial cell–secreted M-CSF served as a chemoattractant for macrophage recruitment. Here, we identified the gene encoding M-CSF as a target of miR-128. Because the level miR-128 in colonic epithelial cells was specifically increased after exposure to strain SE2472, the levels of epithelial cellular M-CSF and secreted M-CSF by these epithelial cells would decrease. Therefore, the M-CSF–mediated subsequent recruitment of macrophages would be attenuated. In conclusion, the present study provides a novel miRNA-based mechanism that may play a part in modulating Salmonella immune evasion during the infection process.
The regulatory role of miR-128 in M-CSF expression and M-CSF recruitment of macrophages was further validated in a mouse infection model. The results from PEI delivery of anti–miR-128 ASO demonstrated a link between the induction of epithelial miR-128 and the virulence of the Salmonella strain. When colonic epithelial miR-128 was depleted by anti–miR-128 ASO (Figure 6B), the infection of strain SE2472 in mouse small intestine and colon tissues (Figure 6A) and in spleen and liver (Figure 6C) was significantly attenuated. In this model, infection with the virulent Salmonella strain specifically increased epithelial cell miR-128 expression, which, in turn, downregulated the level of M-CSF secreted from epithelial cells and consequently reduced M-CSF–mediated macrophage recruitment. In addition, reactive oxygen species and NO production was further increased in strain SE2472–infected mice when treated with anti–miR-128 ASO (Figure 7D–F), indicating that activation of macrophages may be occur correspondingly. Although the cytokines TNF-α and IL-6 (as controls) were also induced during Salmonella infection, they were not affected by treatment with anti–miR-128 ASO or pre–miR-128, which is in agreement with the finding that these cytokines are not targeted by miR-128 (data not shown). Because mouse infection by virulent Salmonella can be effectively attenuated by PEI delivery of anti–miR-128 ASO, delivery of anti–miR-128 ASO may be a novel approach for controlling Salmonella infection.
In conclusion, the present study provided the first evidence that Salmonella infection can suppress the host immune responses through upregulating host cell miR-128, which, in turn, decreases the secretion of M-CSF by host cells and the M-CSF–mediated recruitment of macrophages.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment. We thank Dr Jieming Pan (Yangzhou University, Jiangsu, China), for providing Salmonella strains.
Financial support. This work was supported by the National Basic Research Program of China (grants 2012CB517603 and 2011CB504803863), the National Natural Science Foundation of China (grants 30988003, 30890044, 30800946, 30871019, 30890032, 31071232, and 31000323), the Research Foundation for Doctoral Programs in China (grants 20100091120026 and 20100091120023), and the Natural Science Foundation of Jiangsu Province (grant BK2011013), and Fundamental Research Funds for the Central Universities (grants 1117020803 and 1095020823).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ben Aissa R, Al-Gallas N. Molecular typing of Salmonella enterica serovars Enteritidis, Corvallis, Anatum and Typhimurium from food and human stool samples in Tunisia, 2001–2004. Epidemiol Infect. 2008;136:468–75. doi: 10.1017/S0950268807008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graziani C, Mughini-Gras L, Owczarek S, Dionisi A, Luzzi I, Busani L. Distribution of Salmonella enterica isolates from human cases in Italy, 1980 to 2011. Euro Surveill. 2013;18:1–9. [PubMed] [Google Scholar]
- 3.Agbor TA, McCormick BA. Salmonella effectors: important players modulating host cell function during infection. Cell Microbiol. 2011;13:1858–69. doi: 10.1111/j.1462-5822.2011.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallstrom K, McCormick BA. Salmonella interaction with and passage through the intestinal mucosa: through the lens of the organism. Front Microbiol. 2011;2:88. doi: 10.3389/fmicb.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miao EA, Leaf IA, Treuting PM, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–42. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchiya K, Nikai T. Salmonella enterica serovar Typhimurium infection induces cyclooxygenase 2 expression in macrophages: involvement of Salmonella pathogenicity island 2. Infect Immun. 2004;72:6860–9. doi: 10.1128/IAI.72.12.6860-6869.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otero K, Turnbull IR, Poliani PL, et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat Immunol. 2009;10:734–43. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 10.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Tsitsiou E, Lindsay MA. microRNAs and the immune response. Curr Opin Pharmacol. 2009;9:514–20. doi: 10.1016/j.coph.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson E, Tremaroli V, Lee YS, et al. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut. 2012;61:1124–31. doi: 10.1136/gutjnl-2011-301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masotti A. Interplays between gut microbiota and gene expression regulation by miRNAs. Front Cell Infect Microbiol. 2012;2:137. doi: 10.3389/fcimb.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang P, Hou J, Lin L, et al. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185:6226–33. doi: 10.4049/jimmunol.1000491. [DOI] [PubMed] [Google Scholar]
- 15.Schulte LN, Eulalio A, Mollenkopf HJ, Reinhardt R, Vogel J. Analysis of the host microRNA response to Salmonella uncovers the control of major cytokines by the let-7 family. EMBO J. 2011;30:1977–1989. doi: 10.1038/emboj.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su J, Gong H, Lai J, Main A, Lu S. The potassium transporter Trk and external potassium modulate Salmonella enterica protein secretion and virulence. Infect Immun. 2009;77:667–75. doi: 10.1128/IAI.01027-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu S, Killoran PB, Riley LW. Association of Salmonella enterica serovar enteritidis yafD with resistance to chicken egg albumen. Infect Immun. 2003;71:6734–41. doi: 10.1128/IAI.71.12.6734-6741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsang WP, Ho FY, Fung KP, Kong SK, Kwok TT. p53-R175H mutant gains new function in regulation of doxorubicin-induced apoptosis. Int J Cancer. 2005;114:331–6. doi: 10.1002/ijc.20818. [DOI] [PubMed] [Google Scholar]
- 19.Smith PD, Smythies LE, Shen R, Greenwell-Wild T, Gliozzi M, Wahl SM. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4:31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanamura T, Asakura E, Tanabe T. Macrophage colony-stimulating factor (M-CSF) augments cytokine induction by lipopolysaccharide (LPS)-stimulation and by bacterial infections in mice. Immunopharmacology. 1997;37:15–23. doi: 10.1016/s0162-3109(96)00166-x. [DOI] [PubMed] [Google Scholar]
- 21.Vedham V, Phee H, Coggeshall KM. Vav activation and function as a rac guanine nucleotide exchange factor in macrophage colony-stimulating factor-induced macrophage chemotaxis. Mol Cell Biol. 2005;25:4211–20. doi: 10.1128/MCB.25.10.4211-4220.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shelley CS, Teodoridis JM, Park H, Farokhzad OC, Bottinger EP, Arnaout MA. During differentiation of the monocytic cell line U937, Pur alpha mediates induction of the CD11c beta 2 integrin gene promoter. J Immunol. 2002;168:3887–93. doi: 10.4049/jimmunol.168.8.3887. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–33. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 24.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou R, Hu G, Liu J, Gong AY, Drescher KM, Chen XM. NF-kappaB p65-dependent transactivation of miRNA genes following Cryptosporidium parvum infection stimulates epithelial cell immune responses. PLoS Pathog. 2009;5:e1000681. doi: 10.1371/journal.ppat.1000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Xiao B, Tang B, et al. Up-regulated microRNA-146a negatively modulate Helicobacter pylori-induced inflammatory response in human gastric epithelial cells. Microbes Infect. 2010;12:854–63. doi: 10.1016/j.micinf.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Zuo L, Huang Z, Dong L, et al. Targeting delivery of anti-TNFalpha oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut. 2010;59:470–9. doi: 10.1136/gut.2009.184556. [DOI] [PubMed] [Google Scholar]
- 28.Dalmasso G, Nguyen HT, Yan Y, et al. Microbiota modulate host gene expression via microRNAs. PLoS One. 2011;6:e19293. doi: 10.1371/journal.pone.0019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaser A, Niederreiter L, Blumberg RS. Genetically determined epithelial dysfunction and its consequences for microflora-host interactions. Cell Mol Life Sci. 2011;68:3643–9. doi: 10.1007/s00018-011-0827-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh Y, Kaul V, Mehra A, et al. Mycobacterium tuberculosis controls microRNA-99b (miR-99b) expression in infected murine dendritic cells to modulate host immunity. J Biol Chem. 2013;288:5056–61. doi: 10.1074/jbc.C112.439778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Chao T, Li R, et al. MicroRNA-128 inhibits glioma cells proliferation by targeting transcription factor E2F3a. J Mol Med (Berl) 2009;87:43–51. doi: 10.1007/s00109-008-0403-6. [DOI] [PubMed] [Google Scholar]
- 32.Evangelisti C, Florian MC, Massimi I, et al. MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB J. 2009;23:4276–87. doi: 10.1096/fj.09-134965. [DOI] [PubMed] [Google Scholar]
- 33.Woo HH, Laszlo CF, Greco S, Chambers SK. Regulation of colony stimulating factor-1 expression and ovarian cancer cell behavior in vitro by miR-128 and miR-152. Mol Cancer. 2012;11:58. doi: 10.1186/1476-4598-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diacovich L, Gorvel JP. Bacterial manipulation of innate immunity to promote infection. Nat Rev Microbiol. 2010;8:117–28. doi: 10.1038/nrmicro2295. [DOI] [PubMed] [Google Scholar]
- 35.McGhie EJ, Brawn LC, Hume PJ, Humphreys D, Koronakis V. Salmonella takes control: effector-driven manipulation of the host. Curr Opin Microbiol. 2009;12:117–24. doi: 10.1016/j.mib.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoi AY, Hickey MJ, Hall P, et al. Macrophage migration inhibitory factor deficiency attenuates macrophage recruitment, glomerulonephritis, and lethality in MRL/lpr mice. J Immunol. 2006;177:5687–96. doi: 10.4049/jimmunol.177.8.5687. [DOI] [PubMed] [Google Scholar]
- 37.Khan WI, Motomura Y, Wang H, et al. Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol. 2006;291:G803–11. doi: 10.1152/ajpgi.00069.2006. [DOI] [PubMed] [Google Scholar]
- 38.DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J Clin Invest. 1998;101:1693–8. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.